Abstract
Spores of Bacillus subtilis have a thick outer layer of relatively insoluble protein called the coat, which protects spores against a number of treatments and may also play roles in spore germination. However, elucidation of precise roles of the coat in spore properties has been hampered by the inability to prepare spores lacking all or most coat material. In this work, we show that spores of a strain with mutations in both the cotE and gerE genes, which encode proteins involved in coat assembly and expression of genes encoding coat proteins, respectively, lack most extractable coat protein as seen by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, as well as the great majority of the coat as seen by atomic force microscopy. However, the cotE gerE spores did retain a thin layer of insoluble coat material that was most easily seen by microscopy following digestion of these spores with lysozyme. These severely coat-deficient spores germinated relatively normally with nutrients and even better with dodecylamine but not with a 1:1 chelate of Ca2+ and dipicolinic acid. These spores were also quite resistant to wet heat, to mechanical disruption, and to treatment with detergents at an elevated temperature and pH but were exquisitely sensitive to killing by sodium hypochlorite. These results provide new insight into the role of the coat layer in spore properties.
Spores of various Bacillus species are metabolically dormant and extremely resistant to a variety of harsh treatments, including heat, radiation, and many toxic chemicals (37). This extreme resistance is the main reason that spores are major causative agents of food spoilage and food-borne disease and why spores of Bacillus anthracis are a potential biological warfare agent. Spore resistance is due to a variety of factors, but a significant one is the spore coat. The coat is the outer layer of spores of a number of Bacillus species and consists primarily of protein, with >70 different individual proteins in the coat of Bacillus subtilis spores, many of which are cross-linked (7, 8, 10). Most of the latter proteins are components of the coats only, although a few coat proteins also have significant roles in coat assembly (7, 8, 10, 12). The coat layer is only semipermeable, generally allowing passage of molecules of <5 kDa to interior layers, in particular the spore's inner membrane, where receptors that sense the presence of nutrient molecules that trigger spore germination are located (7, 10, 36). Because of its permeability properties, the spore coat is very important in preventing access of exogenous lytic enzymes such as lysozyme from gaining access to the spore's peptidoglycan (PG) cortex, located beneath the coat layer, and the germ cell wall beneath the cortex (7, 10, 13). This is important in spore survival, since hydrolysis of cortex and germ cell wall PG by lysozyme can cause spore lysis. The coat is also important in the resistance of spores to many, albeit not all, reactive chemicals (37). The precise mechanism whereby the coat protects against reactive chemicals is not known, but coat proteins may react with and detoxify such chemicals before they can gain access to and damage more sensitive targets further in the spore's interior. The spore coat also appears to contain enzymes such as superoxide dismutase and perhaps catalase that may assist in detoxification of some reactive toxic chemicals, as well as the CotA laccase, which can contribute to spore hydrogen peroxide resistance (7, 10).
In addition to a role in spore resistance, the spore coat has been suggested to play roles in spore germination. Thus, some proteins suggested to be important in allowing permeation of nutrient germinant molecules into the interior regions of the spore are coat proteins (36). At least one enzyme, CwlJ, important in lysis of the B. subtilis spore's PG cortex during germination is also in the spore coat, perhaps at the cortex-coat boundary (2, 4). Indeed, proper assembly of CwlJ in B. subtilis spores requires the coat protein GerQ (30). Some of the second redundant B. subtilis spore cortex-lytic enzyme, SleB, also appears to be located at the coat-cortex boundary (4).
Most studies probing the importance of the coat in various spore properties have used B. subtilis spores with coats that are defective because of either chemical removal of much coat protein by extraction with detergents at high pH or the lack of CotE, a protein essential for assembly of many coat proteins as well as the outer coat layer (7, 10). However, both cotE and chemically decoated spores retain much coat protein, most notably as an insoluble “rind” that is also extremely resistant to a variety of digestive enzymes (7, 10, 13). Therefore, it would be most helpful if spores lacking all or almost all coat proteins were available to more rigorously test the role of the coat in spore properties. GerE is a DNA binding protein acting in the mother cell compartment late in sporulation which, in addition to having other activities, positively regulates the expression of genes coding for a number of proteins in the spore coat that are resistant to extraction at a high pH with detergent plus a reducing agent; these proteins constitute the insoluble fraction of the spore coat (8, 10). gerE mutants also make spores in which much of the coat can be readily lost, and it has been reported that cotE gerE spores appear to lack visible coats (6, 21, 38). Consequently, in this communication we describe the generation of a cotE gerE strain and the properties of the cotE gerE spores that appear to lack the majority of coat proteins.
MATERIALS AND METHODS
Strains used and spore preparation.
The strains used in this work are isogenic derivatives of strain PS832, a prototrophic laboratory derivative of strain 168. Strain PS533 (35) (wild type) carries plasmid pUB110, which encodes resistance to kanamycin (Kmr; 10 μg/ml). PS3328 (24) carries a tetracycline resistance (Tcr; 10 μg/ml) cassette replacing the majority of the cotE coding sequence. Strain PS4149 carries a spectinomycin resistance (Spr; 100 μg/ml) cassette replacing much of the gerE coding sequence (see below), and strain PS4150 carries the cotE and gerE deletion mutations from strains PS3328 and PS4149.
