Abstract
Spatial control of proteolysis is emerging as a common feature of regulatory networks in bacteria. In the spore-forming bacterium Bacillus subtilis, the peptidase ClpP can associate with any of three ATPases: ClpC, ClpE, and ClpX. Here, we report that ClpCP, ClpEP, and ClpXP localize in foci often near the poles of growing cells and that ClpP and the ATPase are each capable of polar localization independently of the other component. A region of ClpC containing an AAA domain was necessary and sufficient for polar localization. We also report that ClpCP and ClpXP proteases differentially localize to the forespore and mother cell compartments of the sporangium during spore formation. Moreover, model substrates for each protease created by appending recognition sequences for ClpCP or ClpXP to the green fluorescent protein were preferentially eliminated from the forespore or the mother cell, respectively. Biased accumulation of ClpCP in the forespore may contribute to the cell-specific activation of the transcription factor σF by preferential ClpCP-dependent degradation of the anti-σF factor SpoIIAB.
Proteolysis is an integral feature of many regulatory circuits in bacteria, such as those governing genetic competence in Bacillus subtilis, stress responses in Escherichia coli, and the cell cycle in Caulobacter crescentus (1, 6, 21, 31, 37). Precise temporal and spatial control of proteolysis in response to extracellular or intracellular signals is critical to the proper functioning of these systems. An important family of proteases involved in gene regulatory circuits (and the subject of this report) is two-chain enzymes consisting of the peptidase ClpP and one of several different ATPase subunits (e.g., ClpA, ClpC, ClpE, and ClpX, depending on the bacterium) (for a review, see reference 12). The ATPase and ClpP each form a multimeric ring, with the two rings stacked on top of each other. The ATPases are gatekeepers that recognize a characteristic class of substrates, which they unfold and translocate into the proteolytic channel created by the ring of ClpP subunits (for a review, see reference 32).
A well-characterized and instructive example of the involvement of a ClpP family member in a regulatory circuit is ClpXP in C. crescentus, which degrades the master regulator for cell cycle control, CtrA, and does so in a cell cycle-dependent manner (6, 17, 31). Interestingly, both ClpXP and its CtrA substrate colocalize at the cell pole at a particular time in the cell cycle, coincident with the degradation of CtrA (25, 30). Periodic localization of ClpXP to the pole is dependent on a recently identified protein called CpdR (16).
Here we are concerned with the involvement of ClpCP and ClpXP in sporulation in B. subtilis, a complex developmental process that is triggered by nutrient limitation. A hallmark of sporulation is the formation of an asymmetrically positioned division septum that divides the developing cell or sporangium into a small compartment known as the forespore (or prespore), which is destined to become the spore, and a large chamber known as the mother cell (for a review, see reference 35). Initially, the forespore and the mother cell lie side by side but later in development the mother cell engulfs the forespore, pinching it off as a free protoplast. Ultimately, the mother cell lyses, liberating the mature spore. The forespore and the mother cell exhibit dissimilar programs of gene expression, which are set in motion just after the formation of the polar septum by the activation in the forespore of the transcription factor σF and in the mother cell by the activation of σE. A specific role for ClpCP in sporulation was previously proposed based on the observation that the anti-σF factor SpoIIAB undergoes degradation in a ClpCP-dependent manner (28). Necessary and sufficient for this degradation is the C-terminal sequence leucine-cysteine-asparagine (LCN), which is unique to SpoIIAB and a conserved feature of SpoIIAB orthologs (28, 29).
Here we report on the subcellular localization of ClpCP, ClpEP, and ClpXP during growth and the subcellular localization and compartmentalization of ClpCP and ClpXP during sporulation. We show that all three proteases localize as foci near a cell pole during growth and that ClpP and its cognate ATPases do not depend on each other for this localization. We also present evidence that, in the case of ClpC, a region of the protein that contains a C-terminal-proximal AAA domain was necessary and sufficient for localization. During sporulation, ClpCP and ClpXP continued to localize as polar foci, but evidence indicates that the ClpCP foci were confined to the forespore whereas the ClpXP foci were preferentially localized to the mother cell. Finally, we demonstrate preferential degradation in the forespore of the green fluorescent protein (GFP) that had been appended with LCN and preferential degradation of GFP appended with a recognition sequence for ClpXP (alanine-alanine-valine [AAV]) in the mother cell. Preferential degradation of SpoIIAB via its LCN tag may contribute to the forespore-specific activation of σF.
MATERIALS AND METHODS
Strain construction.
All strains used in this study are listed in Table 1. All oligonucleotide primers used for cloning are listed in Table 2. E. coli DH5α was the host for cloning all plasmids. Either electrocompetent or chemical-competent cells of E. coli were used for transformation of ligated constructs. The one-step method was used for transformation of B. subtilis strains with either integration plasmids or chromosomal DNA (41).
TABLE 1.
List of strains and plasmids
| Strain or plasmid | Genotypea | Reference or source |
|---|---|---|
| Strains | ||
| B. subtilis | ||
| PY79 | Prototrophic derivative of B. subtilis 168 | 46 |
| BJK166 | clpC-gfp spc | This study |
| BJK207 | clpP-gfp spc | This study |
| BJK210 | clpX-gfp spc | This study |
| LAS363 | clpP-linker-cfp cat | Gift of Lyle Simmons |
| LAS58 | clpX-yfp cat | Gift of Lyle Simmons |
| BJK485 | clpX-yfp cat clpP-linker-cfp spc | This study |
| BJK478 | clpP-linker-cfp spc | This study |
| BJK493 | clpC-yfp spc | This study |
| BJK499 | clpC-yfp spc clpP-linker-cfp cat | This study |
| BJK200 | clpP::erm clpC-gfp spc | This study |
| BJK242 | clpC::tet clpP-gfp spc | This study |
| BJK243 | clpC::tet clpX-gfp spc | This study |
| BJK234 | clpX::kan clpP-gfp spc | This study |
| BJK235 | clpX::kan clpC-gfp spc | This study |
| BJK370 | clpP::erm clpX-gfp spc | This study |
| RL2774 | clpC::tet | 28 |
| RL2259 | clpP::erm | 28 |
| BJK230 | clpX::kan | This study |
| BJK285 | clpE-gfp spc | This study |
| BJK424 | spx::erm | This study |
| BJK446 | clpC::cat | This study |
| BJK465 | spx::erm clpE-gfp spc clpC::tet clpX::kan | This study |
| BJK474 | spx::erm clpP-gfp spc clpE::tet clpC::cat clpX::kan | This study |
| BJK552 | clpC(1-640)-gfp spc | This study |
| BJK317 | clpC(1-452)-gfp spc | This study |
| BJK359 | amyE::Phyperspank-clpC(144-810)-gfp spc | This study |
| BJK506 | amyE::Phyperspank-clpC(453-810)-gfp spc | This study |
| BJK315 | clpC(1-143)-gfp spc | This study |
| BJK311 | clpX(1-57)-gfp spc | This study |
| BJK509 | clpCΩRBS-gfp-LCN kan | This study |
| BJK510 | clpCΩRBS-gfp kan | This study |
| BJK456 | amyE::Phyperspank-gfp-AAV spc | This study |
| BJK458 | amyE::Phyerspank-gfp spc | This study |
| E. coli | ||
| DH5α | Cloning host | Laboratory stock |
| BJK476 | Phyperspank-clpC-gfp bla | This study |
| BJK477 | Phyperspank-clpP-gfp bla | This study |
| BJK505 | Phyperspank-clpX-gfp bla | This study |
| BJK504 | Phyperspank-clpC(453-810)-gfp bla | This study |
| Plasmids | ||
| pDR111 | Phyperspankspc bla | Gift of David Rudner |
| pKL147 | gfp spc bla | 22 |
| pDG780 | kan bla | 13 |
| pAH52 | erm | Gift of Amy Hitchcock-Camp |
| pAC225 | cat | Gift of Arnaud Chastanet |
RBS, ribosome binding site.
