Abstract
To elucidate the biosynthetic pathways of carotenoids, especially myxol 2′-glycosides, in cyanobacteria, Anabaena sp. strain PCC 7120 (also known as Nostoc sp. strain PCC 7120) and Synechocystis sp. strain PCC 6803 deletion mutants lacking selected proposed carotenoid biosynthesis enzymes and GDP-fucose synthase (WcaG), which is required for myxol 2′-fucoside production, were analyzed. The carotenoids in these mutants were identified using high-performance liquid chromatography, field desorption mass spectrometry, and 1H nuclear magnetic resonance. The wcaG (all4826) deletion mutant of Anabaena sp. strain PCC 7120 produced myxol 2′-rhamnoside and 4-ketomyxol 2′-rhamnoside as polar carotenoids instead of the myxol 2′-fucoside and 4-ketomyxol 2′-fucoside produced by the wild type. Deletion of the corresponding gene in Synechocystis sp. strain PCC 6803 (sll1213; 79% amino acid sequence identity with the Anabaena sp. strain PCC 7120 gene product) produced free myxol instead of the myxol 2′-dimethyl-fucoside produced by the wild type. Free myxol might correspond to the unknown component observed previously in the same mutant (H. E. Mohamed, A. M. L. van de Meene, R. W. Roberson, and W. F. J. Vermaas, J. Bacteriol. 187:6883-6892, 2005). These results indicate that in Anabaena sp. strain PCC 7120, but not in Synechocystis sp. strain PCC 6803, rhamnose can be substituted for fucose in myxol glycoside. The β-carotene hydroxylase orthologue (CrtR, Alr4009) of Anabaena sp. strain PCC 7120 catalyzed the transformation of deoxymyxol and deoxymyxol 2′-fucoside to myxol and myxol 2′-fucoside, respectively, but not the β-carotene-to-zeaxanthin reaction, whereas CrtR from Synechocystis sp. strain PCC 6803 catalyzed both reactions. Thus, the substrate specificities or substrate availabilities of both fucosyltransferase and CrtR were different in these species. The biosynthetic pathways of carotenoids in Anabaena sp. strain PCC 7120 are discussed.
Cyanobacteria synthesize carotenoids, as do all phototrophic organisms. The carotenoids in cyanobacteria are not limited to just the common carotenoids, such as β-carotene, but also include some unique ketocarotenoids and carotenoid glycosides, which are not found in higher plants (6). It has become evident from several recent studies that these unique carotenoids are different in different cyanobacterial species, and cyanobacteria can be classified into several groups based on their unique carotenoids (39). Members of the first group, which includes the genera Anabaena and Nostoc, contain β-carotene, keto derivatives of β-carotene such as echinenone, and myxol glycosides, but little or no zeaxanthin. Members of the second group, including Synechocystis sp. strain PCC 6803 and Thermosynechococcus elongatus strain BP-1, contain these carotenoids as well as zeaxanthin. Members of the third group, which includes the genera Synechococcus and Prochlorococcus, contain β-carotene, zeaxanthin, and nostoxanthin but lack both ketocarotenoids and carotenoid glycosides. Prochlorococcus also contains α-carotene, and the lycopene cyclases in these genera have homology to those of plants.
We recently identified the molecular structures of carotenoids in some Anabaena and Nostoc strains, and we proposed carotenogenesis pathways and genes (37, 38). In these genera, the major carotenoids were β-carotene and echinenone, and the polar carotenoids were myxol and 4-ketomyxol glycosides. These glycosides are limited to cyanobacteria and have not been reported for any other bacteria (6), whereas free myxol has been found in a strain of cyanobacteria, Anabaena variabilis ATCC 29413 (38), and in only two other bacteria, both of which are marine members of the Flavobacteriaceae, strain P99-31 (= MBIC 03313) (43) and strain YM6-073 (= MBIC 06409) (30). In Anabaena sp. strain PCC 7120 (also known as Nostoc sp. strain PCC 7120), the myxol and 4-ketomyxol glycosides are (3R,2′S)-myxol 2′-fucoside and (3S,2′S)-4-ketomyxol 2′-fucoside, respectively. The glycoside moiety of these compounds is α-l-fucose, although it was previously believed that rhamnose was the common glycosylating sugar in cyanobacteria (37, 39). We also found that A. variabilis ATCC 29413, a strain that is phylogenetically close to Anabaena sp. strain PCC 7120, contains free myxol and 4-hydroxymyxol but lacks myxol glycosides (38), while another strain of A. variabilis, IAM M-3, contains myxol 2′-fucoside and 4-ketomyxol 2′-fucoside (37).
The carotenoids in Synechocystis sp. strain PCC 6803 include β-carotene, echinenone, and zeaxanthin, as well as the myxol glycoside (3R,2′S)-myxol 2′-(2,4-di-O-methyl-α-l-fucoside) (36). Deletion mutants with mutations related to myxol 2′-dimethyl-fucoside synthesis have been produced (27-29), but the biosynthetic pathways of carotenoids, especially myxol glycosides, in cyanobacteria have not been fully characterized yet.
The functional identification of carotenogenesis enzymes is furthest along in Synechocystis sp. strain PCC 6803. In this strain, phytoene synthase (CrtB) produces phytoene (21), phytoene desaturase (CrtP) catalyzes a two-step desaturation to produce ζ-carotene (20), ζ-carotene desaturase (CrtQb) catalyzes another two-step desaturation to produce lycopene (4), and poly-cis-neurosporene and lycopene produced during desaturation are changed to all-trans forms by cis-carotene isomerase (CrtH) (5, 23). β-Carotene is converted to zeaxanthin by β-carotene hydroxylase (CrtR) (15, 22) and is converted to echinenone by β-carotene ketolase (CrtO) (11, 16). CrtR also converts deoxymyxol 2′-dimethyl-fucoside to myxol 2′-dimethyl-fucoside (15). A GDP-fucose synthase (WcaG) mutant cannot synthesize myxol 2′-dimethyl-fucoside (28). On the other hand, in Anabaena sp. strain PCC 7120, only the following three enzymes have been functionally identified: CrtQa (17, 18), whose amino acid sequence shows no similarity to other cyanobacterial CrtQb amino acid sequences, and two distinct β-carotene ketolases for conversion of β-carotene to echinenone (CrtO) and conversion of myxol 2′-fucoside to 4-ketomyxol 2′-fucoside (CrtW) (26). Myxol-synthesizing enzymes and fucosyltransferase have not been identified yet.
Fucosylation of myxol is catalyzed by fucosyltransferase, and this enzyme requires a nucleotide sugar, GDP-l-fucose, as a donor of fucose. GDP-l-fucose is known to be synthesized from GDP-mannose (2). GDP-mannose 4,6-dehydratase (EC 4.2.1.47) dehydrates GDP-mannose to form GDP-4-dehydro-6-deoxy-d-mannose. The next two steps, epimerization and an NADPH-dependent reduction with resulting formation of GDP-fucose, are catalyzed by the bifunctional enzyme GDP-fucose synthase (EC 1.1.1.271). The genes encoding GDP-mannose 4,6-dehydratase and GDP-fucose synthase have been cloned from several organisms, and the genes from Escherichia coli are designated gmd and wcaG, respectively (25, 32). Homologous gmd and wcaG genes have been found in Anabaena sp. strain PCC 7120 (all4828 and all4826, respectively) and Synechocystis sp. strain PCC 6803 (sll1212 and sll1213, respectively), using gene designations from the CyanoBase database (http://bacteria.kazusa.or.jp/cyanobase/).