For construction of strain PS4149, the DNA region from bp −1 to −282 relative to the translation start site of gerE was PCR amplified from chromosomal DNA of strain PS832 using primers with additional base pairs that included an EcoRI site in the forward primer and a PstI site in the reverse primer (all primer sequences are available upon request). The PCR product was purified, digested with EcoRI and PstI, and cloned between the EcoRI and PstI sites in plasmid pJL74 (18) to generate plasmid pJL74gerE1. A second DNA fragment encompassing bp +181 to +469 relative to the gerE translation start site was PCR amplified from chromosomal DNA of strain PS832 using primers with additional base pairs, including a NotI site in the forward primer and a BamHI site in the reverse primer. This PCR product was purified and cloned between the NotI and BamHI sites in plasmid pJL74gerE1, giving plasmid pJL74gerE2 with fragments upstream and downstream of gerE flanking a Spr resistance cassette. Cloning of the correct fragments and the proper orientation of these fragments in pJL74gerE2 were confirmed by PCR. Plasmid pJL74gerE2 was linearized with XhoI and used to transform strain PS832 to Spr. The replacement of much of the gerE coding sequence with a Spr cassette in one Spr transformant, termed strain PS4149, was confirmed by PCR. Chromosomal DNA from strain PS4149 was then used to transform strain PS3328 to a Spr Tcr phenotype, and the expected loss of much of the gerE coding sequence in one transformant (termed strain PS4150) was again confirmed by PCR.
Spores of all strains were prepared at 37°C on 2× SG medium agar plates without antibiotics as described previously (23). After 2 to 3 days at 37°C, plates were held at 23°C for 2 to 4 days to allow further lysis of growing or sporulating cells, and then spores were scraped from plates and purified as described previously (23). All spores used in this work were free (>98%) of growing or sporulating cells or cell debris. In some cases, spores were purified even further by centrifugation through a solution of 50% Nycodenz, as described previously, to remove cell debris (5).
Analysis of spore coat proteins.
Coat proteins were extracted from spores at an optical density at 600 nm (OD600) of ∼25 for 2 h at 70°C in 1% sodium dodecyl sulfate (SDS)-0.1 M NaOH-0.1 M NaCl-0.1 M dithiothreitol (1). The decoated spores were washed as described previously (1), the material extracted was run on SDS-polyacrylamide gel electrophoresis (PAGE) using a 10% acrylamide gel (14), and the gel was stained with Coomassie brilliant blue.
Chemically decoated wild-type spores and intact gerE, cotE, and cotE gerE spores at an OD600 of ∼10 in 1 ml of 20 mM Tris-HCl (pH 8) were digested with hen egg white lysozyme (20 μg/ml) for 1 h at 37°C, followed by addition of pancreatic DNase to 10 μg/ml and further incubation for 20 min to reduce the viscosity of the extract. The extracts were then centrifuged in a microcentrifuge for 5 min, the pellet fraction was washed twice with 20 mM Tris-HCl (pH 8), and the final pellets were prepared for either differential interference contrast (DIC) microscopy or electron microscopy (EM) as described below. In other experiments, this final pellet was suspended in 0.5 ml of 25 mM Tris-HCl buffer (pH 8) and incubated for 6 h at 37°C with 50 μg/ml pronase or boiled for 20 min with 1% SDS.
Analysis of spore properties.
Atomic force microscopy (AFM) on intact spores was carried out and images were collected using a Nanoscope IV AFM (Veeco Instruments, Santa Barbara, CA) operated in tapping mode as described previously (26-28). For DIC microscopy, pellets from lysozyme-digested spores obtained as described above were suspended in 25 to 50 μl of 25 mM KPO4 (pH 7.4)-0.1 M NaCl, 5-μl aliquots were applied to agarose-coated slides, and the slides were imaged using DIC optics on a Zeiss LSM510 laser scanning microscope with a 63-by-1.4 numerical aperture planapochromat lens. For EM, pellets from lysozyme-digested spores obtained as described above were fixed, processed, sectioned, and photographed as described previously (9).
The core wet densities of spores with or without prior decoating were determined as described previously (19, 29) by banding in equilibrium density gradients of Nycodenz with intact wild-type spores labeled with ruthenium red added to all density gradients as an internal standard to correct for slight differences between different gradients (39; K. Griffiths and P. Setlow, unpublished results).
Spore resistance to wet heat at ∼90°C was determined using spores in water at an OD600 of 1. At various times, the heated spores were diluted 1/100 in 23°C water and then diluted further, aliquots were spotted on LB medium (25) agar plates containing an appropriate antibiotic, plates were incubated for ∼24 h at 37°C, and colonies were counted. Further incubation gave no increase in colony numbers.
Spore resistance to sodium hypochlorite was determined essentially as described previously (40) by incubation of spores at an OD600 of 1 at 23°C in 50 mM KPO4 buffer (pH 7.0) plus a 1/105 dilution of commercial sodium hypochlorite (Sigma; 10 to 13% available chlorine). At various times, aliquots were diluted 1/10 in 1% sodium thiosulfate; this dilution was incubated for at least 10 min at 23°C, samples were diluted further in water, and numbers of survivors were determined as described above. Spore resistance to mechanical abrasion in liquid was determined as described previously (11).
Spore germination.
Spores were germinated following heat activation (30 min; 75°C) of spores at an OD600 of 10 in water. Spores were germinated at an OD600 of ∼1 and at 37°C in 25 mM Tris-HCl (pH 8)-1 mM l-alanine. The progress of germination was assessed by measuring the OD600 of the cultures and was also checked by phase-contrast microscopy. Analysis of spore germination with dodecylamine did not use heat-shocked spores and was carried out with spores at an OD600 of ∼2 at 40°C in 20 mM KPO4 buffer (pH 7.5)-1 mM dodecylamine (33). Germination of spores with dodecylamine was assessed by measuring the OD270 of the supernatant fluid from 1-ml aliquots of the germinating culture, which monitors the release of dipicolinic acid (DPA) from the spores, an early event in spore germination. Analysis of spore germination with the 1:1 chelate of Ca2+ and DPA (Ca-DPA) again used spores that were not heat shocked and was carried out in 60 mM Ca-DPA-25 mM Tris-HCl (pH 8) at 23°C (24). The progress of spore germination was assessed by phase-contrast microscopy. Spore germination was also assessed by the ability of the spores of the various strains to form colonies on nutrient plates with appropriate antibiotics as described above.
