Abstract
Symbiotic N2 fixation in Bradyrhizobium japonicum is controlled by a complex transcription factor network. Part of it is a hierarchically arranged cascade in which the two-component regulatory system FixLJ, in response to a moderate decrease in oxygen concentration, activates the fixK2 gene. The FixK2 protein then activates not only a number of genes essential for microoxic respiration in symbiosis (fixNOQP and fixGHIS) but also further regulatory genes (rpoN1, nnrR, and fixK1). The results of transcriptome analyses described here have led to a comprehensive and expanded definition of the FixJ, FixK2, and FixK1 regulons, which, respectively, consist of 26, 204, and 29 genes specifically regulated in microoxically grown cells. Most of these genes are subject to positive control. Particular attention was addressed to the FixK2-dependent genes, which included a bioinformatics search for putative FixK2 binding sites on DNA (FixK2 boxes). Using an in vitro transcription assay with RNA polymerase holoenzyme and purified FixK2 as the activator, we validated as direct targets eight new genes. Interestingly, the adjacent but divergently oriented fixK1 and cycS genes shared the same FixK2 box for the activation of transcription in both directions. This recognition site may also be a direct target for the FixK1 protein, because activation of the cycS promoter required an intact fixK1 gene and either microoxic or anoxic, denitrifying conditions. We present evidence that cycS codes for a c-type cytochrome which is important, but not essential, for nitrate respiration. Two other, unexpected results emerged from this study: (i) specifically FixK1 seemed to exert a negative control on genes that are normally activated by the N2 fixation-specific transcription factor NifA, and (ii) a larger number of genes are expressed in a FixK2-dependent manner in endosymbiotic bacteroids than in culture-grown cells, pointing to a possible symbiosis-specific control.
Members of several genera of the alphaproteobacteria, collectively named “the rhizobia,” are capable of living not only in soil or in laboratory culture (free-living) but also facultatively within the infected cells of legume root nodules (endosymbiotic). Bacteroids—as they are called in the symbiotic state—fix molecular nitrogen as a nitrogen source for the host plant. Within root nodules, the rhizobia encounter oxygen-limiting conditions (microoxia) which trigger the expression of specific genes. Microoxia has been clearly recognized as a key factor that drives the synthesis and activity of nitrogenase, the enzyme that converts N2 to ammonium (6, 19, 24, 25).
In the soybean symbiont Bradyrhizobium japonicum, a sophisticated regulatory network consisting of two linked regulatory cascades coordinates the expression of genes required for microaerobic respiration (the FixLJ-FixK2 cascade) and for nitrogen fixation (the RegSR-NifA cascade). In these two cascades, different oxygen-sensing mechanisms are responsible for a stepwise activation of downstream events (63). In the RegSR-NifA cascade, the low-oxygen-responsive NifA protein activates the transcription of essential symbiotic nitrogen fixation genes at an oxygen concentration at or below 0.5% oxygen in the gas phase over a culture. In contrast, only a moderate decrease of the ambient oxygen concentration, to 5%, in the gas phase over a culture is already sufficient to trigger ATP-dependent autophosphorylation of the deoxygenated FixL hemoprotein in the FixLJ-FixK2 cascade and subsequent transfer of the phosphoryl group to the cognate response regulator FixJ (31, 33). Phosphorylated FixJ then activates the expression of the fixK2 gene. The FixK2 protein, in turn, plays a dual role in that it downregulates, directly or indirectly, the expression of its own gene (53) and acts as a transcriptional activator of genes for adaptation to microoxia, such as the fixNOQP genes for the cbb3-type high-affinity terminal oxidase, an enzyme that allows bacteroid respiration inside root nodules (53, 57).
A comparison of the regulatory circuits operating in B. japonicum with those in other rhizobial species reveals differences in the connectivity and subordination of the regulatory players FixLJ, FixK, and NifA (19). In Azorhizobium caulinodans, the nifA gene is directly regulated by FixK, whereas in Sinorhizobium meliloti, FixJ is the master regulator that directly controls both nifA and fixK (25). In B. japonicum, the only known FixJ target is fixK2, whose product in turn activates the regulatory protein genes fixK1, rpoN1, and nnrR, thus expanding the downstream end of the cascade (47, 49, 53) to compose, for instance, a FixLJ-FixK2-NnrR cascade (47).
