Abstract
The periplasmic multicopper oxidase (CueO) is involved in copper homeostasis and protection against oxidative stress. Here, we show that the deletion of cueO in uropathogenic Escherichia coli increases its colonization of the urinary tract despite its increased sensitivity to hydrogen peroxide. The cueO deletion mutant accumulated iron with increased efficiency compared to its parent strain; this may account for its advantage in the iron-limited environment of the urinary tract.
Urinary tract infections (UTI) are among the most common infectious diseases of humans and a major cause of morbidity. It is estimated that 40 to 50% of healthy adult women have experienced at least one UTI episode in their lifetime (6). Uropathogenic Escherichia coli (UPEC) is the cause of the majority (>80%) of UTI in humans. UPEC isolates exhibit a high degree of genetic diversity due to the possession of specialized virulence genes located on pathogenicity islands (21). Although no single virulence factor is uniquely definitive of UPEC, its ability to cause symptomatic UTI is enhanced by adhesins (e.g., type 1 and P fimbriae) and toxins (e.g., hemolysin) (16, 38). Another important virulence property of UPEC is its ability to sequester iron. The concentration of soluble iron is very low in urine and represents an important growth-limiting factor for bacteria. UPEC possesses multiple mechanisms to acquire iron, including the production of siderophores, such as aerobactin and enterobactin (and the glycosylated enterobactin derivative, salmochelin), and the direct utilization of host iron compounds (particularly heme or hemoglobin) (1, 3, 10, 23, 25, 26). UPEC mutants deleted in these processes display reduced virulence in the mouse urinary tract (32).
The global oxidative stress response regulator OxyR is required for virulence in a mouse model of UTI (14). This indicates that UPEC responds to oxidative stress during infection, consistent with evidence that the attachment of UPEC to the uroepithelium leads to neutrophil recruitment (11, 14). OxyR also acts in concert with Dam methyltransferase to regulate the expression of the antigen 43-encoding flu gene (9, 12, 28). Antigen 43 is an autotransporter protein that promotes aggregation, biofilm formation, and long-term persistence of UPEC in the urinary bladder (15, 36).
The cueO gene encodes a periplasmic multicopper oxidase which is known to be involved in copper homeostasis and protection against oxidative stress. CueO possesses ferroxidase (Fe2+→Fe3+), cuprous oxidase (Cu+→Cu2+), and polyphenol oxidase (oxidation of phenolic compounds, including enterobactin) activities (8, 24, 30). Both Cu+ and Fe2+ generate toxic hydroxyl radicals via the Fenton reaction (Fe2+ or Cu+ + H2O2 → Fe3+ or Cu2+ + OH− + OH). Oxidation of enterobactin by CueO prevents Cu2+ reduction by the reactive catechol groups on enterobactin and has been proposed to form a 2-carboxymuconate derivative in the periplasm that may sequester both copper and iron ions (8) and thereby protect bacteria against metal ion-promoted oxidative stress. In view of the role of CueO in E. coli K-12, we were interested in determining whether it contributes to the pathogenesis of the prototypical UPEC strain CFT073 (19, 37).
CueO is required for copper resistance in UPEC CFT073.
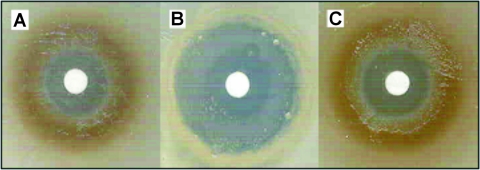
The deletion of cueO renders E. coli K-12 sensitive to CuSO4, a phenotype that is enhanced under conditions of iron limitation in which high concentrations of enterobactin are produced. To assess the role of CueO from E. coli CFT073 in copper tolerance, a cueO deletion strain (CFT073cueO) was constructed by λ red-mediated homologous recombination, as previously described (5). Copper sensitivity was assessed by growing CFT073 and CFT073cueO on Tris-buffered mineral salts agar supplemented with 0.2% glycerol and 0.3% CAS amino acids in the presence of filter discs impregnated with 5 μl of 1 M CuSO4. In this assay, CFT073 was resistant to copper and produced a distinct brown pigment in the region of growth at the periphery of the clearing zone (Fig. 1). In contrast, CFT073cueO was highly sensitive to copper. The copper resistance phenotype of CFT073cueO could be restored by complementation with a plasmid containing the cueO gene (pCueO) (Fig. 1).
FIG. 1.