TABLE 2.
List of primers
| Primer name | Nucleotide sequence (5′ to 3′) |
|---|---|
| OJK193 | CCGGAATTCCGCAATAAATATGTCGGCTTTAACG |
| OJK194 | GCCCTCGAGATTCGTTTTAGCAGTCGTTTTTACGA |
| OJK248 | CCCGAATTCTTGTTCATTTGCGGCGGACGTTTC |
| OJK249 | CCGCTCGAGTGCAGATGTTTTATCTTGGCTTACC |
| OJK250 | CCCGAATTCATGAATTTAATACCTACAGTCATTGAAC |
| OJK251 | CGCCTCGAGCTTTTTGTCTTCTGTGTGAGTCAAAAT |
| OJK358 | CCGGAATTCGACACTGTGATCATCATGACAAGTA |
| OJK359 | GCCCTCGAGTTTTGCTCGCACTTTGATTTTATCATC |
| OJK590 | CCGCTCGAGATGGTGAGCAAGGGCGAGGAG |
| OJK591 | CCCAAGCTTTTACTTGTACAGCTCGTCCATGCC |
| OJK454 | CATGACATTTCTGATTGCACTGGC |
| OJK455 | CAATTCGCCCTATAGTGAGTCGTCATCTTCACTCCTCTAATTAGTAGG |
| OJK456 | CCAGCTTTTGTTCCCTTTAGTGAGGATCGTATCATCAAAAGAAGGCTGA |
| OJK457 | TTCAATTTCCATTTGCCAACCTTCC |
| OJK277 | GCTGAAAACTACTCTCTAGAAGTAG |
| OJK278 | CAATTCGCCCTATAGTGAGTCGTTCTTTCACCCCTTAATCTTGTCTCA |
| OJK279 | CCAGCTTTTGTTCCCTTTAGTGAGGAAGAAGCATTGGTTGCGATCTTAA |
| OJK280 | CTCACTGTAATACGCACTGTCTAAA |
| OJK542 | AGCAAGAAACTTTGAGCATATTCGG |
| OJK543 | CAATTCGCCCTATAGTGAGTCGTGAATTTTTTCTGAACCGAGTCCAAG |
| OJK544 | CCAGCTTTTGTTCCCTTTAGTGAGATTGTTCTTGATGTAGAAGATGGCG |
| OJK546 | GAGCGTTCTTCTAAGAAAATTTCCG |
| OJK449 | GGCGTCGACACATAAGGAGGAACTACTATGTTTAAATTTAACGAGGAAAAAGGA |
| OJK599 | CCCGTCGACAGGAGGATGAATCGATATGGTAGAGGATACGAAGAAATCATGG |
| OJK616 | GCCCTCGAGTGAGCCGATTGGGCGTTTAGGATC |
| OJK380 | GCCGAATTCTTAGGCATAGACTTAGGCATAATAAAA |
| OJK383 | CGGCTCGAGTCCTAGAAGCTGGAGCACCTGCT |
| OJK386 | GCCGAATTCCTTGCTCGTGGCGAACTCCAATG |
| OJK387 | CGGCTCGAGTTGCTCGCGCAGGCGTTGTTCA |
| OJK377 | GCCGAATTCTTATCTCTTTTTCTTGGGGCAATATA |
| OJK378 | CGGCTCGAGTTCTACTTCTTCTTCTGTTCCGAGT |
| OJK446 | GGCGTCGACACATAAGGAGGAACTACTATGAGTAATGAAACAGGATCATCAGCGG |
| OJK606 | CCCGAATTCCTTCCATTCACTTGAGAAAAAACATC |
| OJK607 | CCCAAGCTTTTAATTCGTTTTAGCAGTCGTTTTTAC |
| OJK362 | GGCAAGCTTACATAAGGAGGAACTACTATGAGTAAAGGAGAAGAACTTTTCAC |
| OJK444 | GGCGGATCCTTAATTACAAAGTTTGTATAGTTCATCC |
| OJK445 | GGCGGATCCTTATTTGTATAGTTCATCCATGCCATG |
| OGH1 | CCCAAGCTTAGGAGGAGCATTATGAATTTAATACC |
| OGH2 | CCCGCATGCTTATTTGTATAGTTCATCCATGCCAT |
| OGH4 | CCCGTCGACAGGAGGATGAATCGATATGATGTTT |
To examine Clp localization, C-terminal fusions were built by inserting a portion of the 3′ end of clpC, clpE, clpX, or clpP using the product of the primer set OJK193-OJK194, OJK358-OJK359, OJK248-OJK249, or OJK250-OJK251, respectively, into the gfp-carrying vector pKL147 (22). These pKL147 derivatives were transformed into PY79 via a Campbell-type integration, yielding BJK166, BJK207, BJK210, or BJK285.
The dual-fluorophore-labeled strain BJK499 was built by transforming LAS363 (a gift of Lyle Simmons) with BJK493. BJK493 was built by a three-way ligation of the clpC fragment from OJK193-OJK194 and the yfp gene from OJK590-OJK591 into pKL147 with the gfp gene removed by restriction; the resulting vector was transformed into PY79. BJK485 was built by transforming LAS58 (a gift of Lyle Simmons) with BJK478. BJK478 was derived from LAS363 by replacement of the cat gene with the spc gene.
To assess the interdependence of Clp localization with the other clp genes, BJK166, BJK207, BJK210, and BJK285 were transformed with chromosomal DNA from strains with the appropriate insertion deletions (RL2774, RL2259, BJK446, BJK230, or BJK424), generating BJK200, BJK242, BJK243, BJK235, BJK370, BJK465, and BJK474. Insertion deletions were built using a long-flanking homology method (38) with the following primer sets: spx::erm from OJK454 through OJK457; clpX::kan from OJK277 through OJK280; and clpC::cat from OJK542, OJK543, OJK544, and OJK546. Templates for the antibiotic cassettes are from pAH52 (a gift of Amy Hitchcock-Camp), pDG780 (13), and pAC225 (a gift of Arnaud Chastanet).
