Abstract
The induction of focus formation in low serum and of neoplastic transformation of Syrian hamster embryo cells was examined after the expression of herpes simplex virus type 2 functions. Syrian hamster embryo cells infected at a high multiplicity (5 PFU/cell) with 5-bromo-2'-deoxyuridine-labeled herpes simplex virus type 2 (11% substitution of thymidine residues) were exposed to near UV light irradiation at various times postinfection. This procedure specifically inactivated the viral genome, while having little, if any, effect on the unlabeled cellular DNA. Focus formation in 1% serum and neoplastic transformation were observed in cells exposed to virus inactivated before infection, but the frequency was enhanced (15- to 27-fold) in cells in which the virus was inactivated at 4 to 8 h postinfection. Only 2 to 45 independently isolated foci were capable of establishing tumorigenic lines. The established lines exhibited phenotypic alterations characteristic of a transformed state, including reduced serum requirement, anchorage-independent growth, and tumorigenicity. They retained viral DNA sequences and, even at relatively late passage, expressed viral antigens, including ICP 10.
Full text
PDF


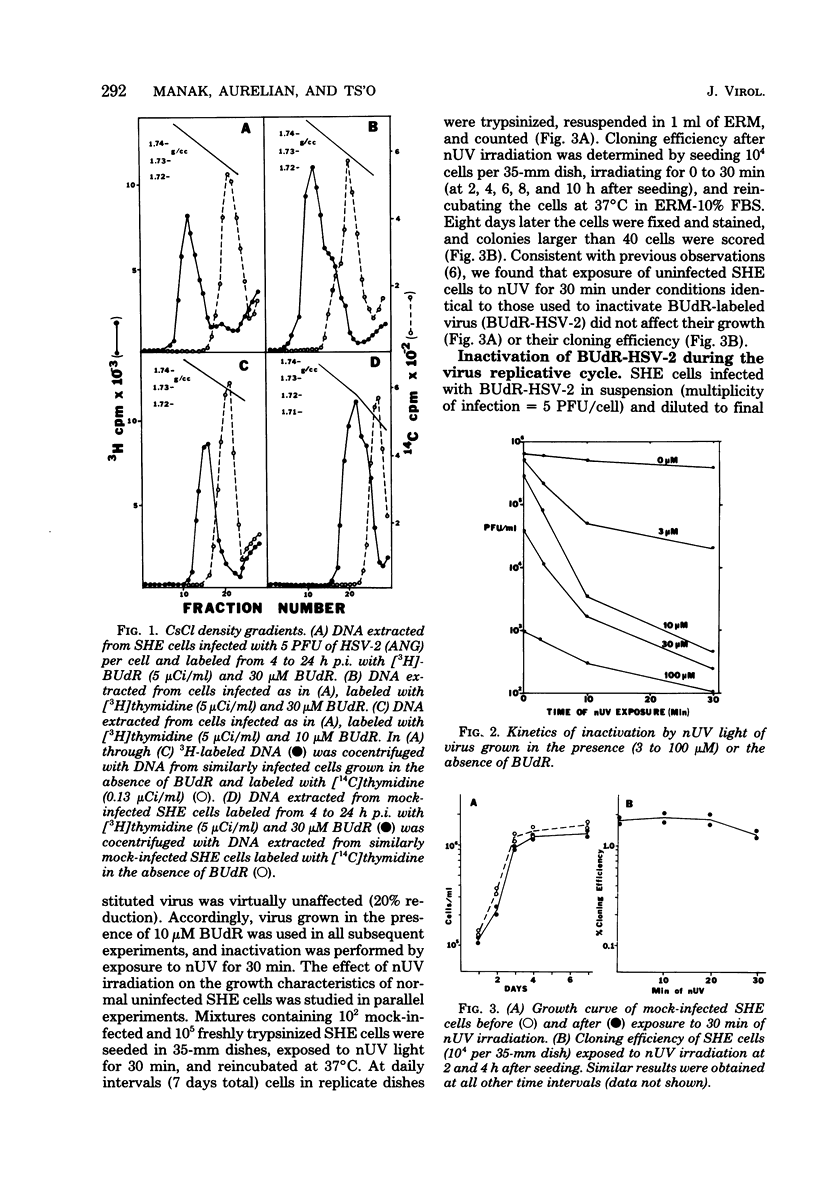
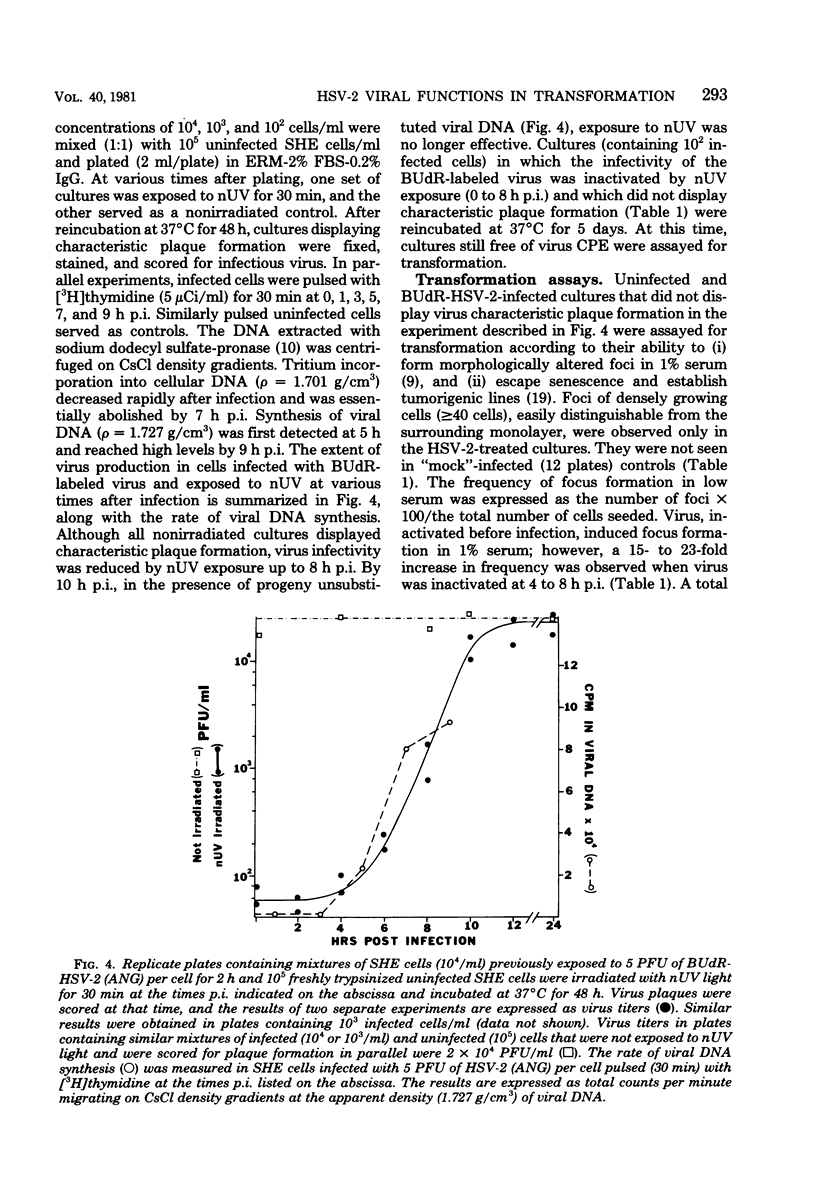




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aurelian L. Demonstration by plaque reduction technique of immunologic relationship between canine herpes virus and herpes simplex virus. Proc Soc Exp Biol Med. 1968 Feb;127(2):485–488. doi: 10.3181/00379727-127-32721. [DOI] [PubMed] [Google Scholar]
- Aurelian L., Smith M. F., Cornish D. J. IgM antibody to a tumor-associated antigen (AG-4) induced by herpes simplex virus type 2: its use in location of the antigen in infected cells. J Natl Cancer Inst. 1976 Mar;56(3):471–477. doi: 10.1093/jnci/56.3.471. [DOI] [PubMed] [Google Scholar]
- Aurelian L., Strnad B. C., Smith M. F. Immunodiagnostic potential of a virus-coded, tumor-associated antigen (AG-4) in cervical cancer. Cancer. 1977 Apr;39(4 Suppl):1834–1849. doi: 10.1002/1097-0142(197704)39:4+<1834::aid-cncr2820390816>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Ts'o P. O. Evidence for the progressive nature of neoplastic transformation in vitro. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3761–3765. doi: 10.1073/pnas.75.8.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Tsutsui T., Ts'o P. O. Neoplastic transformation induced by a direct perturbation of DNA. Nature. 1978 Jul 20;274(5668):229–232. doi: 10.1038/274229a0. [DOI] [PubMed] [Google Scholar]