RESULTS
Generation of severely coat-defective spores.
While spores of strains with mutations in either cotE or gerE have defective coats, as evidenced most notably by the sensitivity of these spores to lysozyme, these spores do retain significant amounts of coat protein (7, 10). However, these two mutations likely result in defective spore coats by different mechanisms: a cotE mutation, which eliminates a protein essential for the assembly of many coat proteins as well as the outer coat layer, and a gerE mutation, which eliminates the expression of a number of coat protein genes, including some coding for proteins in both the inner and outer coat layers, as well as derepressing the expression of other proteins (7, 10, 43). Thus, it seemed worthwhile to examine spores of a cotE gerE strain for the presence of any residual coat, especially since spores of a ΔcotE strain that also carry a point mutation in gerE are reported to lack a visible coat (6, 32, 38). Consequently, we constructed a strain with deletions of both cotE and gerE and examined the spores of this strain for coat material. Strikingly, the spores of such a strain had very little coat protein extractable at a high pH with SDS plus a reducing agent, as determined by SDS-PAGE of extracts from intact spores, in contrast to the large amount of protein extracted from gerE and from cotE spores (Fig. 1). However, there was some protein extracted from the cotE gerE spores (Fig. 1).
FIG. 1.
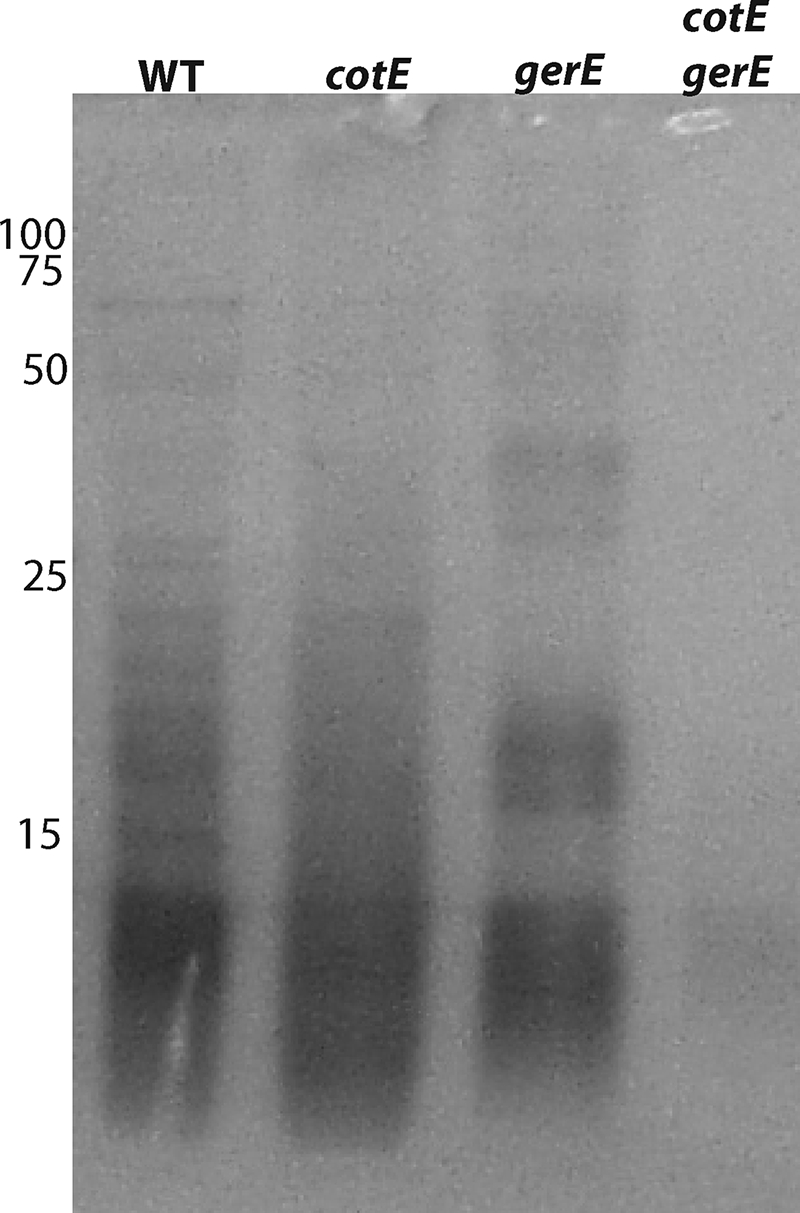
SDS-PAGE of coat extracts from spores of various strains. Purified spores were extracted with decoating solution, an aliquot (10 μl) of the extract was run on SDS-PAGE, and the gel was stained as described in Materials and Methods. The samples were from spores of strains PS533 (wild-type), PS3328 (cotE), PS4149 (gerE), and PS4150 (cotE gerE). The numbers to the left of lane 1 give the migration position of molecular mass standards, in kDa.
Analysis by AFM also showed the presence of significant coat material in cotE and gerE spores, as expected (Fig. 2a to d). However, the great majority of cotE gerE spores appeared to lack the obvious coat material present on wild-type, cotE, and most gerE spores, most notably the prominent outer ridges, outermost amorphous and rodlet layers, and inner coat crystalline layers seen previously (3, 27; M. Plomp, A. M. Carroll, P. Setlow, and A. J. Malkin, unpublished results), as the outer surfaces of the great majority of the cotE gerE spores appeared to be quite smooth (Fig. 2e and f). Pellets of purified cotE gerE spores were also almost white, in contrast to the brown to salmon color of pellets of wild-type, cotE, and gerE spores (data not shown). In addition, there was a significant amount of darkly pigmented material in the top layer of initial pellets from sporulating cotE gerE cells harvested by centrifugation that was not seen with sporulating cells of the other three strains (data not shown). This pigmented material may be in part coat material that misassembled in the cytoplasm of the sporulating cotE gerE cells and was easily removed during spore purification. Alternatively, this material may be an aggregate that was generated by the action of a misassembled coat protein, perhaps by CotA, which can act as a laccase (10).