The strains BJK476, BJK477, BJK505, and BJK504 were built using the primer pairs OGH1-OGH2, OGH4-OGH2, OJK449-OGH2, and OJK599-OGH2 to amplify the corresponding clp-gfp fragment, which was then inserted into pDR111 (a gift of David Rudner). These pDR111 derivatives were transformed into E. coli DH5α.
The C-terminal ClpC-GFP truncation deletion mutants BJK552, BJK315, and BJK317 were built by inserting a fragment lacking the appropriate C-terminal codons of the clpC gene, using the primer pair OJK386-OJK640, OJK380-OJK383, or OJK386-OJK387, into pKL147. These pKL147 derivatives were transformed into PY79. The N-terminal ClpC-GFP truncation deletion mutants BJK359 and BJK506 were built by inserting a fragment of clpC-gfp lacking the appropriate N-terminal codons from the primer pair OJK446-OGH2 or OJK599-OGH2 into pDR111. These pDR111 derivatives were transformed into PY79. BJK311 was built using the clpX PCR product from OJK377-OJK378 inserted into pKL147; the resulting vector was transformed by Campbell integration into PY79.
BJK509 was built by a three-way ligation of the PCR products OJK606-OJK607 and OJK362-OJK444 into pDG780 and transformation of this vector by Campbell integration into PY79. BJK510 was built by a three-way ligation of the PCR products OJK606-OJK607 and OJK362-OJK445 into pDG780 and transformation of this vector by Campbell integration into PY79.
Growth and media.
E. coli strain DH5α and its derivatives were grown in Luria-Bertani (LB) medium at 37°C. B. subtilis strain PY79 and its derivatives were grown in LB or casein hydrolysate (CH) medium at 37°C. Sporulation was induced either by exhaustion in Difco sporulation medium or by a resuspension method in SM medium as described previously (15). When appropriate, antibiotics were included at the following concentrations: ampicillin, 100 μg/ml; spectinomycin, 100 μg/ml; kanamycin, 10 μg/ml; tetracycline, 10 μg/ml; erythromycin plus lincomycin, 1 μg/ml and 25 μg/ml, respectively; and chloramphenicol, 5 μg/ml. When appropriate, IPTG (isopropyl-β-d-thiogalactopyranoside) was supplemented at a 1 M final concentration.
Fluorescence microscopy.
Cells were imaged with a BX61 Olympus microscope. For GFP imaging, a U-M41001 filter cube from Olympus was used (455- to 495-nm excitation filter, 510- to 555-nm barrier filter). For FM4-64 imaging, a U-MWG2 filter cube from Olympus was used (510- to 550-nm excitation filter, >590-nm barrier filter). For cyan fluorescent protein (CFP) imaging, a 31044v2 filter cube from Chroma was used (455 DCLP, D436/20×, D480/40m). For yellow fluorescent protein (YFP) imaging, a 41029 filter cube from Chroma was used (Q515LP, HQ500/20×, HQ520LP). Cells were spun down at appropriate time points and resuspended in phosphate-buffered saline. When indicated, the vital membrane dye FM4-64 was added. Cells of B. subtilis were immobilized on poly-l-lysine-treated coverslips and placed on glass slides. For imaging of E. coli, cells were immobilized on poly-l-lysine-treated coverslips and placed on glass slides with 1% agarose pads. Images were processed using Adobe Photoshop.
RESULTS
ClpC, ClpX, and ClpP localize as polar foci during growth.
To investigate the subcellular localization of ClpC, ClpE, ClpX, and ClpP, we created fusions at the 3′ end of the coding sequence for each protein to the coding sequence for GFP and integrated each gene fusion into the chromosome at its normal chromosomal position. The synthesis of each gene fusion was under the control of its normal clp gene promoter. The fusions were apparently functional because in no case did the replacement of the wild-type clp gene with the corresponding gene fusion measurably impair growth or sporulation. (Null mutations of clpX and clpP are known to be defective in growth, and clpC, clpX, and clpP mutations are defective in sporulation [11, 26, 28].)
First, we examined the subcellular localization of ClpC-GFP, ClpE-GFP, ClpX-GFP, and ClpP-GFP during exponential growth. We failed to detect a signal with ClpE-GFP, a point to which we return below; however, the other three proteins were all visible as foci, typically located at or near the cell poles (Fig. 1A to C). Occasionally, foci were seen at the midcell position, an example of which can be seen in Fig. 1B. Of cells containing ClpC-GFP, 31% (n = 261) harbored foci. Among these foci, 80% were at or near the pole, 11% were at the midcell position, and 9% could not confidently be assigned to either the pole or the midcell. Of cells containing ClpX-GFP, 70% (n = 254) exhibited foci, with 76% of these foci at or near the pole, 19% at the midcell position, and 5% that could not confidently be assigned. Finally, of cells containing ClpP-GFP, 62% (n = 232) displayed foci, with 80% of the foci at or near the pole, 14% at the midcell position, and 6% that could not be assigned.
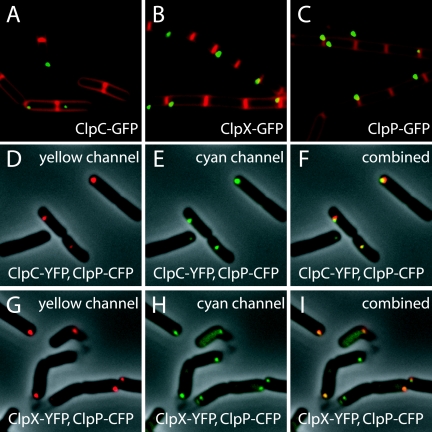
FIG. 1.
Subcellular localization of ClpC, ClpX, and ClpP. The coding sequence for GFP was fused to the coding sequence for ClpC (A; BJK166), ClpX (B; BJK210), or ClpP (C; BJK207), and images were captured from cells harboring the fusions by using fluorescence microscopy during exponential growth in LB medium. Membranes were stained with the dye FM4-64. Panels D to F show cells from a strain (BJK499) harboring both clpC-yfp and clpP-cfp. The cells were grown on LB plates and imaged by fluorescence microscopy in the yellow channel (false-colored red) (D), the cyan channel (false-colored green) (E), and merged (F). The yellow and cyan fluorescence signals were overlaid with the phase-contrast image. Panels G to I show cells from a strain (BJK485) harboring both clpX-yfp and clpP-cfp. Cells were taken from plates and imaged with fluorescence microscopy in the yellow channel (false-colored red) (G), the cyan channel (false-colored green) (H), and merged (I). The yellow and cyan fluorescence signals were overlaid with the phase-contrast image.