Elucidation of the cyanobacterial carotenogenesis pathways and comparison of these pathways in different species are essential for understanding the diversity of carotenoids. Although Anabaena sp. strain PCC 7120 and Synechocystis sp. strain PCC 6803 are two of the most commonly investigated cyanobacteria and their genome sequences are complete, their carotenogenesis pathways appear to have interesting differences that may lead to insights regarding carotenoid biogenesis. We previously proposed carotenogenesis genes for these species based on their genome sequences, specific mutants, and the identification of carotenoids in wild-type and mutant strains (27-29, 36, 37, 39). In this study, we constructed and/or analyzed deletion mutants with mutations in proposed crt genes and wcaG. An unexpected result was the difference in substrate specificities or substrate availabilities of carotenogenesis enzymes between Anabaena sp. strain PCC 7120 and Synechocystis sp. strain PCC 6803.
MATERIALS AND METHODS
Bacterial species and cultivation.
Anabaena sp. strain PCC 7120 (also known as Nostoc sp. strain PCC 7120) and mutants of this strain were grown in BG-11 medium with continuous shaking (110 rpm) at 26 to 28°C under continuous illumination with white fluorescent light (30 to 40 μmol photons·m−2·s−1 photosynthetically active radiation) for 2 weeks as described previously (37). Cultured cells in the stationary state were collected by centrifugation and stored at −30°C. Synechocystis sp. strain PCC 6803 and a mutant of this strain were cultured as described previously (28), and freeze-dried cells were shipped to Japan for carotenoid analysis.
E. coli strains were maintained in Luria-Bertani medium at 37°C. E. coli strain XL1-Blue MRF′ (Stratagene, United States) was used for plasmid maintenance, and strain HB101 containing pRL623 (10) was used as a conjugal donor.
Antibiotics were added at the following concentrations: for Anabaena mutants, 5 μg/ml erythromycin and 30 μg/ml neomycin; and for E. coli strains, 100 μg/ml ampicillin, 100 μg/ml chloramphenicol, 100 μg/ml erythromycin, and 50 μg/ml kanamycin.
Construction of deletion mutants.
Sequence information for the genomic DNA from Anabaena sp. strain PCC 7120 (14) and Synechocystis sp. strain PCC 6803 (13) are available in the CyanoBase database.
Mutants of Anabaena sp. strain PCC 7120 were constructed by triparental mating (8, 10) as described elsewhere (24). The target genes used in this study are summarized in Table 1, and the locations of the interruptions or deletions in the mutants are shown in Fig. 1.
TABLE 1.
Predicted cyanobacterial orthologues of carotenoid biosynthesis genesa
| Gene | Enzyme | Orthologue in Anabaena sp. strain PCC 7120 | Orthologue in Synechocystis sp. strain PCC 6803c |
|---|---|---|---|
| wcaGb | GDP-l-Fucose synthase | all4826 | sll1213 |
| crtR | β-Carotene hydroxylase | alr4009 | sll1468 |
| crtD | Methoxyneurosporene desaturase | all5123 | slr1293 |
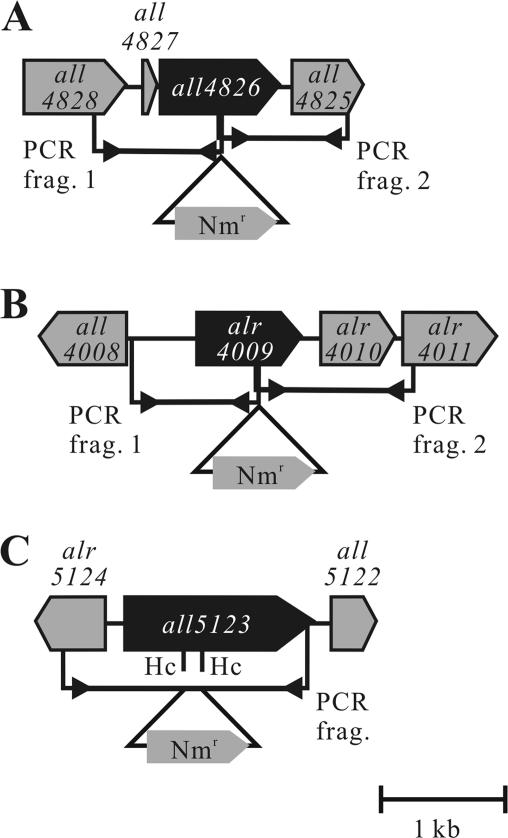
FIG. 1.
Gene constructs used for generation of mutants: interruption-deletion strategy used for double-crossover mutants with mutations in (A) all4826, (B) alr4009, and (C) all5123. The target genes are indicated by black boxes. PCR frag., PCR fragment(s); Hc, HincII site.
Genomic DNA was isolated from Anabaena sp. strain PCC 7120 by the glass bead method (40). Appropriate parts of the target genes and flanking regions (Fig. 1) were amplified by PCR using KOD-Plus polymerase (Toyobo, Japan) and the primers described in Table S1 in the supplemental material. The all4826 and alr4009 double-crossover mutants were obtained by using the same protocol that was used for construction of the crtO and crtW deletion mutants described previously (26).
To obtain the all5123 double-crossover mutant, an approximately 2-kb PCR product of the gene was ligated into the EcoRV site of pBluescript II SK(+) (Stratagene) to form p5123. The resulting plasmid was partially digested with HincII, and a 1.1-kb SmaI fragment containing a neomycin resistance (Nmr) cassette from pRL648 (9) was inserted into the digested site. The SacI-XhoI fragment containing the disrupted all5123 gene was ligated into the corresponding sites in pRL271 (3), a nonreplicating vector used for triparental mating.
Construction of the Synechocystis sp. strain PCC 6803 mutant lacking wcaG (sll1213) has been described previously (28).
Analysis and identification of carotenoids.
The organic solvent-soluble pigments were extracted from cells of Anabaena sp. strain PCC 7120 and Synechocystis sp. strain PCC 6803 using an acetone-methanol mixture (7:2, vol/vol) and were analyzed by high-performance liquid chromatography (HPLC) using an apparatus equipped with a μBondapak C18 column (8 by 100 mm; RCM type; Waters, United States) that was eluted with methanol-water (9:1, vol/vol) for 20 min and then with 100% methanol at a rate of 2.0 ml/min (37).
The major polar carotenoids were isolated and purified as follows. We extracted the pigments with acetone-methanol (7:2, vol/vol) using an ultrasonicator and then evaporated the solvent. The pigments were loaded onto a column of Silica Gel 60 (Merck, Germany). First β-carotene was eluted with n-hexane, and then chlorophyll a and nonpolar carotenoids were eluted with n-hexane-acetone (8:2, vol/vol). Finally, the polar carotenoids were eluted with acetone-methanol (9:1, vol/vol). The last fraction was then loaded onto a column of DEAE-Toyopearl 650 M (Tosoh, Japan), and carotenoids were eluted with n-hexane-acetone (1:1, vol/vol), but chlorophyll a and polar lipids remained on the column. The polar carotenoids were purified by silica gel thin-layer chromatography (Merck, Germany) using dichloromethane-ethyl acetate-acetone-methanol (2:4:2:1, vol/vol/vol/vol) as the eluent and were finally collected from the HPLC apparatus described above (37). Acetylation of the polar carotenoids was carried out as described previously (35).
Spectroscopic analysis.