- Ben-Porat T., Tokazewski S. A. Replication of herpesvirus DNA. II. Sedimentation characteristics of newly synthesized DNA. Virology. 1977 Jun 15;79(2):292–301. doi: 10.1016/0042-6822(77)90356-7. [DOI] [PubMed] [Google Scholar]
- Brandsma J., Miller G. Nucleic acid spot hybridization: rapid quantitative screening of lymphoid cell lines for Epstein-Barr viral DNA. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6851–6855. doi: 10.1073/pnas.77.11.6851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casto B. C., Janosko N., DiPaolo J. A. Development of a focus assay model for transformation of hamster cells in vitro by chemical carcinogens. Cancer Res. 1977 Oct;37(10):3508–3515. [PubMed] [Google Scholar]
- Chen M. S., Ward D. C., Prusoff W. H. Specific herpes simplex virus-induced incorporation of 5-iodo-5'-amino-2',5'-dideoxyuridine into deoxyribonucleic acid. J Biol Chem. 1976 Aug 25;251(16):4833–4838. [PubMed] [Google Scholar]
- Cleaver J. E. Repair replication and degradation of bromouracil-substituted DNA in mammalian cells after irradiation with ultraviolet light. Biophys J. 1968 Jul;8(7):775–791. doi: 10.1016/S0006-3495(68)86520-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R. Topoinhibition and serum requirement of transformed and untransformed cells. Nature. 1970 Aug 22;227(5260):802–806. doi: 10.1038/227802a0. [DOI] [PubMed] [Google Scholar]
- Eckhart W. Complementation between temperature-sensitive (ts) and host range nontransforming (hr-t) mutants of polyoma virus. Virology. 1977 Apr;77(2):589–597. doi: 10.1016/0042-6822(77)90484-6. [DOI] [PubMed] [Google Scholar]
- Graham F. L., Harrison T., Williams J. Defective transforming capacity of adenovirus type 5 host-range mutants. Virology. 1978 May 1;86(1):10–21. doi: 10.1016/0042-6822(78)90003-x. [DOI] [PubMed] [Google Scholar]
- Hirsch I., Roubal J., Vonka V. Replicating DNA of herpes simplex virus type 1. Intervirology. 1976;7(3):155–175. doi: 10.1159/000149949. [DOI] [PubMed] [Google Scholar]
- Honess R. W., Roizman B. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J Virol. 1974 Jul;14(1):8–19. doi: 10.1128/jvi.14.1.8-19.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson F. The lesions produced by ultraviolet light in DNA containing 5-bromouracil. Q Rev Biophys. 1973 May;6(2):201–246. doi: 10.1017/s0033583500001141. [DOI] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Neoplastic transformation of cultured Syrian hamster embryo cells by DNA of herpes simplex virus type 2. J Virol. 1979 Apr;30(1):404–409. doi: 10.1128/jvi.30.1.404-409.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jariwalla R. J., Aurelian L., Ts'o P. O. Tumorigenic transformation induced by a specific fragment of DNA from herpes simplex virus type 2. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2279–2283. doi: 10.1073/pnas.77.4.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN A. S. STUDIES ON THE REPLICATING POOL OF VIRAL DNA IN CELLS INFECTED WITH PSEUDORABIES VIRUS. Virology. 1964 Sep;24:19–25. doi: 10.1016/0042-6822(64)90143-6. [DOI] [PubMed] [Google Scholar]