FIG. 2.
AFM analyses of spores of various strains. Purified spores of various strains were analyzed by AFM and photographed as described in Materials and Methods. The spores analyzed were from strains PS533 (wild-type) (a), PS3328 (cotE) (b), PS4149 (gerE) (c and d), and PS4150 (cotE gerE) (e and f). Bars, 500 nm. The asterisks (e) indicate a few spores that retained obvious coat material.
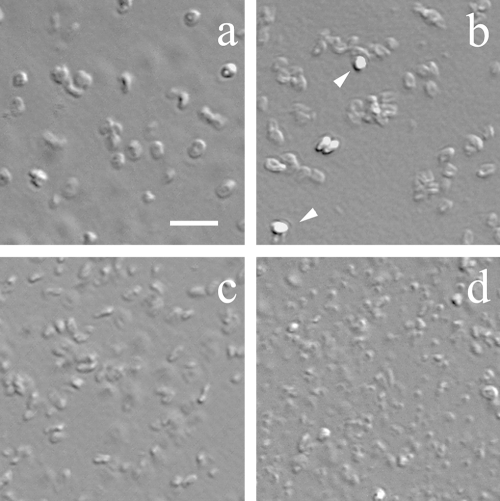
As a further check on the presence of coat material, in particular the presence of the insoluble material that has been termed a rind and is seen with cotE or decoated wild-type spores treated with lysozyme (12), the spores of these two strains as well as gerE spores were treated with lysozyme, and the insoluble material was purified and examined by EM and DIC microscopy, the latter a light microscopic technique similar in many respects to phase-contrast microscopy (Fig. 3 and 4). As expected (7, 10, 12), DIC microscopy of material remaining after centrifugation and washing of lysozyme digests revealed that the lysozyme digestion left behind an insoluble rind that is almost certainly derived largely from the insoluble proteins in the spore coat (Fig. 3a to c). Surprisingly, there was also what appeared to be rind material left behind after lysozyme digestion of cotE gerE spores (Fig. 3d), and this material was not destroyed by digestion with pronase or boiling with SDS (data not shown). The sizes of the rinds from wild-type and cotE spores appeared to be similar in DIC microscopy (Fig. 3a and b), but the gerE spore rinds appeared to be smaller, with the cotE gerE spore rinds appearing even smaller (Fig. 3c and d).
FIG. 3.
DIC micrographs of spores with or without coat defects after digestion with lysozyme and DNase. Spores of strains PS533 (wild-type) (a), PS3328 (cotE) (b), PS4149 (gerE) (c), and PS4150 (cotE gerE) (d) were digested, and the material remaining after digestion was examined by DIC microscopy as described in Materials and Methods. The PS533 spores had been chemically decoated prior to lysozyme treatment, and the arrowheads (b) point to a few spores that were not digested with lysozyme. Bar, 20 μm (all images are at the same magnification).
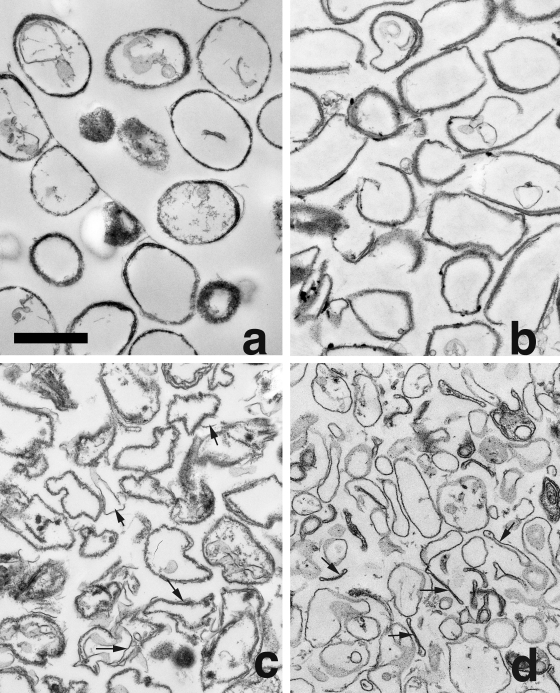
FIG. 4.
Electron micrographs of rind material generated by lysozyme and DNase digestion of spores with or without coat defects. Spores of strains PS533 (wild-type) (a), PS3328 (cotE) (b), PS4149 (gerE) (c), and PS4150 (cotE gerE) (d) were digested, and rind material was isolated, fixed, and examined by EM as described in Materials and Methods. Bars, 1 μm (all figures are at the same magnification). Arrows indicate thin rind material in gerE spores (c) and cotE gerE rinds that collapsed upon themselves (d).
EM of the rind material from the cotE and decoated wild-type spores revealed relatively thick rinds that largely retained the shape of the intact spores (Fig. 4a and b). Most rinds generated from gerE spores were similar in thickness to the rinds from the cotE and decoated wild-type spores (Fig. 4c). However, the gerE spore rinds appeared less rigid, as many were deformed (Fig. 4c); this deformation is most likely the reason that the gerE spore rinds appeared to be smaller than wild-type and cotE spore rinds by DIC microscopy. Surprisingly, EM of insoluble material remaining after lysozyme digestion of cotE gerE spores indicated that these spores did have rinds (Fig. 4d). However, these rinds were significantly thinner than those from cotE, gerE, or decoated wild-type spores, and again, almost all of the cotE gerE rinds did not retain the spore shape, with many having collapsed almost completely (Fig. 4d). Again, this is most likely the reason that the cotE gerE spore rinds appeared to be smaller than rinds from other types of spores in DIC microscopy. In any event, it appears that while cotE gerE spores have much less coat material than wild-type, cotE, or gerE spores, they do retain some coat protein.