We measured the absorption spectra of the pigments using an MCPD-3600 photodiode array detector (Otsuka Electronics, Japan) attached to the HPLC apparatus described above (37). For quantitative analysis, the molar extinction coefficients at the maximum wavelengths of the carotenoids were assumed to be the same. The relative molecular masses of the carotenoids were determined using an M-2500 double-focusing gas chromatograph-mass spectrometer (Hitachi, Japan) equipped with a field desorption apparatus (33). The 1H nuclear magnetic resonance (NMR) (500 MHz) spectra of the carotenoids in CDCl3 at 24°C were determined using the UNITY INOVA-500 system (Varian, United States) (37).
RESULTS AND DISCUSSION
Deletion mutants of Anabaena sp. strain PCC 7120 with mutations in carotenogenesis genes.
We inactivated proposed carotenogenesis genes shown in Table 1 by triparental mating. The genes of the mutants obtained are shown in Fig. 1.
Double-crossover recombination using the conditionally lethal sacB gene (7) was used to replace the wild-type gene with the mutated gene, and the target gene was interrupted by an antibiotic resistance cassette in the mutant. Using an Nmr marker and constructs shown in Fig. 1, we obtained all4826, alr4009, and all5123 double-crossover mutants and confirmed that there was full segregation of mutants with the appropriate disrupted target genes by PCR (see Fig. S1 in the supplemental material).
Carotenoids in wild-type Anabaena sp. strain PCC 7120 and Synechocystis sp. strain PCC 6803.
The HPLC elution profiles of the pigments of Anabaena sp. strain PCC 7120 (Fig. 2A) and Synechocystis sp. strain PCC 6803 (Fig. 3A) had characteristic carotenoid and chlorophyll a peaks. The major carotenoids of Anabaena sp. strain PCC 7120 have been identified as β-carotene, echinenone, canthaxanthin, (3R,2′S)-myxol 2′-α-l-fucoside, and (3S,2′S)-4-ketomyxol 2′-α-l-fucoside (Table 2 and Fig. 4) (37). The major carotenoids of Synechocystis sp. strain PCC 6803 are β-carotene, (3R,3′R)-zeaxanthin, echinenone, (3′R)-3′-hydroxyechinenone, and (3R,2′S)-myxol 2′-(2,4-di-O-methyl-α-l-fucoside) (36).
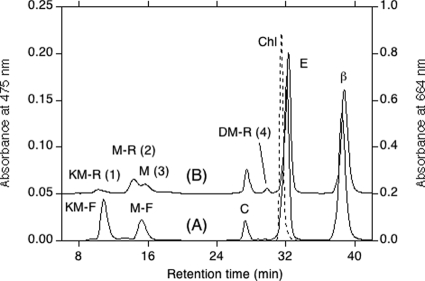
FIG. 2.
HPLC elution profiles of pigments extracted from Anabaena sp. wild-type strain PCC 7120 (profile A) and the wcaG deletion mutant (profile B). The eluent was methanol-water (9:1, vol/vol) for the first 20 min and then 100% methanol at a rate of 2.0 ml/min. The absorbance at 475 nm (solid lines) and the absorbance at 664 nm (dashed line) are shown. The numbers in parentheses are peak numbers. KM-F, 4-ketomyxol 2′-fucoside; M-F, myxol 2′-fucoside; C, canthaxanthin; E, echinenone; β, β-carotene; Chl, chlorophyll a; KM-R, 4-ketomyxol 2′-rhamnoside; M-R, myxol 2′-rhamnoside; M, myxol; DM-R, deoxymyxol 2′-rhamnoside.
FIG. 3.
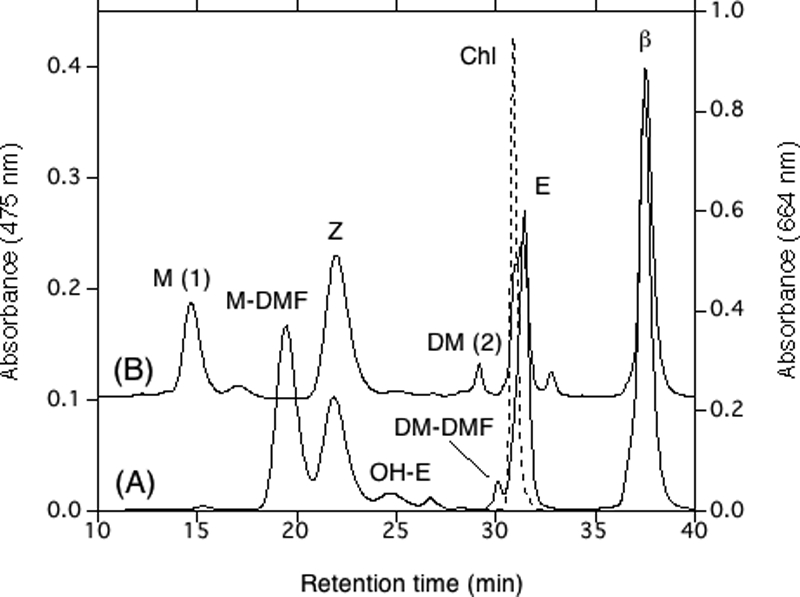
HPLC elution profiles of pigments extracted from Synechocystis sp. wild-type strain PCC 6803 (profile A) and the wcaG deletion mutant (profile B). The eluent was methanol-water (9:1, vol/vol) for the first 20 min and then 100% methanol at a rate of 2.0 ml/min. The absorbance at 475 nm (solid lines) and the absorbance at 664 nm (dashed line) are shown. The numbers in parentheses are peak numbers. M-DMF, myxol 2′-dimethyfucoside; Z, zeaxanthin; OH-E, 3′-hydroxyechinenone; DM-DMF, deoxymyxol 2′-dimethylfucoside; E, echinenone; β, β-carotene; Chl, chlorophyll a; M, myxol; DM, deoxymyxol.
TABLE 2.
Carotenoid composition of Anabaena sp. wild-type strain PCC 7120 and mutants of this strain
| Carotenoid | mol% of total carotenoids in:
|
||
|---|---|---|---|
| Wild type | Δall4826 (ΔwcaG) mutant | Δalr4009 (ΔcrtR) mutant | |
| Myxol 2′-fucoside | 7 | ||
| 4-Ketomyxol 2′-fucoside | 12 | ||
| Myxol | 3 | ||
| Myxol 2′-rhamnoside | 5 | ||
| 4-Ketomyxol 2′-rhamnoside | 2 | ||
| Deoxymyxol 2′-rhamnoside | 1 | ||
| Deoxymyxol 2′-fucoside | 10 | ||
| 4-Ketodeoxymyxol 2′-fucoside | 5 | ||
| β-Carotene | 41 | 41 | 48 |
| Echinenone | 37 | 41 | 36 |
| Canthaxanthin | 4 | 6 | 2 |
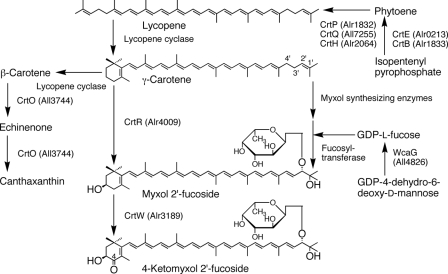
FIG. 4.
Biosynthetic pathways for carotenoids and their enzymes in Anabaena sp. strain PCC 7120. See the text for details.
wcaG deletion mutant of Anabaena sp. strain PCC 7120.