- Kieff E. D., Bachenheimer S. L., Roizman B. Size, composition, and structure of the deoxyribonucleic acid of herpes simplex virus subtypes 1 and 2. J Virol. 1971 Aug;8(2):125–132. doi: 10.1128/jvi.8.2.125-132.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura S., Flannery V. L., Levy B., Schaffer P. A. Oncogenic transformation of primary hamster cells by herpes simplex virus type 2 (hsv-2) and an hsv-2 temperature-sensitive mutant. Int J Cancer. 1975 May 15;15(5):786–798. doi: 10.1002/ijc.2910150510. [DOI] [PubMed] [Google Scholar]
- Kucera L. S., Edwards I. Herpes simplex virus type 2 functions expressed during stimulation of human cell DNA synthesis. J Virol. 1979 Jan;29(1):83–90. doi: 10.1128/jvi.29.1.83-90.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiden J. M., Frenkel N., Rapp F. Identification of the herpes simplex virus DNA sequences present in six herpes simplex virus thymidine kinase-transformed mouse cell lines. J Virol. 1980 Jan;33(1):272–285. doi: 10.1128/jvi.33.1.272-285.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk D. C., Bick M. D. Determination of 5'-bromodeoxyuridine in DNA by buoyant density. Anal Biochem. 1977 Feb;77(2):346–349. doi: 10.1016/0003-2697(77)90247-0. [DOI] [PubMed] [Google Scholar]
- MACPHERSON I., MONTAGNIER L. AGAR SUSPENSION CULTURE FOR THE SELECTIVE ASSAY OF CELLS TRANSFORMED BY POLYOMA VIRUS. Virology. 1964 Jun;23:291–294. doi: 10.1016/0042-6822(64)90301-0. [DOI] [PubMed] [Google Scholar]
- Macnab J. C., Visser L., Jamieson A. T., Hay J. Specific viral antigens in rat cells transformed by herpes simplex virus type 2 and in rat tumors induced by inoculation of transformed cells. Cancer Res. 1980 Jun;40(6):2074–2079. [PubMed] [Google Scholar]
- Meuth M., Green H. Induction of a deoxycytidineless state in cultured mammalian cells by bromodeoxyuridine. Cell. 1974 Jun;2(2):109–112. doi: 10.1016/0092-8674(74)90099-3. [DOI] [PubMed] [Google Scholar]
- Pignatti P. F., Cassai E., Meneguzzi G., Chenciner N., Milanesi G. Herpes simplex virus DNA isolation from infected cells with a novel procedure. Virology. 1979 Feb;93(1):260–264. doi: 10.1016/0042-6822(79)90295-2. [DOI] [PubMed] [Google Scholar]