Viability and germination of coat-deficient spores.
An obvious question about spores that lack most coat material is whether these spores are viable. Analysis of the viability of the cotE gerE spores on nutrient plates indicated that these spores had essentially the same viability as wild-type spores and that both gerE and cotE spores also had similar viability (Table 1).
TABLE 1.
Viability of spores of various strainsa
| Strain | CFU/ml at OD600 of 1.0
|
|
|---|---|---|
| Intact | Decoated | |
| PS533 (wild type) | 1.2·108 | 1.2·108 |
| PS3228 (cotE) | 1.4·108 | 1.3·108 |
| PS4149 (gerE) | 1.1·108 | 1.0·108 |
| PS4150 (cotE gerE) | 1.3·108 | 9·107 |
Aliquots of purified intact or chemically decoated spores were spotted on plates with appropriate antibiotics, plates were incubated, and colonies were counted as described in Materials and Methods.
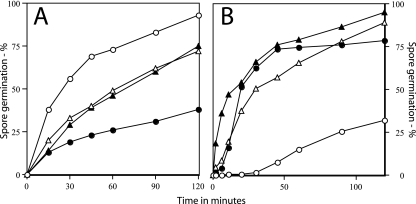
The nearly identical viability of spores of these four strains indicated that these spores could germinate with comparable efficiencies. However, the viability data did not indicate how fast these various spores germinated. Consequently, we measured the germination of these spores with the nutrient germinant l-alanine by monitoring the OD600 of germinating cultures (Fig. 5A). As reported previously, the germination of both cotE and gerE spores was slower than that of wild-type spores (7, 21, 43). Germination of the cotE gerE spores was also slower than that of wild-type spores but was actually faster than that of cotE spores. The observation that cotE gerE spores germinated only about twofold more slowly than wild-type spores with l-alanine was also obtained by monitoring spore germination by measuring the release of the dormant spore's large depot of Ca-DPA, an early event in spore germination (data not shown).
FIG. 5.
l-Alanine (A) and dodecylamine (B) germination of spores with or without coat proteins. (A) Spores of various strains were heat shocked and germinated with l-alanine, and the OD600 of cultures was monitored to assess spore germination, as described in Materials and Methods. The OD600 falls ≥60% upon completion of wild-type spore germination, and this value was used to calculate the degree of spore germination at various time points. (B) Spores of various strains were incubated with dodecylamine, and DPA release was monitored to assess spore germination, as described in Materials and Methods. ○, PS533 (wild-type); •, PS3328 (cotE); ▵, PS4149 (gerE); ▴ PS4150 (cotE gerE).
In addition to specific nutrients, spores can also be triggered to germinate by a number of nonnutrient agents; two of these are the cationic surfactant dodecylamine and exogenous Ca-DPA. When endogenous Ca-DPA release was monitored to assess spore germination, cotE gerE spores germinated significantly faster with dodecylamine than did wild-type spores, as did cotE and gerE spores (Fig. 5B). In contrast, while wild-type spores germinated >90% in 2 h with exogenous Ca-DPA, as determined by phase-contrast microscopy, ≤5% of cotE gerE spores germinated in 2 h with Ca-DPA (data not shown). Similarly, cotE and gerE spores also did not germinate with exogenous Ca-DPA (data not shown). The lack of germination of cotE spores with Ca-DPA was not unexpected, since CwlJ, the enzyme whose action on the spore cortex is triggered by Ca-DPA, does not assemble into the spore coat in cotE strains (2, 4, 30). Presumably this is also the case in gerE strains.
Resistance properties of cotE gerE spores.
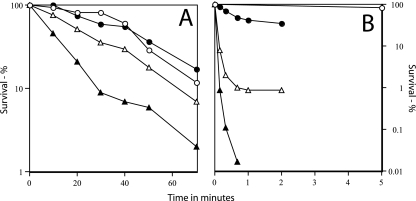
While the cotE gerE spores germinated reasonably well with several germinants, it was possible that loss of most of the coat might drastically alter the resistance properties of these spores. However, there was only a relatively small effect on spore resistance to wet heat due to loss of most of the spore coat, as the D88°C value (time for inactivation of 90% of spores) decreased only about threefold in cotE gerE spores compared to the value for wild-type spores (Fig. 6A). The heat resistances of cotE and gerE spores were identical to that of wild-type spores and slightly less, respectively (Fig. 6A).
FIG. 6.
Resistance of spores with and without coat defects to moist heat (A) and sodium hypochlorite (B). Spores of various strains were incubated in water at 88°C or sodium hypochlorite, and spore viability was determined as described in Materials and Methods. ○, PS533 (wild-type); •, PS3328 (cotE); ▵, PS4149 (gerE); ▴, PS4150 (cotE gerE).
A major factor determining the resistance of spores of B. subtilis and other Bacillus species to moist heat is the water content of the spore core, with a higher core water content giving spores with lower moist-heat resistance (37). Determination of the wet density of the core of the spores of these four strains by equilibrium density gradient centrifugation of decoated spores indicated that spores of all four strains had very similar core wet densities, and thus essentially identical core water contents, although the core water content of the cotE gerE spores was the highest of the spores of the four strains examined, consistent with their lower wet-heat resistance (Table 2).
TABLE 2.
Core wet density of spores of strains with coat defectsa
| Strain | Core wet density, g/mlb |
|---|---|
| PS533 (wild type) | 1.382 (34) |
| PS3328 (cotE) | 1.380 (34) |
| PS4149 (gerE) | 1.379 (35) |
| PS4150 (cotE gerE) | 1.376 (36) |
Purified spores of various strains were decoated, and their core wet densities were determined as described in Materials and Methods.
Values in parentheses are percentages of core wet weight that were water, calculated as described previously (20).