GDP-l-fucose is synthesized from GDP-4-dehydro-6-deoxy-d-mannose by GDP-fucose synthase, which is WcaG in E. coli (25, 32). Anabaena sp. strain PCC 7120 contains a wcaG-like gene, all4826, whose product shows 35% identity to E. coli WcaG at the amino acid level. GDP-fucose may be a substrate of myxol 2′-fucoside.
We produced a wcaG deletion mutant of Anabaena sp. strain PCC 7120. Complete segregation of this mutant was confirmed by PCR (see Fig. S1A in the supplemental material), and its growth rate was similar to that of the wild type under the growth conditions used. The content of β-carotene, echinenone, and canthaxanthin, identified based on HPLC retention times and absorption spectra, in the mutant was similar to that in the wild type (Fig. 2B and Table 2). Free myxol, identified based on the same HPLC retention time and the same absorption spectra as myxol from A. variabilis ATCC 29413 (38) and a relative molecular mass of 584, was also found (Fig. 2B, peak 3).
Two HPLC polar peaks (Fig. 2B, peaks 1 and 2) had slightly shorter HPLC retention times but the same absorption spectra as 4-ketomyxol 2′-fucoside and myxol 2′-fucoside from the wild type, respectively. The relative molecular masses of the peak 1 and 2 carotenoids were 744 and 730, respectively. 1H NMR analysis of the two polar carotenoids indicated that the carotenoid moieties were 4-ketomyxol and myxol, respectively (Table 3). Since the chemical shifts of H-3" and H-4" of their glycoside moieties overlapped, the carotenoids were acetylated. The 1H NMR analysis data, including chemical shifts, multiplicity, and coupling constants, indicated that the glycoside moiety was α-l-rhamnoside (Table 3). The stereochemistry of the carotenoid moieties was expected to be the same as that of the wild type (37), since the mutation did not affect carotenoid moieties. Consequently, the peak 1 and 2 components were identified as (3S,2′S)-4-ketomyxol 2′-α-l-rhamnoside and (3R,2′S)-myxol 2′-α-l-rhamnoside, respectively (Fig. 5). The peak 4 component might have been deoxymyxol 2′-α-l-rhamnoside. Therefore, the carotenoid composition of the mutant was identical to that of Anabaena sp. wild-type strain PCC 7120, except for replacement of fucoside by rhamnoside and the presence of free myxol in the mutant (Table 2). Since the combined content of myxol and its derivatives of the WcaG mutant was about 60% that of the wild type, the myxol branch of carotenogenesis (Fig. 4) may have been repressed in the mutant.
TABLE 3.
1H NMR spectral data for myxol glycoside, acetylated myxol glycoside, 4-ketomyxol glycoside, and acetylated 4-ketomycol glycoside in CDCl3
| Position | Myxol glycoside
|
Acetylated myxol glycoside
|
4-Ketomyxol glycoside
|
Acetylated 4-ketomyxol glycoside
|
||||
|---|---|---|---|---|---|---|---|---|
| δ (ppm) | Multiplicity (J [Hz])a | δ (ppm) | Multiplicity (J [Hz])a | δ (ppm) | Multiplicity (J [Hz])a | δ (ppm) | Multiplicity (J [Hz])a | |
| 2ax | 1.48 | dd (12, 12) | 1.58 | dd (12, 12) | 1.81 | dd (13, 13) | 2.01 | dd (17, 10) |
| 2eq | 1.77 | ddd (12, 4, 1.5) | 1.78 | ddd (12, 4, 1.5) | 2.15 | dd (13, 5.5) | 2.07 | ddd (17, 5, 1.5) |
| 3 | 4.00 | m | 5.06 | m | 4.32 | dd | 5.54 | m |
| 4ax | 2.05 | dd (17, 10) | 2.11 | dd (17, 10) | NAb | NA | ||
| 4eq | 2.39 | ddd (17, 5, 1.5) | 2.45 | ddd (17, 5, 1.5) | NA | NA | ||
| 7 | 6.11 | d (16) | 6.11 | d (16) | 6.22 | d (16) | 6.21 | d (16) |
| 8 | 6.13 | d (16) | 6.13 | d (16) | 6.43 | d (16) | 6.41 | d (16) |
| 10 | 6.16 | d (11.5) | 6.16 | d (11.5) | 6.30 | d (11.5) | 6.30 | d (11.5) |
| 11 | 6.64 | dd (15.5, 11.5) | 6.64 | dd (15.5, 11.5) | 6.66 | dd (15.5, 11.5) | 6.66 | dd (15.5, 11.5) |
| 12 | 6.35 | d (15.5) | 6.35 | d (15.5) | 6.45 | d (15.5) | 6.45 | d (15.5) |
| 14 | 6.25 | m | 6.25 | m | 6.30 | m | 6.30 | m |
| 15 | 6.63 | m | 6.63 | m | 6.63 | m | 6.63 | m |
| 16 | 1.07 | s | 1.11 | s | 1.32 | s | 1.35 | s |
| 17 | 1.07 | s | 1.08 | s | 1.21 | s | 1.23 | s |
| 18 | 1.74 | s | 1.72 | s | 1.94 | s | 1.90 | s |
| 19 | 1.97 | s | 1.97 | s | 2.00 | s | 2.01 | s |
| 20 | 1.98 | s | 1.97 | s | 1.99 | s | 1.99 | s |
| 2′ | 3.92 | d (9) | 3.85 | d (9) | 3.92 | d (9) | 3.85 | d (9) |
| 3′ | 5.73 | dd (15.5, 9) | 5.69 | dd (15.5, 9) | 5.73 | dd (15.5, 9) | 5.69 | dd (15.5, 9) |
| 4′ | 6.40 | d (15.5) | 6.30 | d (15.5) | 6.40 | d (15.5) | 6.30 | d (15.5) |
| 6′ | 6.20 | d (11.5) | 6.20 | d (11.5) | 6.20 | d (11.5) | 6.20 | d (11.5) |
| 7′ | 6.56 | dd (15.5, 11.5) | 6.56 | dd (15.5, 11.5) | 6.56 | dd (15.5, 11.5) | 6.56 | dd (15.5, 11.5) |
| 8′ | 6.40 | d (15.5) | 6.40 | d (15.5) | 6.40 | d (15.5) | 6.40 | d (15.5) |
| 10′ | 6.27 | d (11.5) | 6.27 | d (11.5) | 6.27 | d (11.5) | 6.27 | d (11.5) |
| 11′ | 6.64 | dd (15.5, 11.5) | 6.64 | dd (15.5, 11.5) | 6.64 | dd (15.5, 11.5) | 6.64 | dd (15.5, 11.5) |
| 12′ | 6.31 | d (15.5) | 6.31 | d (15.5) | 6.31 | d (15.5) | 6.31 | d (15.5) |
| 14′ | 6.25 | m | 6.25 | m | 6.30 | m | 6.30 | m |
| 15′ | 6.63 | m | 6.63 | m | 6.63 | m | 6.63 | m |
| 16′ | 1.21 | s | 1.22 | s | 1.21 | s | 1.22 | s |
| 17′ | 1.21 | s | 1.22 | s | 1.21 | s | 1.22 | s |
| 18′ | 1.93 | s | 1.93 | s | 1.93 | s | 1.93 | s |
| 19′ | 1.98 | s | 1.97 | s | 1.97 | s | 1.97 | s |
| 20′ | 1.99 | s | 1.99 | s | 1.99 | s | 1.99 | s |
| 1" | 4.57 | br.s | 4.66 | br.s | 4.57 | br.s | 4.66 | br.s |
| 2" | 4.07 | br.s | 5.50 | d (3) | 4.06 | br.s | 5.50 | d (3) |
| 3" | ∼3.43 | m | 5.01 | dd (10, 3) | ∼3.43 | m | 5.01 | dd (10, 3) |
| 4" | ∼3.43 | m | 5.06 | dd (10, 10) | ∼3.43 | m | 5.06 | dd (10, 10) |
| 5" | 3.23 | qd (6.5, 6) | 3.47 | dq (10, 6.5) | 3.23 | qd (6.5, 6) | 3.47 | dq (10, 6.5) |
| 6" | 1.32 | d (6.5) | 1.22 | d (6.5) | 1.32 | d (6.5) | 1.22 | d (6.5) |
| Acetyl | NA | 2.05 | s | NA | 2.05 | s | ||
| 2.17 | s | 2.17 | s | |||||
| 2.19 | s | 2.19 | s | |||||
| 2.21 | s | 2.21 | s | |||||
s, singlet; br.s, broad singlet; d, doublet; q, quartet; m, multiplet; J, coupling constants.