Next, we sought to examine whether ClpC and ClpX colocalized with their partner protein ClpP. To do so, we created fusions to YFP for ClpC and ClpX and a fusion to CFP for ClpP. The YFP fusions appeared to be fully functional, but creating a functional ClpP-CFP fusion proved to be challenging. However, by inserting a 23-residue linker between ClpP and CFP (see Materials and Methods), we succeeded in building a fusion that caused only a mild growth defect. Using cells producing both kinds of fusions, we observed substantial colocalization of ClpP-CFP foci with foci from ClpC-YFP (Fig. 1D to F) and likewise with foci from ClpX-YFP (Fig. 1G to I).
ClpC, ClpX, and ClpP localizations are not interdependent.
We next wished to determine whether the localization of ClpP was interdependent with the ClpC or ClpX ATPases. Clp-GFP localization was investigated systematically in single gene deletions of clpC, clpX, and clpP. As shown in Fig. 2A, no single gene deletion disrupted the polar localization of the remaining two Clp-GFP proteins. There are two possible explanations for this result. In the first model, ClpP localization depends on its partner ATPase for polar localization. However, since ClpP can interact with either ClpC or ClpX, the absence of one is compensated for by the other. In the second model, ClpC, ClpX, and ClpP are each capable of polar localization independently. To distinguish between the two models, we sought to construct a strain with a double deletion of both clpC and clpX. Such a double deletion mutant is known to be inviable (20), but we reasoned it might be possible to create the double mutant by the use of a suppressor mutation in spx. It was known from previous work that spx can suppress the growth and competence defects of clpX or clpP mutants (27). Indeed, a clpC clpX spx triple mutant was viable. Interestingly, ClpP-GFP was able to localize as polar foci in the double deletion mutant for the ATPase genes (data not shown, but see below).
FIG. 2.
Polar localizations of ClpP and its ATPases are not interdependent. Panel A shows the effects of the indicated clp mutations on the localization of the indicated Clp-GFP fusions. The strains used were BJK243 (a), BJK235 (b), BJK200 (c), BJK242 (d), BJK234 (e), and BJK370 (f). Panel B shows the localization of the indicated Clp-GFP fusions in the presence of no mutation or the indicated multiple mutations. The strains used were BJK285 (a), BJK465 (b), and BJK474 (c). Panel C shows the localization of fusions of GFP to the indicated B. subtilis Clp proteins produced in E. coli. The gene fusions for the proteins were expressed from an IPTG-inducible promoter, and the images were captured 30 min after the addition of IPTG (1 mM final concentration) during exponential-phase growth in CH medium. Membranes were stained with the dye FM4-64 and overlaid with the GFP signal. The strains used were BJK476 (a), BJK477 (b), and BJK505 (c).
As mentioned above, ClpE-GFP was not readily detectable by fluorescence microscopy [Fig. 2B(a)], likely due to its low abundance in non-heat-shocked cells (5). Interestingly, however, ClpE-GFP could be seen as bright polar foci in the clpC clpX spx triple mutant [Fig. 2B(b)]. Perhaps the absence of the other two ATPases upregulated the synthesis of ClpE. In any event, the use of the triple mutant allowed us to conclude that ClpE molecules also coalesce into discrete foci.
Finally, we built a quadruple mutant with deletions of spx and the genes for all three ATPases: clpC, clpX, and clpE. As observed in Fig. 2B(c), ClpP-GFP exhibited a clear polar localization signal in the absence of its three cognate ATPases. Taken together, we conclude that ClpC-GFP, ClpX-GFP, and ClpP-GFP localizations are not interdependent.
B. subtilis Clp proteins localize as polar foci when produced in E. coli.
Next, we asked whether the Clp proteins would display polar localization in a heterologous host, such as E. coli. clpC-gfp, clpX-gfp, and clpP-gfp were cloned into a vector containing an IPTG-inducible promoter and transformed into E. coli. ClpC-GFP, ClpX-GFP, and ClpP-GFP were each able to localize to the poles when artificially expressed in E. coli [Fig. 2C(a) through (c)]. This result suggests either that the B. subtilis Clp proteins are intrinsically able to recognize a shared hallmark of bacterial poles or that the distantly related E. coli has a conserved anchor protein at its pole, perhaps used for its own endogenous Clp proteins.
The region of ClpC containing the second ATPase domain is necessary and sufficient for polar localization.
We next sought to identify the domain(s) of ClpC responsible for polar localization by creating truncated proteins. ClpC, which is 810 residues long, can be roughly divided into five domains: two N domains in tandem, followed by an AAA domain, a linker domain with homology to the UVR family, and finally a second AAA domain (Fig. 3A) (7). Initially, we built truncated versions of ClpC that lacked amino acids from the C-terminal end by fusing the coding sequence for gfp to copies of clpC harboring deletions from the 3′ end of the coding sequence. The truncation constructs were integrated into the chromosome at the normal position of clpC. The synthesis of each fusion was under the control of the native clpC promoter. We found that removal of 170 amino acids from the C terminus did not prevent ClpC-GFP from localizing to the poles, whereas a longer truncation removing the C-terminal AAA domain impaired localization severely [Fig. 3B(a-c)].
FIG. 3.
An AAA-containing domain of ClpC is sufficient for polar focus formation. Panel A shows the anatomy of Clp ATPases and the end points of the indicated truncations of ClpC. The ATPases have one or two nucleotide-binding domains designated AAA1 or AAA2, with AAA1 domains more similar to each other than to AAA2 domains. Additional domains are labeled as follows: N, Clp N domain; L, UVR linker domain; Z, zinc binding domain. Horizontal lines indicate truncations from the N-terminal or C-terminal direction, with short vertical lines designating the end points. Panel B shows the localization pattern of GFP fused to the indicated truncations of ClpC. The gene fusions were expressed from either the native promoter (a to c) or from IPTG-inducible promoters (d to f), and images were captured 30 min after the addition of IPTG (1 mM final concentration) to cells in the exponential phase of growth in CH medium. Fluorescence from staining with the dye FM4-64 was overlaid with the signal from GFP. The cells were B. subtilis, except for those in image f, in which the indicated ClpC truncation was artificially produced in E. coli by use of an IPTG-inducible promoter. The strains were BJK166 (a), BJK552 (b), BJK317 (c), BJK359 (d), BJK506 (e), and BJK504 (f).
We then created deletions of the clpC-gfp gene fusion that extended in from the 5′ end of the coding sequence. These deletion-mutated gene fusions were placed under the control of an IPTG-inducible promoter and integrated into the chromosome at the amyE locus. As observed in Fig. 3B(d) and (e), removal of the tandem N domains, the N-terminal-proximal AAA domain, and the UVR linker region (residues 1 to 452) had little or no effect on polar localization. The same truncated protein (lacking residues 1 to 452) was also capable of polar localization when produced artificially in E. coli [Fig. 3B(f)]. Taken together, we infer that the region in common between the N-terminal truncations and the C-terminal truncation (residues 453 to 640), which contains the second (C-terminal-proximal) AAA domain, is responsible for localization of ClpC-GFP. An appealing aspect of this model is that the sole AAA domain of ClpX, as well as the C-terminal-proximal AAA domain of ClpE, is homologous to the C-terminal-proximal AAA domain of ClpC. We hypothesize that in all cases the AAA domain is responsible for Clp proteins to coalesce into foci that are preferentially positioned near the cell pole.