While there were no large differences in the resistance of wild-type and coatless spores to moist heat, this was expected not to be the case for resistance to many toxic chemicals. Previous work has shown that coat proteins are important in protecting spores against a number of chemicals, including sodium hypochlorite, chlorine dioxide, and ozone, and cotE or chemically decoated wild-type spores are much more sensitive to these agents than are intact wild-type spores (37, 40, 41). This was also seen in the current work when extremely dilute sodium hypochlorite was used, as cotE spores were more sensitive than wild-type spores, and gerE spores were even more sensitive (Fig. 6B). However, the cotE gerE spores were even more sensitive than the gerE spores, with <0.01% survival after 1 min of treatment. The killing of the cotE gerE spores by this sodium hypochlorite concentration was actually slightly faster than the killing of vegetative cells of strain PS533 (data not shown). In contrast to the greater sensitivity to sodium hypochlorite of the spores with various coat defects, these coat-defective spores were resistant to treatment with SDS at a high pH and temperature, as this treatment, which is used to remove much spore coat protein, had only a minimal effect on the viability of even cotE gerE spores (Table 1).
In addition to having resistance to moist heat and chemicals, dormant spores are also more resistant to mechanical disruption than are germinated spores or growing cells (11). The factors providing spore resistance to mechanical disruption are not known, but one such factor has been suggested to be the highly insoluble rind of coat protein, which is retained even in cotE spores. However, examination of spore killing by mechanical disruption, a process that has been shown to be due almost certainly to actual disruption of the spores (11), indicated that spores of the wild-type, cotE, gerE, and cotE gerE strains exhibited almost identical rates of killing (data not shown).
DISCUSSION
The cotE gerE spores described in this report appear to be the most severely coat-defective yet stable B. subtilis spores reported to date. The spore coat's normal outer layers as well as the more inner rodlet layer are absent from these cotE gerE spores, and they retain little detergent-extractable coat protein, although they do contain some. This observation is consistent with previous work that reported the extraction of several coat proteins from cotE gerE spores, although the gerE mutation used in the previous work is most likely a point mutation (22, 32, 38). It is, however, notable that the amount of detergent-extractable coat protein in our cotE gerE spores is significantly less than that reported by others. Whether this is due to differences in the gerE mutations used or to differences in the B. subtilis strains, sporulation conditions, or spore purification regimens is not clear. Indeed, B. subtilis spores prepared on plates, as was done in this work, are reported to have less extractable coat protein than spores prepared in liquid, the method used for spore preparation in much other work (31). In any event, the most notable finding about our cotE gerE spores is that they appear to retain a very thin layer of detergent-insoluble protein that is resistant to protease digestion, although this structure does not appear to be as rigid as the wild-type spore coat. Presumably, much of the thin coat remnant in these cotE gerE spores is composed of cross-linked proteins, although the identity of these proteins is not clear. The combination of a cotE and a gerE mutation almost certainly does not block synthesis of a number of coat proteins, although the lack of GerE abolishes synthesis of some (8, 10, 42, 43). Indeed, we observed that pigmented material likely derived from or generated by misassembled coat protein accumulated in cultures of fully sporulated cotE gerE cells, but this material was not associated with the mature spores. Presumably the combination of the cotE and gerE mutations prevented the adherence of the normal outer layers of the coat to the spore, either because the coat misassembles or because it lacks some essential protein(s) or an essential coat modification crucial for adherence. This is clearly a topic for future investigation, as is the precise composition of the insoluble coat material remaining in cotE gerE spores.
It was notable that the cotE gerE spores germinated reasonably well with the nutrient germinant l-alanine, suggesting that most coat protein is not essential for nutrient germination. Previous work has indicated that a gerE mutation has major effects on spore germination with nutrients (6, 7, 10, 21), but we did not see this with either our gerE or cotE gerE spores. The reason(s) for the better germination of our gerE spores is not clear, although we note that (i) the genetic background for our strains is different from that used previously, and (ii) the gerE mutation used in our work is a deletion mutation, in contrast to the gerE point mutation used in other work. However, the cotE gerE spores did not germinate with Ca-DPA. The explanation for this result is most likely that the cortex lytic enzyme CwlJ is absent from cotE gerE spores, as it is from cotE spores. CwlJ is essential for B. subtilis spore germination with Ca-DPA (2, 24). Presumably the second redundant cortex lytic enzyme, SleB, remains in the cotE gerE spores and is sufficient for the completion of their germination (4), although the absence of CwlJ from these spores may be at least in part the reason for their slower germination with l-alanine. Since gerE spores also did not germinate with Ca-DPA, we expect that these spores also lack CwlJ.
In contrast to their slower germination with l-alanine, the cotE gerE spores actually released DPA faster than wild-type spores when germination was triggered by the cationic surfactant dodecylamine. The reason for this is not completely clear, but it may be simply that the hydrophobic dodecylamine has an easier time accessing the spore's inner membrane, the likely site where this chemical triggers spore germination, in cotE gerE spores, which have only a very thin layer of coat material, than in wild-type, cotE, and gerE spores, which have a much thicker coat layer (33).
The relatively normal resistance of the cotE gerE spores to moist heat was not unexpected, as the spore coats are not thought to play a major role in spore resistance to moist heat. However, the combination of the absence of most of the coat from these spores and their relatively normal core water content is consistent with the idea that any restraining action of the spore coat is not essential for the reduction in core water content late in spore development, which is the major cause of spore resistance to wet heat. The absence of any effect of the much thinner and evidently less rigid coat in cotE gerE spores on spore resistance to mechanical disruption further indicates that the coat is not of major important in this resistance property either, and by the process of elimination, this must be due at least in part to the spore's thick PG cortex. The extremely high sensitivity of the cotE gerE spores to hypochlorite, however, indicates that the coat is extremely important in the resistance to this and probably other chemicals. Indeed, the cotE gerE spores were at least as sensitive to this agent as were growing cells. The precise coat component responsible for spore resistance to hypochlorite and other reactive chemicals is not known, and it may be due simply to the normally large amount of protein that can detoxify reactive chemicals by nonspecific reactions with them.