NA, not available.
FIG. 5.
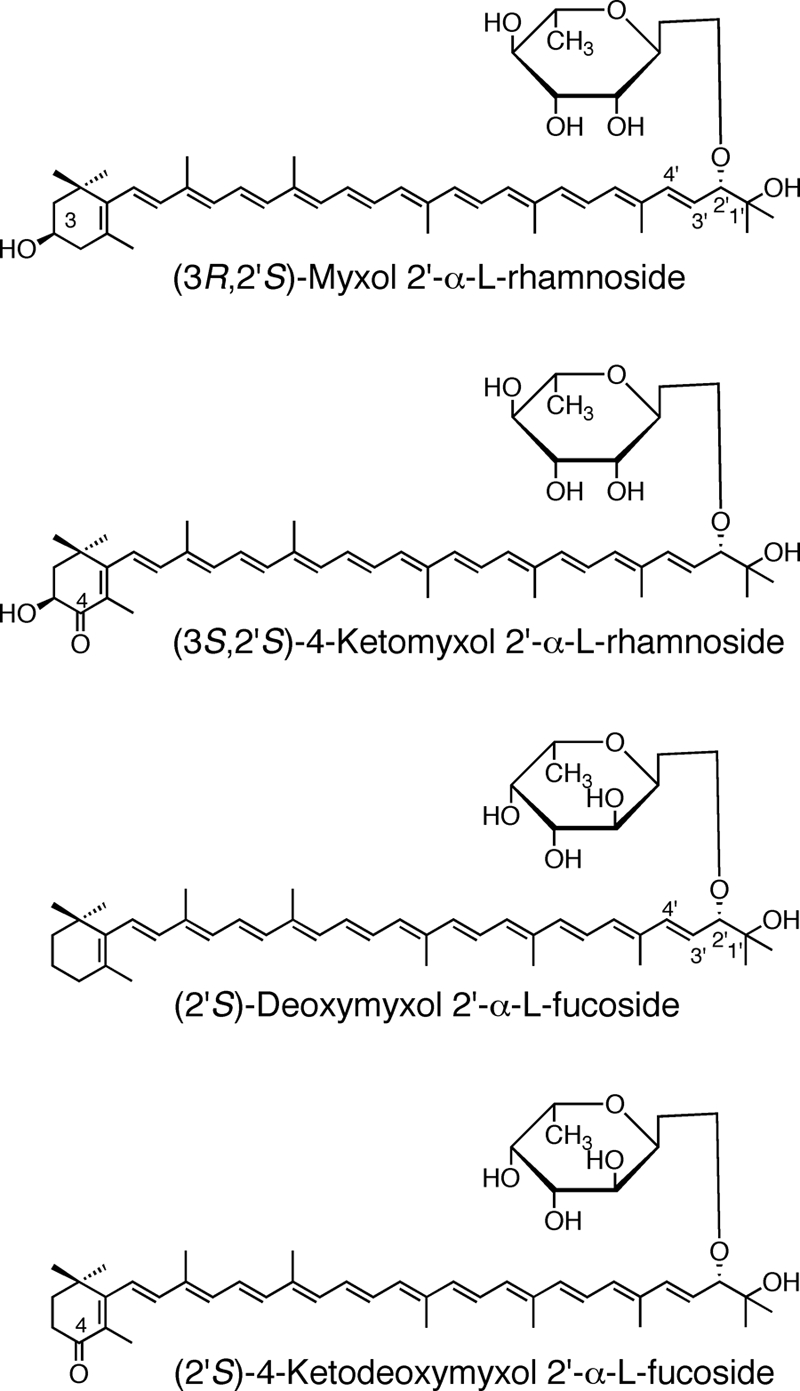
Structure of selected carotenoid glycosides.
wcaG deletion mutant of Synechocystis sp. strain PCC 6803.
Synechocystis sp. strain PCC 6803 also contains a wcaG-like gene, sll1213. The Sll1213 amino acid sequence is 79% identical to the Anabaena sp. strain PCC 7120 protein and 34% identical to WcaG of E. coli. The sll1213 deletion mutant was investigated (28), and we confirmed that the unknown peak described by Mohamed et al. (28), which corresponded to a main polar peak (Fig. 3B, peak 1), was free myxol based on its retention time in HPLC and absorption spectrum compared to the retention time and absorption spectrum of free myxol from A. variabilis ATCC 29413 (38) and based on the relative molecular mass, 584. A small peak (Fig. 3B, peak 2) was identified as deoxymyxol, and a minor peak that eluted just after peak 1 (Fig. 3B) might have been cis-myxol. Both assignments were based on the HPLC retention times and absorption spectra. We did not find a detectable amount of myxol glycoside by silica gel thin-layer chromatography, although Mohamed et al. (28) found myxol glycosides in extracts from the same mutant.
In Synechocystis sp. strain PCC 6803, WcaG has been reported to be required for normal cell wall structure and thylakoid organization (28). This may be due to presence of free myxol instead of myxol 2′-dimethyl-fucoside. On the other hand, the growth of the wcaG deletion mutant of Anabaena sp. strain PCC 7120 was similar to the growth of the wild-type strain, suggesting that myxol 2′-rhamnoside can functionally substitute for myxol 2′-fucoside.
Mutation of the E. coli wcaG-like genes, all4826 and sll1213, in the two cyanobacteria is expected to result in the absence of GDP-fucose, the native substrate for fucosyltransferase to transfer this compound to myxol. In the Synechocystis sp. strain PCC 6803 mutant, fucosyltransferase apparently was unable to readily link other sugars to myxol, and free myxol accumulated. In the Anabaena sp. strain PCC 7120 mutant, GDP-rhamnose could also serve as the substrate of fucosyltransferase, implying that the substrate specificity of this enzyme was broad. This resulted in the formation of myxol 2′-α-l-rhamnoside and 4-ketomyxol 2′-α-l-rhamnoside, and some myxol remained in the free form. Consequently, either the substrate specificity of the fucosyltransferase from Synechocystis sp. strain PCC 6803 is different from the substrate specificity of the fucosyltransferase from Anabaena sp. strain PCC 7120, or Synechocystis sp. strain PCC 6803 does not readily make GDP-rhamnose that can be recognized by the enzyme.