ClpC, ClpX, and ClpP differentially localize during sporulation.
Since ClpC, ClpX, and ClpP are required for sporulation, we examined their subcellular localization after entry into this developmental process (11, 26, 28). Early in sporulation, the developing cell is divided by the formation of a polar septum into a large compartment called the mother cell and a small compartment called the forespore (35). As sporulation proceeds, the membranes of the polar septum migrate around the forespore, eventually fully engulfing it as a free protoplast within the mother cell.
We found that ClpX-GFP and ClpP-GFP had similar localization patterns, displaying a large focus clearly visible in the mother cell (Fig. 4A and B). The large focus accumulated either near the polar septum or near the other end of the mother cell. Often, a significantly smaller focus can be detected abutting the forespore membranes (Fig. 4A and B).
FIG. 4.
Subcellular localization of ClpC, ClpX, and ClpP during sporulation. Panel A shows the subcellular localization of ClpX-GFP (a; BJK210) and ClpP-GFP (b; BJK207) 3 h after the induction of sporulation by resuspension. Arrows highlight large foci in the mother cell. Arrowheads highlight smaller foci frequently found on the forespore. Panel B shows a time course of ClpC-GFP (BJK166) during sporulation. Cells were imaged at 0.5 (a), 2 (b), 3 (c), and 5 (d) hours after the induction of sporulation by resuspension. In image b, the arrow highlights a spirallike focus pattern in the mother cell, and the arrowhead highlights foci tracking with the engulfing membranes. In image c, arrows highlight a diffuse GFP signal in the mother cell while arrowheads highlight foci associated with the forespore. In image d, arrowheads highlight delocalized foci in the forespore. All images were captured by fluorescence microscopy. Membranes were stained with the dye FM4-64 and overlaid with the GFP signal. Also shown in the panels are interpretive cartoons.
In contrast, ClpC-GFP exhibited a dynamic localization pattern during sporulation. ClpC-GFP switched from polar foci to a diffuse pattern of fluorescence at the onset of sporulation. At the beginning of engulfment, when the septal membranes start to migrate around the forespore, ClpC-GFP was visible as a spirallike structure in the mother cell [Fig. 4B(b)]. Additionally, ClpC-GFP foci appeared to track with the engulfing membranes. Strikingly, at later times, ClpC-GFP appeared as a discrete focus abutting the forespore membrane at the distal pole [Fig. 4B(c)] while the GFP signal in the mother cell remained diffuse. Finally, late in sporulation, the ClpC-GFP focus abutting the forespore delocalized [Fig. 4B(d)].
ClpC and ClpX differentially accumulate in the forespore and the mother cell, respectively.
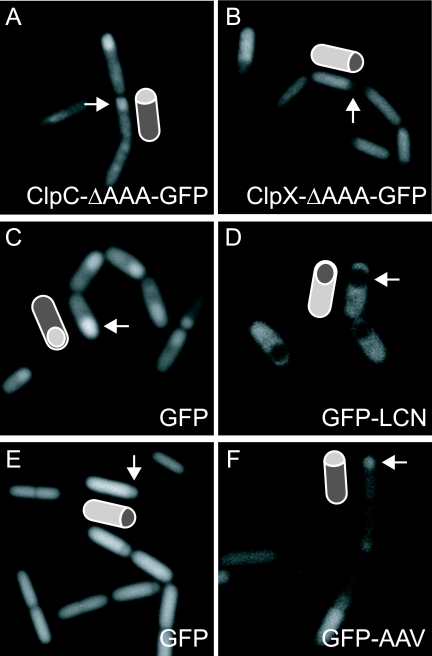
It was tempting to hypothesize that the localization patterns of ClpC-GFP, ClpX-GFP, and ClpP-GFP observed during sporulation represented differential localization of the proteolytic complexes, with ClpXP being enriched in the mother cell and ClpCP enriched in the forespore. To investigate this hypothesis further, we used GFP fusions to C-terminal truncations of ClpC and ClpX that could not coalesce into foci. We therefore expected that fluorescence would accumulate throughout one compartment or the other. Indeed, fluorescence from the GFP fusion to truncated ClpC accumulated preferentially in the forespore and fluorescence from the fusion to truncated ClpX accumulated preferentially in the mother cell (Fig. 5A and B).
FIG. 5.
ClpCP and ClpXP exhibit compartment-specific biases in localization and activity during sporulation. Panels A and B show that the N-terminal portion of ClpC or ClpX fused to GFP accumulates in the forespore or the mother cell, respectively. The strains used were BJK315 (A) and BJK311 (B). Images were taken using fluorescence microscopy 2 h after initiation of sporulation by resuspension. Panels C and D show the pattern of accumulation of GFP (C; BJK510) or GFP-LCN (D; BJK456) produced from copies of the corresponding coding sequences that had been introduced into the chromosome just downstream of the native locus for clpC. Images were captured at hour 3 after resuspension in SM medium. Panels E and F show the pattern of accumulation of GFP (E; BJK458) or GFP-LCN (F; BJK456) produced under the control of an IPTG-inducible promoter. Images were captured at hour 1.5 after resuspension. Also shown in the panels are interpretative cartoons, with arrows identifying the forespores.
The four-gene operon that contains clpC at its terminus is reported to contain a promoter for the forespore-specific transcription factor σF. This putative promoter is located just upstream of the second member of the operon, mcsA (39). It therefore seemed possible that, at least in the case of ClpC, differential accumulation was occurring at the transcriptional level. To investigate this, we created a transcriptional fusion by inserting the coding sequence for gfp together with a ribosome binding site sequence just downstream of clpC in the operon. Once again, fluorescence was seen to accumulate preferentially in the forespore (Fig. 5C). Taken together, the results indicate that ClpC and ClpX differentially accumulate in the forespore and the mother cell, respectively, and that at least in the case of ClpC, compartmentalization is occurring at the level of transcription.
ClpCP preferentially degrades substrates in the forespore.