One additional outcome of the identification of these stable and severely coat-defective cotE gerE B. subtilis spores is that such spores provided an excellent reagent for determining the reason that spores often give faint peripheral staining with stains that are thought to be relatively specific for nucleic acids or membranes (15, 20, 34), as this could well be due to adsorption of these stains to coat protein. In addition, the relative lack of coat proteins from cotE gerE spores may allow the identification of the spore components responsible for the spore's significant autofluorescence, a property that has been suggested may be useful for spore detection (16, 17). Recent work has indicated that the cotE gerE spores described in this work do indeed exhibit decreased peripheral staining with a number of reagents and, perhaps more importantly, exhibit greatly decreased autofluorescence (19a). The latter property may make these coat-defective spores useful in studies of the location of fluorescent reporter proteins fused to extremely low-abundance proteins in spores, such as the germinant receptors in the spore's inner membrane (36).
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM-19698) and the Army Research Office to P.S. The Center for Cell Analysis and Modeling at the University of Connecticut Health Center is supported by NIH RR022232. Part of this work was performed under the auspices of the U.S. Department of Energy by Lawrence Livermore National Laboratory under contract number DE-AC52-07NA27344.
We are grateful to Art Hand for assistance with EM, to Keren Griffiths for advice and reagents for the analysis of spore core water content, to Michael Mallozzi and Adam Driks for helpful advice, and to one reviewer for helpful suggestions.
Footnotes
Published ahead of print on 22 August 2008.
REFERENCES
- 1.Bagyan, I., M. Noback, S. Bron, M. Paidhungat, and P. Setlow. 1998. Characterization of yhcN, a new forespore-specific gene of Bacillus subtilis. Gene 212179-188. [DOI] [PubMed] [Google Scholar]
- 2.Bagyan, I., and P. Setlow. 2002. Localization of the cortex lytic enzyme CwlJ in spores of Bacillus subtilis. J. Bacteriol. 1841289-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chada, V. G., E. A. Sanstad, R. Wang, and A. Driks. 2003. Morphogenesis of Bacillus spore surfaces. J. Bacteriol. 1856255-6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chirakkal, H., M. O'Rourke, A. Atrih, S. J. Foster, and A. Moir. 2002. Analysis of spore cortex lytic enzymes and related proteins in Bacillus subtilis endospore germination. Microbiology 1482383-2392. [DOI] [PubMed] [Google Scholar]
- 5.Coleman, W. H., D. Chen, Y.-q. Li, A. E. Cowan, and P. Setlow. 2007. How moist heat kills spores of Bacillus subtilis. J. Bacteriol. 1898458-8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Driks, A., S. Roels, B. Beall, C. P. Moran, Jr., and R. Losick. 1994. Subcellular localization of proteins involved in the assembly of the spore coat of Bacillus subtilis. Genes Dev. 8234-244. [DOI] [PubMed] [Google Scholar]
- 7.Driks, A. 1999. Bacillus subtilis spore coat. Microbiol. Mol. Biol. Rev. 631-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driks, A. 2002. Maximum shields: the assembly and function of the bacterial spore coat. Trends Microbiol. 10251-254. [DOI] [PubMed] [Google Scholar]
- 9.Hand, A. R. 1995. Electron microscopy, p. 205-260. In J. A. Glasel and M. P. Deutscher (ed.), Introduction to biophysical methods for protein and nucleic acid research. Academic Press, New York, N.Y.
- 10.Henriques, A. O., and C. P. Moran, Jr. 2007. Structure, assembly and function of the spore surface layers. Annu. Rev. Microbiol. 61555-588. [DOI] [PubMed] [Google Scholar]
- 11.Jones, C. A., N. L. Padula, and P. Setlow. 2005. Effect of mechanical abrasion on the viability, disruption and germination of spores of Bacillus subtilis. J. Appl. Microbiol. 991484-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim, H., M. Hahn, P. Grabowski, D. C. McPherson, M. M. Otte, R. Wang, C. C. Ferguson, P. Eichenberger, and A. Driks. 2006. The Bacillus subtilis spore coat protein interaction network. Mol. Microbiol. 59487-502. [DOI] [PubMed] [Google Scholar]
- 13.Klobutcher, L. A., K. Ragkousi, and P. Setlow. 2006. The Bacillus subtilis spore coat provides “eat resistance” during phagosomal predation by the protozoan Tetrahymena thermophila. Proc. Natl. Acad. Sci. USA 103165-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 15.Laflamme, C., J. Ho, M. Veillette, M.-C. deLatremoille, D. Verrault, A. Meriaux, and C. Duchaine. 2005. Flow cytometry analysis of germinating Bacillus spores, using membrane potential dye. Arch. Microbiol. 183107-112. [DOI] [PubMed] [Google Scholar]
- 16.Laflamme, C., D. Verreault, J. Ho, and C. Duchaine. 2006. Flow cytometry sorting protocol of Bacillus spore using ultraviolet laser and autofluorescence as main sorting criterion. J. Fluoresc. 16733-737. [DOI] [PubMed] [Google Scholar]
- 17.Laflamme, C., D. Verreault, S. Lavigne, L. Trudel, J. Ho, and C. Duchaine. 2005. Autofluorescence as a viability marker for detection of bacterial spores. Front. Biosci. 101647-1653. [DOI] [PubMed] [Google Scholar]
- 18.LeDeaux, J. R., and A. D. Grossman. 1995. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to sporulation sensor kinases KinA and KinB in Bacillus subtilis. J. Bacteriol. 177166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lindsay, J. A., T. C. Beaman, and P. Gerhardt. 1985. Protoplast water content of bacterial spores determined by buoyant density gradient sedimentation. J. Bacteriol. 163735-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19a.Magge, A., B. Setlow, A. E. Cowan, and P. Setlow. 2008. Analysis of dye binding by and membrane potential in spores of Bacillus species. J. appl. Microbiol., in press. [DOI] [PMC free article] [PubMed]
- 20.Melly, E., A. E. Cowan, and P. Setlow. 2002. Studies on the mechanism of killing of Bacillus subtilis spores by hydrogen peroxide. J. Appl. Microbiol. 93316-325. [DOI] [PubMed] [Google Scholar]
- 21.Moir, A. 1981. Germination properties of a spore coat-defective mutant of Bacillus subtilis. J. Bacteriol. 1461106-1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moir, A., D. A. Lafferty, and D. A. Smith. 1979. Genetic analysis of spore germination mutants of Bacillus subtilis 168: the correlation of phenotype with map location. J. Gen. Microbiol. 111165-180. [DOI] [PubMed] [Google Scholar]
- 23.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Chichester, England.