Alternate explanations for the results observed are possible as well. One possibility is that Anabaena sp. strain PCC 7120 naturally contains a rhamnosyltransferase that can transfer a GDP-rhamnose to myxol but that in the wild type its presence is functionally obscured by the fucosyltransferase. However, in the mutant the absence of the substrate of fucosyltransferase, GDP-fucose, and the presence of sufficient myxol may cause the rhamnosyltransferase activity to become apparent. Moreover, the rhamnosyltransferase activity in the mutant may be due to an enzyme that does not use myxol as its primary substrate. Yet another possibility is that WcaG actually is an isomerase catalyzing conversion of myxol 2′-rhamnoside to myxol 2′-fucoside, but then myxol 2′-rhamnoside should also have been produced in the Synechocystis sp. strain PCC 6803 mutant. Moreover, WcaG does not exhibit sequence similarity to isomerases. In any case, final resolution of this issue must await identification of glycosyltransferases recognizing myxol in cyanobacteria, but the alternate explanations seem less likely than the conclusions described above.
There is possible precedence for extended substrate specificity of glycosyltransferases: since Oscillatoria limnetica produces both myxol 2′-α-l-fucoside and myxol 2′-α-l-chinovoside and Spirulina platensis produces both oscillol 2,2′-di(α-l-fucoside) and oscillol 2,2′-di(α-l-chinovoside) (1), the substrates of glycosyltransferase in these two species might be both fucose and chinovose, or both fucosyltransferase and chinovosyltransferase might be present.
crtR deletion mutant of Anabaena sp. strain PCC 7120.
β-Carotene hydroxylases, CrtZ in bacteria and CrtR in plants, are known to catalyze the conversion of β-carotene to zeaxanthin; crtR homologues are also found in cyanobacteria (39). In Synechocystis sp. strain PCC 6803, CrtR (Sll1468) catalyzes not only conversion of β-carotene to zeaxanthin but also conversion of deoxymyxol 2′-dimethyl-fucoside to myxol 2′-dimethyl-fucoside and conversion of echinenone to 3′-hydroxyechinenone (15, 22, 36). CrtR also was found to catalyze conversion of deoxymyxol to myxol based on the presence of free myxol in the wcaG mutant.
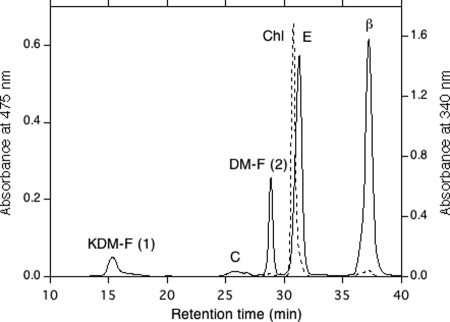
Anabaena sp. strain PCC 7120 contains a gene, alr4009, which is similar to sll1468. Alr4009 is 68% identical to Sll1468 from Synechocystis sp. strain PCC 6803. We produced the alr4009 deletion mutant, and its complete segregation was confirmed by PCR (see Fig. S1B in the supplemental material). Under the conditions used the growth rate of the mutant was similar to that of the wild type. The HPLC elution profiles of the pigments of the mutant (Fig. 6) showed that the peak corresponding to 4-ketomyxol 2′-fucoside in the wild type (Fig. 2A) was not present, the absorption spectrum of the myxol 2′-fucoside peak had changed to the ketomyxol-type spectrum (Fig. 6, peak 1, and Fig. 7), and a new peak with the myxol-type spectrum (Fig. 6, peak 2, and Fig. 7) eluted later. The relative molecular masses of peaks 1 and 2 were 728 and 714, respectively. The stereochemistry of the carotenoid moieties might have been identical to that of the wild type (37). Therefore, the peak 1 and 2 components most likely were (2′S)-4-ketodeoxymyxol 2′-fucoside and (2′S)-deoxymyxol 2′-fucoside (Fig. 5), respectively. The former compound is a novel carotenoid glycoside in organisms (6), and its IUPAC-IUB semisystematic name is (2′S)-1′-hydroxy-2′-(α-l-fucopyranosyloxy)-3′,4′-didehydro-1′,2′-dihydro-β,ψ-caroten-4-one.
FIG. 6.
HPLC elution profile of pigments extracted from the crtR deletion mutant of Anabaena sp. strain PCC 7120. The eluent was methanol-water (9:1, vol/vol) for the first 20 min and then 100% methanol at a rate of 2.0 ml/min. The absorbance at 475 nm (solid line) and the absorbance at 340 nm (dashed line) are shown. The numbers in parentheses are peak numbers. KDM-F, 4-ketodeoxymyxol 2′-fucoside; DM-F, deoxymyxol 2′-fucoside; C, canthaxanthin; E, echinenone; β, β-carotene; Chl, chlorophyll a.
FIG. 7.
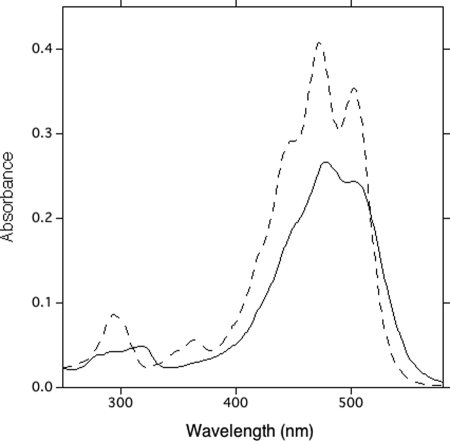
Absorption spectra of two carotenoids, 4-ketodeoxymyxol 2′-fucoside (Fig. 6, peak 1) (dashed line) and deoxymyxol 2′-fucoside (Fig. 6, peak 2) (solid line), in HPLC eluent.
In Anabaena sp. strain PCC 7120, CrtR appeared to specifically convert deoxymyxol 2′-fucoside to myxol 2′-fucoside rather than β-carotene to zeaxanthin, judging from the small amount of zeaxanthin (<1%) but the large amount of β-carotene present in cells (Table 2). Furthermore, CrtR also catalyzed the conversion of deoxymyxol to myxol based on the presence of free myxol in the wcaG mutant. Similar characteristics of CrtR have been found in A. variabilis strains ATCC 29413 and IAM M-3 and Nostoc punctiforme PCC 73102 (37-39). On the other hand, CrtR of Synechocystis sp. strain PCC 6803 readily converts β-carotene to zeaxanthin and deoxymyxol and deoxymyxol 2′-fucoside to myxol and myxol 2′-fucoside, respectively (15, 22). Thus, the substrate specificities or substrate availabilities of CrtR appear to be different in the Nostoc/Anabaena strains and Synechocystis.
all5123 deletion mutant of Anabaena sp. strain PCC 7120.
Based on the structural similarity between the right half of myxol (Fig. 4) and spirilloxanthin from purple bacteria, methoxyneurosporene desaturase (3,4-dehydrogenase, CrtD) of Rhodobacter (34) may play a role in the synthesis of myxol. A crtD homologue from marine bacterium strain P99-3 (= MBIC 03313; previously Flavobacterium sp.), which produces free myxol with the same stereochemistry (43), codes for an enzyme involved in myxol biosynthesis (41). In a Synechocystis sp. strain PCC 6803 mutant lacking the crtD homologue, slr1293, carotenogenesis and especially myxol synthesis were affected (27).