Copper sensitivities of CFT073 (A), CFT073cueO (B), and CFT073cueO(pCueO) (C). Overnight cultures were diluted 1/10,000 and spread plated onto solidified Tris-buffered mineral salts media. Discs containing 5 μl of 1 M CuSO4 were placed on plates and incubated overnight. CFT073cueO displayed increased sensitivity to copper.
Deletion of cueO promotes colonization of the mouse bladder and shedding in urine.
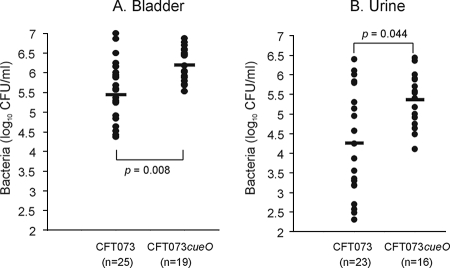
An established mouse model of UTI was employed to examine the role of cueO in UPEC virulence (36). Briefly, female C57BL/6 mice (8 to 10 weeks old) were catheterized using a sterile Teflon catheter by inserting the device directly into the bladder through the urethra. An inoculum of 25 μl, containing 5 × 108 CFU of CFT073 or CFT073cueO in phosphate-buffered saline (PBS), was instilled directly into the bladder by using a 1-ml tuberculin syringe attached to the catheter. Mice were euthanized at 18 h after challenge by cervical dislocation; bladders and kidneys were then excised aseptically, weighed, and homogenized in PBS for colony counts. Urine samples were also collected from each mouse prior to euthanasia for quantitative colony counts. Compared to CFT073, CFT073cueO colonized the mouse bladder in significantly higher numbers in this infection model (Fig. 2A). This also correlated with increased shedding of CFT073cueO in urine compared to that of CFT073 (Fig. 2B). No colonization of the kidneys was observed for CFT073 or for CFT073cueO; this is consistent with previous data from our laboratory using C57BL/6 mice (36).
FIG. 2.
CFT073 and CFT073cueO colonization of C57BL/6 mice bladders (A) and shedding in urine (B). Data for individual mice are expressed as the total number of CFU per 0.1 g of bladder tissue or as the total number of CFU per ml urine. The number of mice used for each experiment (n) is indicated. The data represent a compilation of the results for three individual experiments. Statistical analysis was performed using independent sample t test within the SPSS v9.0.2 software package.
Deletion of cueO does not affect type 1 fimbria expression, adhesive capacity, or growth in urine.
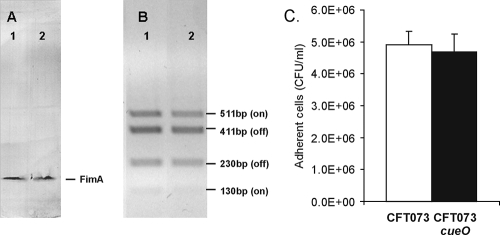
Expression of type 1 fimbriae significantly enhances the attachment of UPEC to uroepithelial cells and the subsequent colonization of the mouse bladder (2, 4, 20). We compared the levels of type 1 fimbria expression in CFT073 and in CFT073cueO by using a combination of standard techniques. First, the abilities of CFT073 and CFT073cueO to cause mannose-sensitive agglutination of yeast cells were examined. There was no difference in the agglutination titers for the two strains (data not shown). Second, the amounts of FimA produced by the two strains were compared by Western blot analysis using a polyclonal serum raised against purified type 1 fimbriae. There was no difference in the amounts of FimA produced by the two strains (Fig. 3A). Third, a PCR-based assay was employed to determine the orientation of the phase-variable fimA promoter (7, 29). There was no difference in the amounts of “on” and “off” fragments amplified from both strains (Fig. 3B). Finally, we compared the abilities of CFT073 and CFT073cueO to adhere to HeLa epithelial cells as previously described (35). In these assays, CFT073 and CFT073cueO displayed equivalent adherence levels (Fig. 3C). Taken together, the data suggest that the expression levels of type 1 fimbriae were the same in CFT073 and CFT073cueO.
FIG. 3.