- 24.Paidhungat, M., K. Ragkousi, and P. Setlow. 2001. Genetic requirements for induction of germination of spores of Bacillus subtilis by Ca2+-dipicolinate. J. Bacteriol. 1834886-4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., and P. Setlow. 2000. Role of Ger− proteins in nutrient and nonnutrient triggering of spore germination in Bacillus subtilis. J. Bacteriol. 1822513-2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Plomp, M., T. J. Leighton, K. E. Wheeler, H. D. Hill, and A. J. Malkin. 2007. In vitro high-resolution structural dynamics of single germinating bacterial spores. Proc. Natl. Acad. Sci. USA 1049644-9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Plomp, M., T. J. Leighton, K. E. Wheeler, and A. J. Malkin. 2005. The high-resolution architecture and structural dynamics of Bacillus spores. Biophys. J. 88603-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Plomp, M., J. M. McCaffery, I. Cheong, X. Huang, C. Bettegowda, K. W. Kinzler, S. Zhou, B. Vogelstein, and A. J. Malkin. 2007. Spore coat architecture of Clostridium novyi NT spores. J. Bacteriol. 1896457-6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Popham, D. L., S. Sengupta, and P. Setlow. 1995. Heat, hydrogen peroxide, and UV resistance of Bacillus subtilis spores with increased core water content and with or without major DNA binding proteins. Appl. Environ. Microbiol. 613633-3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ragkousi, K., P. Eichenberger, C. Van Ooij, and P. Setlow. 2003. Identification of a new gene essential for germination of Bacillus subtilis spores with Ca2+-dipicolinate. J. Bacteriol. 1852315-2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rose, R., B. Setlow, A. Monroe, M. Mallozzi, A. Driks, and P. Setlow. 2007. Comparison of the properties of Bacillus subtilis spores made in liquid or on agar plates. J. Appl. Microbiol. 103691-699. [DOI] [PubMed] [Google Scholar]
- 32.Serrano, M., R. Zilhão, E. Ricca, A. J. Ozin, C. P. Moran, Jr., and A. O. Henriques. 1999. A Bacillus subtilis secreted protein with a role in endospore coat assembly and function. J. Bacteriol. 1813632-3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Setlow, B., A. E. Cowan, and P. Setlow. 2003. Germination of spores of Bacillus subtilis with dodecylamine. J. Appl. Microbiol. 95637-648. [DOI] [PubMed] [Google Scholar]
- 34.Setlow, B., C. A. Loshon, P. C. Genest, A. E. Cowan, C. Setlow, and P. Setlow. 2002. Mechanisms of killing of spores of Bacillus subtilis by acid, alkali and ethanol. J. Appl. Microbiol. 92362-375. [DOI] [PubMed] [Google Scholar]
- 35.Setlow, B., and P. Setlow. 1996. Role of DNA repair in Bacillus subtilis spore resistance. J. Bacteriol. 1783486-3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6550-556. [DOI] [PubMed] [Google Scholar]
- 37.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to radiation, heat and chemicals. J. Appl. Microbiol. 101514-525. [DOI] [PubMed] [Google Scholar]
- 38.Seyler, R. W., A. O. Henriques, A. J. Ozin, and C. P. Moran, Jr. 1997. Assembly and interactions of cotJ-encoded proteins, constituents of the inner layers of the Bacillus subtilis spore coat. Mol. Microbiol. 25955-966. [DOI] [PubMed] [Google Scholar]
- 39.Waller, L. N., N. Fox, K. F. Fox, A. Fox, and R. L. Price. 2004. Ruthenium red staining for ultrastructural visualization of a glycoprotein layer surrounding the spores of Bacillus anthracis and Bacillus subtilis. J. Microbiol. Methods 5823-30. [DOI] [PubMed] [Google Scholar]
- 40.Young, S. B., and P. Setlow. 2003. Mechanisms of killing of Bacillus subtilis spores by hypochlorite and chlorine dioxide. J. Appl. Microbiol. 9554-67. [DOI] [PubMed] [Google Scholar]
- 41.Young, S. B., and P. Setlow. 2004. Mechanisms of Bacillus subtilis spore resistance to and killing by aqueous ozone. J. Appl. Microbiol. 961133-1142. [DOI] [PubMed] [Google Scholar]
- 42.Zhang, J., H. Ichikawa, R. Halberg, L. Kroos, and A. Aronson. 1994. Regulation of the transcription of a cluster of Bacillus subtilis spore coat genes. J. Mol. Biol. 240405-415. [DOI] [PubMed] [Google Scholar]
- 43.Zheng, L., R. Halberg, S. Roels, H. Ichikawa, L. Kroos, and R. Losick. 1992. Sporulation regulatory protein GerE from Bacillus subtilis binds to and can activate or repress transcription from promoters for mother-cell-specific genes. J. Mol. Biol. 2261037-1050. [DOI] [PubMed] [Google Scholar]