All5123 is 66, 28, and 27% identical to CrtD from Synechocystis sp. strain PCC 6803, Rhodobacter sphaeroides, and marine bacterium strain P99-3, respectively. InterPro (http://www.ebi.ac.uk/InterProScan) recognizes a conserved phytoene dehydrogenase domain (IPR008151) near the C terminus of this protein. We produced an all5123 deletion mutant, and its complete segregation was confirmed by PCR (see Fig. S1C in the supplemental material). Its growth was essentially identical to that of the wild type under the conditions used. The HPLC elution profiles of the pigments and the absorption spectra of myxol 2′-fucoside and 4-ketomyxol 2′-fucoside in the mutant also were essentially identical to those of the wild type. This indicates that all5123 is not critical for CrtD function in Anabaena sp. strain PCC 7120, which indicates that All5123 does not have CrtD activity and/or that there is another gene that can functionally complement all5123.
Carotenogenesis in Anabaena sp. strain PCC 7120.
We have proposed biosynthetic pathways for the carotenoids in Anabaena sp. strain PCC 7120 based on identification of carotenoids and the entire nucleotide sequence of its genome (37). Additional data are shown in Fig. 4. The functions of CrtQa (17, 18), CrtO (ketolase converting β-carotene to echinenone), and CrtW (ketolase converting myxol 2′-fucoside to 4-ketomyxol 2′-fucoside) (26) have been confirmed. In this study, we confirmed the functions of CrtR and WcaG but could not find the CrtD activity in all5123 in carotenogenesis. The presence of CrtE, CrtB, CrtP, and CrtH in Anabaena sp. strain PCC 7120 is suggested by sequence similarity (37). So far, lycopene cyclase, some of the myxol-synthesizing enzymes, and fucosyltransferase have not been found. Recently, Maresca et al. (19) reported a new type of functional lycopene cyclase in the green sulfur bacterium Chlorobaculum tepidum and the cyanobacterium Synechococcus sp. strain PCC 7002 (CruA and CruA plus CruP, respectively). Even though cruA orthologues have been found in Anabaena sp. strain PCC 7120 (alr3524) and Synechocystis sp. strain PCC 6803 (sll0147), we have not been able to detect lycopene cyclase activities encoded by these genes in these organisms (M. Mochimaru, H. Masukawa, K. Kondo, M. Ikeuchi, and S. Takaichi, unpublished results). We did not analyze the orthologue of cruP (alr0920) in Anabaena sp. strain PCC 7120. Further research is required to elucidate the function of these genes.
Supplementary Material
Acknowledgments
We thank C. P. Wolk of Michigan State University for the gift of the suicide vectors and H. Sakurai of Waseda University for his help with construction of the Anabaena sp. strain PCC 7120 mutants.
This work was supported in part by Grants-in-Aid for Scientific Research from JSPS (grant 16570038) and the Industrial Technology Research Program in 2005 from NEDO (grant 05A22703a) to S.T.
Footnotes
Published ahead of print on 15 August 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
This paper is dedicated to H. Sakurai on the occasion of his 70th birthday.
REFERENCES
- 1.Aakermann, T., O. M. Skulberg, and S. Liaaen-Jensen. 1992. A comparison of the carotenoids of strains of Oscillatoria and Spirulina (cyanobacteria). Biochem. Syst. Ecol. 20761-769. [Google Scholar]
- 2.Becker, D. J., and J. B. Lowe. 1999. Leukocyte adhesion deficiency type II. Biochim. Biophys. Acta 1455193-204. [DOI] [PubMed] [Google Scholar]
- 3.Black, T. A., Y. Cai, and C. P. Wolk. 1993. Spatial expression and autoregulation of hetR, a gene involved in the control of heterocyst development in Anabaena. Mol. Microbiol. 977-84. [DOI] [PubMed] [Google Scholar]
- 4.Breitenbach, J., B. Fernández-González, A. Vioque, and G. Sandmann. 1998. A higher-plant type ζ-carotene desaturase in the cyanobacterium Synechocystis PCC6803. Plant Mol. Biol. 36725-732. [DOI] [PubMed] [Google Scholar]
- 5.Breitenbach, J., A. Vioque, and G. Sandmann. 2001. Gene sll0033 from Synechocystis 6803 encodes a carotenoid isomerase involved in the biosynthesis of all-E lycopene. Z. Naturforsch. 56 c:915-917. [DOI] [PubMed] [Google Scholar]
- 6.Britton, G., S. Liaaen-Jensen, and H. Pfander. 2004. Carotenoids handbook. Birkhäuser Verlag, Basel, Switzerland.
- 7.Cai, Y., and C. P. Wolk. 1990. Use of a conditionally lethal gene in Anabaena sp. strain PCC 7120 to select for double recombinants and to entrap insertion sequences. J. Bacteriol. 1723138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elhai, J., and C. P. Wolk. 1988. A versatile class of positive-selection vectors based on the nonviability of palindrome-containing plasmids that allows cloning into long polylinkers. Gene 68119-138. [DOI] [PubMed] [Google Scholar]
- 9.Elhai, J., and C. P. Wolk. 1988. Conjugal transfer of DNA to cyanobacteria. Methods Enzymol. 167747-754. [DOI] [PubMed] [Google Scholar]
- 10.Elhai, J., A. Vepritskiy, A. M. Muro-Pastor, E. Flores, and C. P. Wolk. 1997. Reduction of conjugal transfer efficiency by three restriction activities of Anabaena sp. strain PCC 7120. J. Bacteriol. 1791998-2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fernández-González, B., G. Sandmann, and A. Vioque. 1997. A new type of asymmetrically acting β-carotene ketolase is required for the synthesis of echinenone in the cyanobacterium Synechocystis sp. PCC 6803. J. Biol. Chem. 2729728-9733. [DOI] [PubMed] [Google Scholar]
- 12.Reference deleted.