If ClpCP differentially accumulates in the forespore, then there ought to be preferential degradation of its substrates in the small chamber of the sporangium. Previous work by Pan and Losick demonstrated that ClpCP was proteolytically active in both the forespore and the mother cell; however, whether substrates were cleared more rapidly from one compartment or the other was not examined (29). To test for preferential degradation in the forespore, we again inserted a copy of the gene for GFP immediately downstream of clpC. Recall that GFP preferentially accumulates in the forespore compartment with this construct (Fig. 5C). Next, we inserted a modified form of the gene for GFP to which had been appended codons for the residues LCN. We know from earlier work that GFP-LCN is degraded in a ClpCP-dependent manner (29). If ClpCP-mediated proteolysis is indeed greater in the forespore than in the mother cell, then we should observe a loss of the forespore-specific fluorescence when using GFP-LCN. Strikingly, with GFP-LCN, little or no fluorescence was seen in the forespore; instead, only the mother cell exhibited fluorescence (Fig. 5C and D). We interpret this result as indicating that GFP-LCN was indeed rapidly and preferentially degraded in the forespore. Thus, we conclude that ClpCP acts preferentially in the forespore.
ClpXP preferentially degrades substrates in the mother cell.
Next, we investigated whether an analogous relationship exists between ClpXP and the mother cell. The coding sequence for GFP or GFP with the ClpXP-specific degradation tag (AAV) was cloned into a vector containing an IPTG-inducible promoter (2). We found that GFP expressed from the inducible promoter exhibited preferential accumulation in the mother cell in about 55% of the cells (Fig. 5E), a phenomenon commonly observed with inducible promoters under the control of σA, which exhibit preferential transcription in the mother cell (23). However, when we examined the pattern of fluorescence from GFP-AAV, only 3% of the cells retained a mother cell bias. The majority of cells (88%) showed no bias, and a small proportion of cells (9%) displayed a forespore bias, with a stronger signal in the forespore than in the mother cell (Fig. 5F). Cells with a forespore bias were never observed with the untagged GFP. In toto, these results suggest that ClpCP and ClpXP preferentially act in different compartments of the sporangium, with the former biased to the forespore and the latter to the mother cell.
DISCUSSION
We have presented evidence that the ClpCP, ClpEP, and ClpXP proteases localize as foci near the poles of growing cells. Interestingly, it appears that the ClpP peptidase and its cognate ATPase subunits are able to localize to the poles independently of each other. Additionally, we found that when artificially produced in E. coli, ClpC, ClpX, or ClpP of B. subtilis localized to the poles. This suggests either that the Clp proteins are intrinsically able to recognize some common feature of a cell pole or that polar localization is an evolutionarily conserved mechanism. In an accompanying report, Simmons et al. (34) report similar observations on the formation of focal assemblies by Clp proteins.
The mechanism by which the Clp proteins localize near the cell pole is unknown. Conceivably, the proteins self-assemble into supramolecular foci and are then held at or near the pole by a protein anchor. An attractive candidate is DivIVA, a polarly localized protein that is known to anchor the sporulation protein RacA at the poles (3, 24, 36). However, a mutant lacking DivIVA was unimpaired in the localization of ClpC-GFP and ClpP-GFP (data not shown). Moreover, DivIVA does not have an ortholog in E. coli, in which the B. subtilis proteins were able to localize.
Since the Clp proteins were occasionally localized to the midcell position prior to septation, we considered the possibility that the cell division machinery could be responsible for localization. This was an attractive idea because the cell division site becomes the future cell pole. We explored this possibility by creating filaments using cells engineered to produce MciZ, an inhibitor of the division protein FtsZ (14), during growth, in response to an inducer. The Clp proteins maintained their capacity to localize as foci near the poles of filaments (data not shown). Interestingly, however, foci could also be seen abutting the membrane at regular intervals along the length of a filament. A similar observation was made with rare, filamenting cells of E. coli artificially expressing the B. subtilis Clp-GFP proteins (data not shown). Additionally, an accompanying report by Simmons et al. (34) shows that ClpX and ClpP do not colocalize with condensed, DAPI (4′,6′-diamidino-2-phenylindole)-stained nucleoids. Conceivably, foci of Clp proteins are restricted to the polar region of the cell and at regular intervals in filaments by nucleoid occlusion (4, 42, 43). Nucleoid occlusion would explain why Clp proteins were seen near the poles but not always at the extreme poles and why foci were occasionally observed at the midcell position.
The physiological reason for the polar localization of the proteases is unknown. The ClpCP and ClpXP proteases are involved in several cellular processes, and it is not known which, if any, require polar localization. Perhaps this is a mechanism for spatially regulating degradation by keeping the protease and its substrates separated until an appropriate time. This seems to be the case with the proteolysis of the master regulator of the cell cycle CtrA by ClpXP in C. crescentus (16, 25). CtrA and ClpXP colocalize at the cell pole only at the G1-to-S transition, a time point when CtrA is known to be degraded.
A further intriguing aspect of our findings is that ClpCP and ClpXP become compartmentalized after the stage of sporulation when the sporangium becomes divided into forespore and mother cell compartments. Our results show that ClpCP preferentially accumulates in the forespore and ClpXP in the mother cell. Moreover, artificial substrates appended with the recognition sequences for the two proteases were preferentially depleted from the corresponding compartment (i.e., GFP-LCN from the forespore and GFP-AAV from the mother cell). Previous work showed that the half-life of the ClpCP substrate SpoIIAB during sporulation was 28 min (28). However, the half-life was measured based on the degradation of SpoIIAB in both the mother cell and the forespore. Since it is the forespore-specific degradation of SpoIIAB that is physiologically relevant, the reported half-life may have been an underestimation of the true value. The basis for compartmentalization of ClpXP is unclear, but the presence of a putative promoter recognized by the forespore transcription factor σF upstream of clpC suggests that compartmentalization of ClpCP is mediated at least in part at the transcriptional level (39). Another mechanism that may contribute to the Clp compartmentalization is the transient genetic asymmetry due to the position of the clp genes on the chromosome. During sporulation, polar septation is completed prior to chromosome translocation; the origin-distal two-thirds of the forespore chromosome is temporarily trapped in the mother cell (43, 44). A DNA translocase pumps the remaining two-thirds into the forespore over the next 15 to 20 min, representing a period of transient genetic asymmetry (45). Frequently, genes with forespore functions are on the origin-proximal region of the chromosome while genes with mother cell functions are on the origin-distal region (8). Notably, clpC is on the origin-proximal third of the chromosome while clpX is not. We speculate that differential compartmentalization serves to clear certain proteins in a selective manner from the mother cell or the forespore. In this regard, the demonstration that GFP appended with a terminal sequence (LCN) that is unique to the anti-σF factor SpoIIAB and shown to be necessary and sufficient to promote degradation in a ClpCP-dependent manner suggests that compartmentalization contributes to the cell-specific activation of σF (Fig. 6). That is, it is conceivable that activation of σF in the forespore sets up a self-reinforcing cycle that promotes ClpC synthesis in the forespore and thereby enhances degradation of SpoIIAB in the small chamber of the sporangium. Alternatively, or additionally, preferential degradation of SpoIIAB in the forespore could contribute to the activation of σG, a later-appearing transcription factor in the forespore that is also known to be subject to inhibition by the anti-sigma factor (18, 19).