Expression of type 1 fimbriae and adherence to HeLa cell monolayers. (A) Western blot analysis of whole-cell lysates prepared from CFT073 (lane 1) and CFT073cueO (lane 2) using a type 1 fimbria-specific antibody. The FimA major subunit protein of type 1 fimbriae was detected in equivalent amounts in both strains. (B) fim promoter orientation in CFT073 and CFT073cueO. Lane 1, HinfI-digested CFT073 Pfim PCR product; lane 2, HinfI-digested CFT073cueO Pfim PCR product. Bands at 511 bp and 130 bp indicate the relative proportions of the fim promoter in the “on” orientation, and bands at 411 bp and 230 bp indicate the relative proportions of the fim promoter in the “off” orientation. (C) CFT073 and CFT073cueO adherence to HeLa cell monolayers. Bacteria (5 × 106 CFU) were incubated on confluent HeLa cell monolayers for 2 h. Monolayers were washed three times with PBS, and adherent cells were recovered. Error bars represent standard errors.
In E. coli K-12, deletion of cueO results in increased aggregation, and this phenotype correlates with the enhanced expression of genes encoding antigen 43 and curli (33). CFT073 and CFT073cueO produced equal levels of antigen 43, as determined by Western blot analysis (data not shown). We also examined the growth of CFT073 and CFT073cueO and their concordant production of catechols in human urine as previously described (8, 26). No significant difference was observed between the two strains (data not shown).
CueO is required for hydrogen peroxide resistance.
The generation of hydroxyl radicals from hydrogen peroxide and iron (the Fenton reaction) is thought to be the primary bactericidal activity of hydrogen peroxide as, in the presence of iron chelators, hydrogen peroxide toxicity is greatly reduced (13). CueO has previously been shown to protect Salmonella enterica serovar Typhimurium from peroxide stress (17). Given the significant increase in the colonization of the mouse bladder by CFT073cueO, we tested whether this strain displayed increased susceptibility to hydrogen peroxide stress by using an established protocol (34). Despite its hypercolonization phenotype, CFT073cueO was significantly more sensitive to hydrogen peroxide challenge than CFT073 (Fig. 4). CFT073cueO resistance to hydrogen peroxide could be restored by complementation with plasmid pCueO (Fig. 4).
FIG. 4.
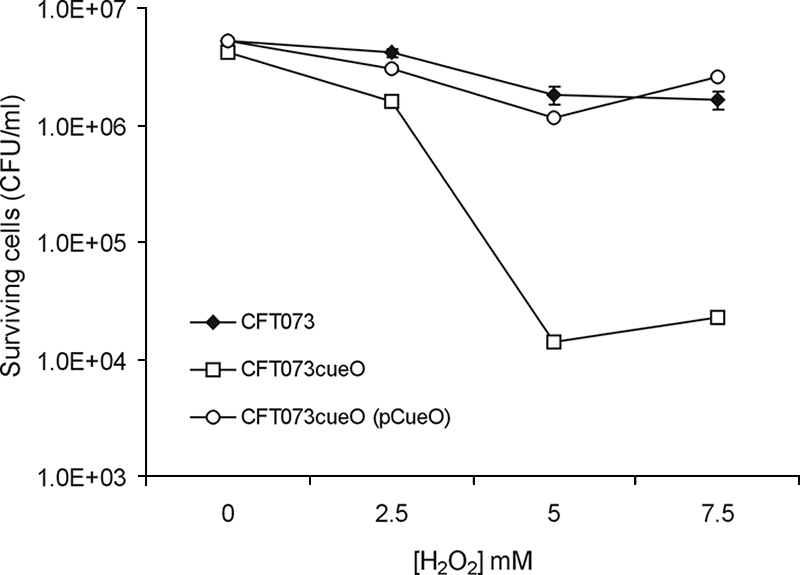
Sensitivities of CFT073, CFT073cueO, and CFT073cueO(pCueO) to H2O2. Approximately 1 × 106 cells for each strain were harvested in mid-exponential growth phase and incubated for 1 h in the presence of 0, 2.5, 5.0, and 7.5 mM H2O2. The reaction was stopped by serial dilution of cells into PBS containing 1,000 U/ml catalase. The number of viable cells remaining after H2O2 treatment was determined by direct colony counts. Error bars represent the standard errors from the results for triplicate assays.
Deletion of cueO increases ferrous iron uptake.
A possible explanation for the hypercolonization phenotype of CFT073cueO is that this strain can take up iron with increased efficiency. Thus, while excessive iron accumulation may be detrimental under conditions of oxidative stress, iron is a limiting nutrient in urine, and enhanced uptake could provide a growth advantage. To compare the abilities of CFT073 and CFT073cueO to take up iron, we performed 55Fe2+ uptake experiments as previously described (18). In these assays, CFT073cueO accumulated 5.5-fold more 55Fe2+ than did CFT073 (Fig. 5). The introduction of plasmid pCueO into CFT073cueO restored the level of 55Fe2+ accumulated to approximately wild-type levels (Fig. 5).