- 13.Kaneko, T., S. Sato, H. Kotani, A. Tanaka, E. Asamizu, Y. Nakamura, N. Miyajima, M. Hirosawa, M. Sugiura, S. Sasamoto, T. Kimura, T. Hosouchi, A. Matsuno, A. Muraki, N. Nakazaki, K. Naruo, S. Okumura, S. Shimpo, C. Takeuchi, T. Wada, A. Watanabe, M. Yamada, M. Yasuda, and S. Tabata. 1996. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC 6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 3109-136. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko, T., Y. Nakamura, C. P. Wolk, T. Kuritz, S. Sasamoto, A. Watanabe, M. Iriguchi, A. Ishikawa, K. Kawashima, T. Kimura, Y. Kishida, M. Kohara, M. Matsumoto, A. Matsuno, A. Muraki, N. Nakazaki, S. Shimpo, M. Sugimoto, M. Takazawa, M. Yamada, M. Yasuda, and S. Tabata. 2001. Complete genomic sequence of the filamentous nitrogen-fixing cyanobacterium Anabaena sp. strain PCC 7120. DNA Res. 8205-213. [DOI] [PubMed] [Google Scholar]
- 15.Lagarde, D., and W. Vermaas. 1999. The zeaxanthin biosynthesis enzyme β-carotene hydroxylase is involved in myxoxanthophyll synthesis in Synechocystis sp. PCC 6803. FEBS Lett. 454247-251. [DOI] [PubMed] [Google Scholar]
- 16.Lagarde, D., L. Beuf, and W. Vermaas. 2000. Increased production of zeaxanthin and other pigments by application of genetic engineering techniques to Synechocystis sp strain PCC 6803. Appl. Environ. Microbiol. 6664-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linden, H., A. Vioque, and G. Sandmann. 1993. Isolation of a carotenoid biosynthesis gene coding for ζ-carotene desaturase from Anabaena PCC 7120 by heterologous complementation. FEMS Microbiol. Lett. 10699-104. [Google Scholar]
- 18.Linden, H., N. Misawa, T. Saito, and G. Sandmann. 1994. A novel carotenoid biosynthesis gene coding for ζ-carotene desaturase: functional expression, sequence and phylogenetic origin. Plant Mol. Biol. 24369-379. [DOI] [PubMed] [Google Scholar]
- 19.Maresca, J. A., J. E. Graham, M. Wu, J. A. Eisen, and D. A. Bryant. 2007. Identification of a fourth family of lycopene cyclases in photosynthetic bacteria. Proc. Natl. Acad. Sci. USA 10411784-11789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martínez-Férez, I., and A. Vioque. 1992. Nucleotide sequence of the phytoene desaturase gene from Synechocystis sp. PCC 6803 and characterization of a new mutation which confers resistance to the herbicide norflurazon. Plant Mol. Biol. 18981-983. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Férez, I., B. Fernández-González, G. Sandmann, and A. Vioque. 1994. Cloning and expression in Escherichia coli of the gene coding for phytoene synthase from the cyanobacterium Synechocystis sp. PCC6803. Biochim. Biophys. Acta 1218145-152. [DOI] [PubMed] [Google Scholar]
- 22.Masamoto, K., N. Misawa, T. Kaneko, R. Kikuno, and H. Toh. 1998. β-Carotene hydroxylase gene from the cyanobacterium Synechocystis sp. PCC6803. Plant Cell Physiol. 39560-564. [DOI] [PubMed] [Google Scholar]
- 23.Masamoto, K., H. Wada, T. Kaneko, and S. Takaichi. 2001. Identification of a gene required for cis-to-trans carotene isomerization in carotenogenesis of the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 421398-1402. [DOI] [PubMed] [Google Scholar]
- 24.Masukawa, H., M. Mochimaru, and H. Sakurai. 2002. Disruption of the uptake hydrogenase gene, but not of the bidirectional hydrogenase gene, leads to enhanced photobiological hydrogen production by the nitrogen-fixing cyanobacterium Anabaena sp. PCC 7120. Appl. Microbiol. Biotechnol. 58618-624. [DOI] [PubMed] [Google Scholar]
- 25.Mattila, P., J. Räbinä, S. Hortling, J. Helin, and R. Renkonen. 2000. Functional expression of Escherichia coli enzymes synthesizing GDP-l-fucose from inherent GDP-d-mannnose in Saccharomyces cerevisiae. Glycobiology 101041-1047. [DOI] [PubMed] [Google Scholar]
- 26.Mochimaru, M., H. Masukawa, and S. Takaichi. 2005. The cyanobacterium Anabaena sp. PCC 7120 has two distinct β-carotene ketolases: CrtO for echinenone and CrtW for ketomyxol synthesis. FEBS Lett. 5796111-6114. [DOI] [PubMed] [Google Scholar]
- 27.Mohamed, H. E., and W. Vermaas. 2004. Slr1293 in Synechocystis sp. strain PCC 6803 is the C-3′,4′ desaturase (CrtD) involved in myxoxanthophyll biosynthesis. J. Bacteriol. 1865621-5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohamed, H. E., A. M. L. van de Meene, R. W. Roberson, and W. F. J. Vermaas. 2005. Myxoxanthophyll is required for normal cell wall structure and thylakoid organization in the cyanobacterium Synechocystis sp. strain PCC 6803. J. Bacteriol. 1876883-6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohamed, H. E., and W. F. J. Vermaas. 2006. Sll0254 (CrtLdiox) is a bifunctional lycopene cyclase/dioxygenase in cyanobacteria producing myxoxanthophyll. J. Bacteriol. 1883337-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shindo, K., K. Kikuta, A. Suzuki, A. Katsuta, H. Kasai, M. Yasumoto-Hirose, Y. Matuo, N. Misawa, and S. Takaichi. 2007. Rare carotenoids, (3R)-saproxanthin and (3R,2′S)-myxol, isolated from novel marine bacteria (Flavobacteriaceae) and their antioxidative activities. Appl. Microbiol. Biotechnol. 741350-1357. [DOI] [PubMed] [Google Scholar]
- 31.Reference deleted.
- 32.Stevenson, G., K. Andrianopoulos, M. Hobbs, and P. R. Reeves. 1996. Organization of the Escherichia coli K-12 gene cluster responsible for production of the extracellular polysaccaride colanic acid. J. Bacteriol. 1784885-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takaichi, S. 1993. Usefulness of field desorption mass spectrometry in determining molecular masses of carotenoids, natural carotenoid derivatives and their chemical derivatives. Org. Mass Spectrom. 28785-788. [Google Scholar]
- 34.Takaichi, S. Distribution and biosynthesis of carotenoids. In C. N. Hunter, F. Daldal, M. C. Thurnauer, and J. T. Beatty (ed.), The purple phototrophic bacteria. Advances in photosynthesis and respiration, vol. 28, in press. Springer, Dordrecht, The Netherlands.
- 35.Takaichi, S., and J. Ishidsu. 1992. Carotenoid glycoside ester from Rhodococcus rhodochrious. Methods Enzymol. 213366-374. [Google Scholar]
- 36.Takaichi, S., T. Maoka, and K. Masamoto. 2001. Myxoxanthophyll in Synechocystis sp. PCC 6803 is myxol 2′-dimethyl-fucoside, (3R,2′S)-myxol 2′-(2,4-di-O-methyl-α-l-fucoside), not rhamnoside. Plant Cell Physiol. 42756-762. [DOI] [PubMed] [Google Scholar]
- 37.Takaichi, S., M. Mochimaru, T. Maoka, and H. Katoh. 2005. Myxol and 4-ketomyxol 2′-fucosides, not rhamnosides, from Anabaena sp. PCC 7120 and Nostoc punctiforme PCC 73102, and proposal for the biosynthetic pathway of carotenoids. Plant Cell Physiol. 46497-504. [DOI] [PubMed] [Google Scholar]
- 38.Takaichi, S., M. Mochimaru, and T. Maoka. 2006. Presence of free myxol and 4-hydroxymyxol and absence of myxol glycosides in Anabaena variabilis ATCC 29413, and proposal of a biosynthetic pathway of carotenoids. Plant Cell Physiol. 47211-216. [DOI] [PubMed] [Google Scholar]
- 39.Takaichi, S., and M. Mochimaru. 2007. Carotenoids and carotenogenesis in cyanobacteria: unique ketocarotenoids and carotenoid glycosides. Cell. Mol. Life Sci. 642607-2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamagnini, P., O. Troshina, F. Oxelfelt, R. Salema, and P. Lindblad. 1997. Hydrogenases in Nostoc sp. strain PCC 73102, a strain lacking a bidirectional enzyme. Appl. Environ. Microbiol. 631801-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Teramoto, M., N. Rählert, N. Misawa, and G. Sandmann. 2004. 1-Hydroxy monocyclic carotenoid 3,4-dehydrogenase from a marine bacterium that produces myxol. FEBS Lett. 570184-188. [DOI] [PubMed] [Google Scholar]
- 42.Reference deleted.
- 43.Yokoyama, A., and W. Miki. 1995. Isolation of myxol from a marine bacterium Flavobacterium sp. associated with a marine sponge. Fish. Sci. 61684-686. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.