- Rapp F., Turner N., Schaffer P. A. Biochemical transformation by temperature-sensitive mutants of herpes simplex virus type 1. J Virol. 1980 Jun;34(3):704–710. doi: 10.1128/jvi.34.3.704-710.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roufa D. J. 5-bromodeoxyuridine-DNA strand symmetry and the repair of photolytic breaks in Chinese hamster cell chromosomes. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3905–3909. doi: 10.1073/pnas.73.11.3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shlomai J., Friedmann A., Becker Y. Replication intermediates of herpes simplex virus DNA. Virology. 1976 Feb;69(2):647–659. doi: 10.1016/0042-6822(76)90493-1. [DOI] [PubMed] [Google Scholar]
- Stabel S., Doerfler W., Friis R. R. Integration sites of adenovirus type 12 DNA in transformed hamster cells and hamster tumor cells. J Virol. 1980 Oct;36(1):22–40. doi: 10.1128/jvi.36.1.22-40.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strnad B. C., Aurelian L. Proteins of herpesvirus type 2. II. Studies demonstrating a correlation between a tumor-associated antigen (AG-4) and a virion protein. Virology. 1976 Aug;73(1):244–258. doi: 10.1016/0042-6822(76)90078-7. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Aurelian L. Proteins of herpesvirus type 2. III. Isolation and immunologic characterization of a large molecular weight viral protein. Virology. 1978 Jun 15;87(2):401–415. doi: 10.1016/0042-6822(78)90144-7. [DOI] [PubMed] [Google Scholar]
- Strnad B. C., Aurelian L. Proteins of herpesvirus type 2: I. Virion, nonvirion, and antigenic polypeptides in infected cells. Virology. 1976 Feb;69(2):438–452. doi: 10.1016/0042-6822(76)90475-x. [DOI] [PubMed] [Google Scholar]
- Tafler S. W., Setlow P., Levine L. Serological relatedness of bacterial deoxyribonucleic acid polymerases. J Bacteriol. 1973 Jan;113(1):18–23. doi: 10.1128/jb.113.1.18-23.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui T., Barrett J. C., Ts'o P. O. Induction of 6-thioguanine- and ouabain-resistant mutations in synchronized Syrian hamster cell cultures during different periods of the S phase. Mutat Res. 1978 Nov;52(2):255–264. doi: 10.1016/0027-5107(78)90146-x. [DOI] [PubMed] [Google Scholar]
- WASSERMAN E., LEVINE L. Quantitative micro-complement fixation and its use in the study of antigenic structure by specific antigen-antibody inhibition. J Immunol. 1961 Sep;87:290–295. [PubMed] [Google Scholar]
- Walboomers J. M., Schegget J. T. A new method for the isolation of herpes simplex virus type 2 DNA. Virology. 1976 Oct 1;74(1):256–258. doi: 10.1016/0042-6822(76)90151-3. [DOI] [PubMed] [Google Scholar]
- Weinstock R., Sweet R., Weiss M., Cedar H., Axel R. Intragenic DNA spacers interrupt the ovalbumin gene. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1299–1303. doi: 10.1073/pnas.75.3.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]