FIG. 5.
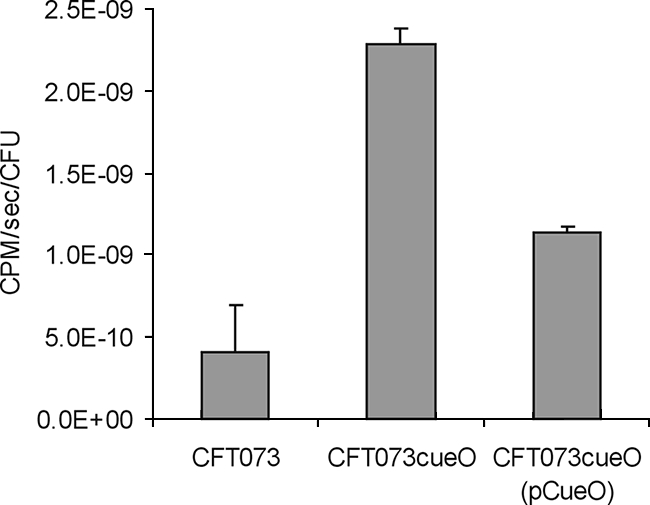
Iron uptake by CFT073, CFT073cueO, and CFT073cueO(pCueO). Strains were incubated in the presence of 100 μM 55Fe2+. The rate of ferrous iron uptake was determined by removing samples at 30, 60, 90, 120, 180, and 300 s and monitoring β emissions by using a Beckman LS3801 scintillation counter. 55Fe2+ uptake rates were normalized to CFU/ml. Data are presented as the mean 55Fe2+ uptake rates per CFU (±standard error) and represent the averages of the results for three independent experiments.
Deletion of cueO does not confer a growth advantage in a systemic infection model.
To examine if the increased colonization observed for CFT073cueO in the mouse bladder was also reflected in colonization of other sites, we tested CFT073 and CFT073cueO in a mouse systemic infection model. Mice were infected with 2 × 107 cells of CFT073 (n = 15) or CFT073cueO (n = 15) by a subcutaneous injection into the abdomen and monitored to assess the clinical effects of infection, as previously described (22). There was no difference in the virulence levels of the two strains (the time taken to kill the mice was approximately 24 h for both strains). Bacterial colony counts were also performed from the liver, spleen, and kidneys of each mouse; there was no significant difference in the abilities of the strains to colonize these organs.
Conclusions.
The results presented herein show that while deletion of cueO in E. coli CFT073 renders the cell sensitive to hydrogen peroxide stress, it also promotes increased uptake of iron. Under the iron-limited conditions encountered in the urinary tract (25, 27, 31, 32), this may represent a competitive advantage, though the consequence may be that it leaves the cell vulnerable to iron-promoted oxidative stress. Recent work by Grass and coworkers (8) has shown that CueO in E. coli K-12 has a high affinity for Fe-enterobactin (Km of 1.5 μM) and that oxidation of the enterobactin precursor 2,3-dihydroxybenzoic acid leads to formation of a polymer capable of chelating copper and iron in the periplasm (8). Production of an iron-chelating polymer in the periplasm of CFT073 under iron-limited conditions may account for the restricted iron uptake observed in this strain; loss of this biological function in the cueO mutant may lead to increased uptake of ferrous iron.
Although the CFT073cueO strain displayed enhanced bladder colonization in comparison to CFT073, this did not translate into increased growth in human urine. Recent transcriptional profiling of UPEC during the formation of intracellular bacterial communities within epithelial cells revealed that the intracellular environment is iron limited and aerobic (23). Increased iron acquisition from damaged epithelial cells could explain the enhanced colonization of CFT073cueO in the mouse bladder. Our results lead us to conclude that CueO is critical for iron and copper homeostasis in UPEC and that it has a key role in maintaining a tightly controlled flux of iron into the cell to avoid oxidative stress. The attachment of UPEC to the uroepithelium induces neutrophil recruitment with a concomitant oxidative burst that would expose UPEC to reactive oxygen species (11). In view of this, the superior colonization of the bladder by the cueO mutant is surprising. However, we note that we employed a short-term infection model, and thus, it is possible that the long-term fitness of CFT073cueO would be diminished in inflamed tissues in which reactive oxygen species cause oxidative stress. This is consistent with our observations that the CFT073cueO mutant is not attenuated in a systemic infection model.