FIG. 6.
Model for a self-reinforcing cycle that contributes to the activation of σF. Panel A summarizes the interrelationships among the anti-σF anti-sigma factor SpoIIAB (AB), the anti-anti-sigma factor SpoIIAA (AA), the inactive, phosphorylated form of SpoIIAA (AA-P), the SpoIIAA-P phosphatase SpoIIE (E), and ClpCP. AB is both an anti-sigma factor and a protein kinase that phosphorylates AA. Unphosphorylated AA can both displace σF from the AB-σF complex and become trapped in an inactive complex with AB (AA-AB). Notice that removal of AB by degradation increases the relative amount of AA, leading to increased disruption of σF-AB and hence increased levels of free and active σF. Panel B depicts a hypothesized self-reinforcing cycle in which σF drives additional synthesis of ClpC, which, in turn, further reduces the levels of the AB anti-sigma factor, leading to yet higher levels of free and active σF.
More speculatively, there could be additional targets for compartment-specific proteolysis. The master regulator of sporulation Spo0A, which is produced at the start of sporulation, becomes a mother cell-specific transcription factor after the stage of asymmetric division (10). The mechanism for this compartmentalization is unknown, but it is conceivable that selective proteolysis of Spo0A or the phosphotransfer protein Spo0F or Spo0B in the forespore could contribute to the elimination of Spo0A activity from the forespore. It has also been suggested that restriction of σE to the mother cell is due to both preferential synthesis in the mother cell under Spo0A control and selective degradation in the forespore (9).
Other ATP-dependent proteases are reported to localize to particular subcellular positions or compartments in B. subtilis. Examples are FtsH, which localizes to the septum, and LonB, which localizes to the cytoplasm of the forespore (33, 40). In the accompanying paper, Simmons et al. (34) additionally show that LonA localized to the nucleoid and that ClpQ localized as small foci coincident with the membranes. Our present findings with ClpP proteases indicate that subcellular localization and compartmentalization are general features of ATP-dependent proteases in B. subtilis and reinforce the view that the spatial control of proteolysis is a pervasive feature of regulatory pathways in bacteria.
Acknowledgments
We thank L. Simmons, A. Grossman, G. Walker, D. Rudner, A. Chastanet, and A. Camp for strains and plasmids and L. Simons, A. Grossman, and G. Walker for sharing their results prior to publication. We thank A. Chastanet, K. Ramamurthi, A. Camp, and D. Rudner for helpful comments and discussions.
This work was supported by NIH grant GM 18568 to R.L.
Footnotes
Published ahead of print on 8 August 2008.
REFERENCES
- 1.Alba, B. M., J. A. Leeds, C. Onufryk, C. Z. Lu, and C. A. Gross. 2002. DegS and YaeL participate sequentially in the cleavage of RseA to activate the sigma(E)-dependent extracytoplasmic stress response. Genes Dev. 162156-2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andersen, J. B., C. Sternberg, L. K. Poulsen, S. P. Bjorn, M. Givskov, and S. Molin. 1998. New unstable variants of green fluorescent protein for studies of transient gene expression in bacteria. Appl. Environ. Microbiol. 642240-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ben-Yehuda, S., M. Fujita, X. S. Liu, B. Gorbatyuk, D. Skoko, J. Yan, J. F. Marko, J. S. Liu, P. Eichenberger, D. Z. Rudner, and R. Losick. 2005. Defining a centromere-like element in Bacillus subtilis by identifying the binding sites for the chromosome-anchoring protein RacA. Mol. Cell 17773-782. [DOI] [PubMed] [Google Scholar]
- 4.Bernhardt, T. G., and P. A. de Boer. 2005. SlmA, a nucleoid-associated, FtsZ binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol. Cell 18555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Derre, I., G. Rapoport, K. Devine, M. Rose, and T. Msadek. 1999. ClpE, a novel type of HSP100 ATPase, is part of the CtsR heat shock regulon of Bacillus subtilis. Mol. Microbiol. 32581-593. [DOI] [PubMed] [Google Scholar]
- 6.Domian, I. J., K. C. Quon, and L. Shapiro. 1997. Cell type-specific phosphorylation and proteolysis of a transcriptional regulator controls the G1-to-S transition in a bacterial cell cycle. Cell 90415-424. [DOI] [PubMed] [Google Scholar]
- 7.Dougan, D. A., A. Mogk, K. Zeth, K. Turgay, and B. Bukau. 2002. AAA+ proteins and substrate recognition, it all depends on their partner in crime. FEBS Lett. 5296-10. [DOI] [PubMed] [Google Scholar]
- 8.Frandsen, N., I. Barak, C. Karmazyn-Campelli, and P. Stragier. 1999. Transient gene asymmetry during sporulation and establishment of cell specificity in Bacillus subtilis. Genes Dev. 13394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita, M., and R. Losick. 2002. An investigation into the compartmentalization of the sporulation transcription factor sigmaE in Bacillus subtilis. Mol. Microbiol. 4327-38. [DOI] [PubMed] [Google Scholar]
- 10.Fujita, M., and R. Losick. 2003. The master regulator for entry into sporulation in Bacillus subtilis becomes a cell-specific transcription factor after asymmetric division. Genes Dev. 171166-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerth, U., E. Kruger, I. Derre, T. Msadek, and M. Hecker. 1998. Stress induction of the Bacillus subtilis clpP gene encoding a homologue of the proteolytic component of the Clp protease and the involvement of ClpP and ClpX in stress tolerance. Mol. Microbiol. 28787-802. [DOI] [PubMed] [Google Scholar]
- 12.Gottesman, S. 2003. Proteolysis in bacterial regulatory circuits. Annu. Rev. Cell Dev. Biol. 19565-587. [DOI] [PubMed] [Google Scholar]
- 13.Guerout-Fleury, A. M., K. Shazand, N. Frandsen, and P. Stragier. 1995. Antibiotic-resistance cassettes for Bacillus subtilis. Gene 167335-336. [DOI] [PubMed] [Google Scholar]
- 14.Handler, A. A., J. E. Lim, and R. Losick. 2008. Peptide inhibitor of cytokinesis during sporulation in Bacillus subtilis. Mol. Microbiol. 68588-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harwood, C., and S. Cutting. 1990. Molecular biological methods for bacillus. John Wiley & Sons, New York, NY.