Acknowledgments
This work was supported by grants from the Australian National Health and Medical Research Council (455914), the Australian Research Council (DP0666852), and the University of Queensland.
Footnotes
Published ahead of print on 22 August 2008.
REFERENCES
- 1.Alteri, C. J., and H. L. Mobley. 2007. Quantitative profile of the uropathogenic Escherichia coli outer membrane proteome during growth in human urine. Infect. Immun. 75:2679-2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bergsten, G., B. Wullt, M. A. Schembri, I. Leijonhufvud, and C. Svanborg. 2007. Do type 1 fimbriae promote inflammation in the human urinary tract? Cell Microbiol. 9:1766-1781. [DOI] [PubMed] [Google Scholar]
- 3.Braun, V. 2003. Iron uptake by Escherichia coli. Front. Biosci. 8:s1409-s1421. [DOI] [PubMed] [Google Scholar]
- 4.Connell, I., W. Agace, P. Klemm, M. Schembri, S. Marild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 939827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Foxman, B. 2002. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Am. J. Med. 113(Suppl. 1A)5S-13S. [DOI] [PubMed] [Google Scholar]
- 7.Gally, D. L., J. Leathart, and I. C. Blomfield. 1996. Interaction of FimB and FimE with the fim switch that controls the phase variation of type 1 fimbriae in Escherichia coli K-12. Mol. Microbiol. 21725-738. [DOI] [PubMed] [Google Scholar]
- 8.Grass, G., K. Thakali, P. E. Klebba, D. Thieme, A. Muller, G. F. Wildner, and C. Rensing. 2004. Linkage between catecholate siderophores and the multicopper oxidase CueO in Escherichia coli. J. Bacteriol. 1865826-5833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haagmans, W., and M. van der Woude. 2000. Phase variation of Ag43 in Escherichia coli: Dam-dependent methylation abrogates OxyR binding and OxyR-mediated repression of transcription. Mol. Microbiol. 35877-887. [DOI] [PubMed] [Google Scholar]
- 10.Hagan, E. C., and H. L. Mobley. 2007. Uropathogenic Escherichia coli outer membrane antigens expressed during urinary tract infection. Infect. Immun. 753941-3949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haraoka, M., L. Hang, B. Frendeus, G. Godaly, M. Burdick, R. Strieter, and C. Svanborg. 1999. Neutrophil recruitment and resistance to urinary tract infection. J. Infect. Dis. 1801220-1229. [DOI] [PubMed] [Google Scholar]
- 12.Henderson, I. R., and P. Owen. 1999. The major phase-variable outer membrane protein of Escherichia coli structurally resembles the immunoglobulin A1 protease class of exported protein and is regulated by a novel mechanism involving Dam and OxyR. J. Bacteriol. 1812132-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imlay, J. A., S. M. Chin, and S. Linn. 1988. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science 240640-642. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, J. R., C. Clabots, and H. Rosen. 2006. Effect of inactivation of the global oxidative stress regulator oxyR on the colonization ability of Escherichia coli O1:K1:H7 in a mouse model of ascending urinary tract infection. Infect. Immun. 74461-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klemm, P., L. Hjerrild, M. Gjermansen, and M. A. Schembri. 2004. Structure-function analysis of the self-recognizing antigen 43 autotransporter protein from Escherichia coli. Mol. Microbiol. 51283-296. [DOI] [PubMed] [Google Scholar]
- 16.Klemm, P., and M. A. Schembri. 2000. Bacterial adhesins: function and structure. Int. J. Med. Microbiol. 29027-35. [DOI] [PubMed] [Google Scholar]
- 17.Lim, S. Y., M. H. Joe, S. S. Song, M. H. Lee, J. W. Foster, Y. K. Park, S. Y. Choi, and I. S. Lee. 2002. cuiD is a crucial gene for survival at high copper environment in Salmonella enterica serovar Typhimurium. Mol. Cells 14177-184. [PubMed] [Google Scholar]
- 18.Makui, H., E. Roig, S. T. Cole, J. D. Helmann, P. Gros, and M. F. Cellier. 2000. Identification of the Escherichia coli K-12 Nramp orthologue (MntH) as a selective divalent metal ion transporter. Mol. Microbiol. 351065-1078. [DOI] [PubMed] [Google Scholar]
- 19.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 581281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulvey, M. A., Y. S. Lopez-Boado, C. L. Wilson, R. Roth, W. C. Parks, J. Heuser, and S. J. Hultgren. 1998. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 2821494-1497. [DOI] [PubMed] [Google Scholar]
- 21.Oelschlaeger, T. A., U. Dobrindt, and J. Hacker. 2002. Pathogenicity islands of uropathogenic E. coli and the evolution of virulence. Int. J. Antimicrob. Agents 19517-521. [DOI] [PubMed] [Google Scholar]
- 22.Picard, B., J. S. Garcia, S. Gouriou, P. Duriez, N. Brahimi, E. Bingen, J. Elion, and E. Denamur. 1999. The link between phylogeny and virulence in Escherichia coli extraintestinal infection. Infect. Immun. 67546-553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reigstad, C. S., S. J. Hultgren, and J. I. Gordon. 2007. Functional genomic studies of uropathogenic Escherichia coli and host urothelial cells when intracellular bacterial communities are assembled. J. Biol. Chem. 28221259-21267. [DOI] [PubMed] [Google Scholar]
- 24.Roberts, S. A., A. Weichsel, G. Grass, K. Thakali, J. T. Hazzard, G. Tollin, C. Rensing, and W. R. Montfort. 2002. Crystal structure and electron transfer kinetics of CueO, a multicopper oxidase required for copper homeostasis in Escherichia coli. Proc. Natl. Acad. Sci. USA 992766-2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roos, V., and P. Klemm. 2006. Global gene expression profiling of the asymptomatic bacteriuria Escherichia coli strain 83972 in the human urinary tract. Infect. Immun. 743565-3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roos, V., G. C. Ulett, M. A. Schembri, and P. Klemm. 2006. The asymptomatic bacteriuria Escherichia coli strain 83972 outcompetes uropathogenic E. coli strains in human urine. Infect. Immun. 74615-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, T. J. Barnard, and J. R. Johnson. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 707156-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schembri, M. A., L. Hjerrild, M. Gjermansen, and P. Klemm. 2003. Differential expression of the Escherichia coli autoaggregation factor antigen 43. J. Bacteriol. 1852236-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schembri, M. A., P. B. Olsen, and P. Klemm. 1998. Orientation-dependent enhancement by H-NS of the activity of the type 1 fimbrial phase switch promoter in Escherichia coli. Mol. Gen. Genet. 259336-344. [DOI] [PubMed] [Google Scholar]
- 30.Singh, S. K., G. Grass, C. Rensing, and W. R. Montfort. 2004. Cuprous oxidase activity of CueO from Escherichia coli. J. Bacteriol. 1867815-7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder, J. A., B. J. Haugen, E. L. Buckles, C. V. Lockatell, D. E. Johnson, M. S. Donnenberg, R. A. Welch, and H. L. Mobley. 2004. Transcriptome of uropathogenic Escherichia coli during urinary tract infection. Infect. Immun. 726373-6381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torres, A. G., P. Redford, R. A. Welch, and S. M. Payne. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 696179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tree, J. J., G. C. Ulett, J. L. Hobman, C. Constantinidou, N. L. Brown, C. Kershaw, M. A. Schembri, M. P. Jennings, and A. G. McEwan. 2007. The multicopper oxidase (CueO) and cell aggregation in Escherichia coli. Environ. Microbiol. 92110-2116. [DOI] [PubMed] [Google Scholar]
- 34.Tseng, H. J., Y. Srikhanta, A. G. McEwan, and M. P. Jennings. 2001. Accumulation of manganese in Neisseria gonorrhoeae correlates with resistance to oxidative killing by superoxide anion and is independent of superoxide dismutase activity. Mol. Microbiol. 401175-1186. [DOI] [PubMed] [Google Scholar]
- 35.Ulett, G. C., J. F. Bohnsack, J. Armstrong, and E. E. Adderson. 2003. Beta-hemolysin-independent induction of apoptosis of macrophages infected with serotype III group B streptococcus. J. Infect. Dis. 1881049-1053. [DOI] [PubMed] [Google Scholar]
- 36.Ulett, G. C., J. Valle, C. Beloin, O. Sherlock, J. M. Ghigo, and M. A. Schembri. 2007. Functional analysis of antigen 43 in uropathogenic Escherichia coli reveals a role in long-term persistence in the urinary tract. Infect. Immun. 753233-3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 9917020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiles, T. J., R. R. Kulesus, and M. A. Mulvey. 2008. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 8511-19. [DOI] [PMC free article] [PubMed] [Google Scholar]