- 16.Iniesta, A. A., P. T. McGrath, A. Reisenauer, H. H. McAdams, and L. Shapiro. 2006. A phospho-signaling pathway controls the localization and activity of a protease complex critical for bacterial cell cycle progression. Proc. Natl. Acad. Sci. USA 10310935-10940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jenal, U., and T. Fuchs. 1998. An essential protease involved in bacterial cell-cycle control. EMBO J. 175658-5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kellner, E. M., A. Decatur, and C. P. Moran, Jr. 1996. Two-stage regulation of an anti-sigma factor determines developmental fate during bacterial endospore formation. Mol. Microbiol. 21913-924. [DOI] [PubMed] [Google Scholar]
- 19.Kirchman, P. A., H. DeGrazia, E. M. Kellner, and C. P. Moran, Jr. 1993. Forespore-specific disappearance of the sigma-factor antagonist spoIIAB: implications for its role in determination of cell fate in Bacillus subtilis. Mol. Microbiol. 8663-671. [DOI] [PubMed] [Google Scholar]
- 20.Krüger, E., E. Witt, S. Ohlmeier, R. Hanschke, and M. Hecker. 2000. The Clp proteases of Bacillus subtilis are directly involved in degradation of misfolded proteins. J. Bacteriol. 1823259-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lange, R., and R. Hengge-Aronis. 1994. The cellular concentration of the sigma S subunit of RNA polymerase in Escherichia coli is controlled at the levels of transcription, translation, and protein stability. Genes Dev. 81600-1612. [DOI] [PubMed] [Google Scholar]
- 22.Lemon, K. P., and A. D. Grossman. 1998. Localization of bacterial DNA polymerase: evidence for a factory model of replication. Science 2821516-1519. [DOI] [PubMed] [Google Scholar]
- 23.Marquis, K. A., B. M. Burton, M. Nollmann, J. L. Ptacin, C. Bustamante, S. Ben-Yehuda, and D. Z. Rudner. 2008. SpoIIIE strips proteins off the DNA during chromosome translocation. Genes Dev. 221786-1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marston, A. L., H. B. Thomaides, D. H. Edwards, M. E. Sharpe, and J. Errington. 1998. Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev. 123419-3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGrath, P. T., A. A. Iniesta, K. R. Ryan, L. Shapiro, and H. H. McAdams. 2006. A dynamically localized protease complex and a polar specificity factor control a cell cycle master regulator. Cell 124535-547. [DOI] [PubMed] [Google Scholar]
- 26.Msadek, T., V. Dartois, F. Kunst, M. L. Herbaud, F. Denizot, and G. Rapoport. 1998. ClpP of Bacillus subtilis is required for competence development, motility, degradative enzyme synthesis, growth at high temperature and sporulation. Mol. Microbiol. 27899-914. [DOI] [PubMed] [Google Scholar]
- 27.Nakano, M. M., F. Hajarizadeh, Y. Zhu, and P. Zuber. 2001. Loss-of-function mutations in yjbD result in ClpX- and ClpP-independent competence development of Bacillus subtilis. Mol. Microbiol. 42383-394. [DOI] [PubMed] [Google Scholar]
- 28.Pan, Q., D. A. Garsin, and R. Losick. 2001. Self-reinforcing activation of a cell-specific transcription factor by proteolysis of an anti-sigma factor in B. subtilis. Mol. Cell 8873-883. [DOI] [PubMed] [Google Scholar]
- 29.Pan, Q., and R. Losick. 2003. Unique degradation signal for ClpCP in Bacillus subtilis. J. Bacteriol. 1855275-5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ryan, K. R., S. Huntwork, and L. Shapiro. 2004. Recruitment of a cytoplasmic response regulator to the cell pole is linked to its cell cycle-regulated proteolysis. Proc. Natl. Acad. Sci. USA 1017415-7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ryan, K. R., E. M. Judd, and L. Shapiro. 2002. The CtrA response regulator essential for Caulobacter crescentus cell-cycle progression requires a bipartite degradation signal for temporally controlled proteolysis. J. Mol. Biol. 324443-455. [DOI] [PubMed] [Google Scholar]
- 32.Sauer, R. T., D. N. Bolon, B. M. Burton, R. E. Burton, J. M. Flynn, R. A. Grant, G. L. Hersch, S. A. Joshi, J. A. Kenniston, I. Levchenko, S. B. Neher, E. S. Oakes, S. M. Siddiqui, D. A. Wah, and T. A. Baker. 2004. Sculpting the proteome with AAA(+) proteases and disassembly machines. Cell 1199-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrano, M., S. Hövel, C. P. Moran, Jr., A. O. Henriques, and U. Völker. 2001. Forespore-specific transcription of the lonB gene during sporulation in Bacillus subtilis. J. Bacteriol. 1832995-3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Simmons, L. A., A. D. Grossman, and G. C. Walker. 2008. Clp and Lon proteases occupy distinct subcellular positions in Bacillus subtilis. J. Bacteriol. 1906758-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stragier, P., and R. Losick. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30297-341. [DOI] [PubMed] [Google Scholar]
- 36.Thomaides, H. B., M. Freeman, M. El Karoui, and J. Errington. 2001. Division site selection protein DivIVA of Bacillus subtilis has a second distinct function in chromosome segregation during sporulation. Genes Dev. 151662-1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turgay, K., J. Hahn, J. Burghoorn, and D. Dubnau. 1998. Competence in Bacillus subtilis is controlled by regulated proteolysis of a transcription factor. EMBO J. 176730-6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wach, A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12259-265. [DOI] [PubMed] [Google Scholar]
- 39.Wang, S. T., B. Setlow, E. M. Conlon, J. L. Lyon, D. Imamura, T. Sato, P. Setlow, R. Losick, and P. Eichenberger. 2006. The forespore line of gene expression in Bacillus subtilis. J. Mol. Biol. 35816-37. [DOI] [PubMed] [Google Scholar]
- 40.Wehrl, W., M. Niederweis, and W. Schumann. 2000. The FtsH protein accumulates at the septum of Bacillus subtilis during cell division and sporulation. J. Bacteriol. 1823870-3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson, G. A., and K. F. Bott. 1968. Nutritional factors influencing the development of competence in the Bacillus subtilis transformation system. J. Bacteriol. 951439-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woldringh, C. L., E. Mulder, P. G. Huls, and N. Vischer. 1991. Toporegulation of bacterial division according to the nucleoid occlusion model. Res. Microbiol. 142309-320. [DOI] [PubMed] [Google Scholar]
- 43.Wu, L. J., and J. Errington. 2004. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell 117915-925. [DOI] [PubMed] [Google Scholar]
- 44.Wu, L. J., and J. Errington. 1998. Use of asymmetric cell division and spoIIIE mutants to probe chromosome orientation and organization in Bacillus subtilis. Mol. Microbiol. 27777-786. [DOI] [PubMed] [Google Scholar]
- 45.Wu, L. J., P. J. Lewis, R. Allmansberger, P. M. Hauser, and J. Errington. 1995. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 91316-1326. [DOI] [PubMed] [Google Scholar]
- 46.Youngman, P., J. B. Perkins, and R. Losick. 1984. Construction of a cloning site near one end of Tn917 into which foreign DNA may be inserted without affecting transposition in Bacillus subtilis or expression of the transposon-borne erm gene. Plasmid 121-9. [DOI] [PubMed] [Google Scholar]