FixK2 is one of the 16 cyclic AMP receptor protein/fumarate and nitrate reduction regulator (CRP/FNR)-type transcriptional regulators whose genes are present in the B. japonicum genome (for a review see references 40 and 48). FixK2 recognizes a palindromic sequence motif (TTG-N8-CAA, termed the FixK2 box) (49) which is located around position −41 upstream of the transcription start site in the regulated promoters. Until now, the expression from their promoters of 14 genes or operons was known to be controlled either directly or indirectly by FixK2. Microaerobically induced targets of FixK2 include the operons fixNOQP (as mentioned above) and fixGHIS (58), both essential for microaerobic respiration; several heme biosynthesis genes (hemA, hemB, hemN1, and hemN2) (15, 27, 55); denitrification genes (napEDABC, nirK, norCBQD, and nosRZDFYLX) (18, 50, 60, 67, 68); and some hydrogen oxidation genes (hup genes) (21). In a cell-free transcription system (in vitro), RNA polymerase, together with purified FixK2, was shown to directly activate transcription from the fixNOQP, fixGHIS, and hemN2 promoters (49).
No target genes had been known so far for the FixK1 protein. Although it is a FixK2 homolog, FixK1 differs from FixK2 in its strong oxygen sensitivity (4). Hence, maximal FixK1 activity in vivo is achieved only in anoxic conditions (nitrate respiration). Despite this difference, however, the FixK2 and FixK1 proteins share a certain functional similarity, because the phenotypic defects of a fixK2 mutant could be partially restored by constitutive fixK1 gene expression (4, 53). The oxygen sensitivity of FixK1 is most likely due to the presence of a cysteine-rich N-terminal extension (missing in FixK2) whereby the FixK1 protein much more closely resembles the oxygen-sensitive Escherichia coli FNR protein in which a [4Fe-4S]2+ cluster is bound to that domain (44, 45; reviewed in reference 39).
In order to expand our knowledge of the regulation mediated by the FixLJ-FixK2-FixK1 cascade, we aimed in this work at a genome-wide transcription profiling of B. japonicum fixJ, fixK2, and fixK1 mutant strains (always in comparison with the wild type), which were grown in free-living microoxic conditions and, in the case of the fixK1 mutant, also in an anoxic condition. The latter condition could not be applied to the fixJ and fixK2 mutants because they are defective in anaerobic nitrate respiration (3, 53). Furthermore, the transcriptomes of ΔfixJ and ΔfixK2 bacteroids from soybean nodules were investigated. Bioinformatics tools used in a FixK2 binding site search, together with in vitro transcription studies of putative targets, have allowed us to identify eight new genes whose expression is directly activated by FixK2. Moreover, novel regulatory interrelations were discovered that may help unravel new facets in the control of the symbiotic and free-living microoxic lifestyles of B. japonicum.
MATERIALS AND METHODS
Bacterial strains, plasmids, and primers.
The bacterial strains and plasmids used in this study are listed in Table 1. A list of oligonucleotides used as primers is available from the authors on request.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant genotype or phenotype | Source or reference |
|---|---|---|
| E. coli strains | ||
| DH5α | supE44 ΔlacU169 (φ80 lacZΔM15) hsdR17 recA1 gyrA96 thi-1 relA1 | BRL, Gaithersburg, MD |
| S17-1 | Smr SprhsdR (RP4-2 kan::Tn7 tet::Mu; integrated into the chromosome) | 64 |
| B. japonicum strains | ||
| 110spc4 | Spr; wild type | 59 |
| 7360 | KmrfixJ::aphII (same orientation) | 3 |
| 9043 | Spr SmrfixK2::Ω | 53 |
| 7454 | KmrfixK1::aphII (same orientation) | 4 |
| 9039K2 | Spr Smr KmrfixK2::Ω fixJ::aphII fixK1c (constitutive expression of fixK1) | 53 |
| 8882 | KmrcycS::aphII (same orientation) | This work |
| 8883 | KmrcycS::aphII (opposite orientation) | This work |
| 8884 | Spr TcrcycS-lacZ chromosomally integrated into 110spc4 | This work |
| 8884K2 | Spr Smr TcrcycS-lacZ chromosomally integrated into 9043 | This work |
| 8884JK2 | Spr Smr Kmr TcrcycS-lacZ chromosomally integrated in 9039K2 | This work |
| Plasmids | ||
| pBluescript SK(+) | Apr cloning vector | Stratagene, La Jolla, CA |
| pGEM-T Easy | Apr cloning vector | Promega, Madison, CA |
| pBSL86 | Apr Kmr | 1 |
| pSUP202pol4 | Tcr (pSUP202) part of the polylinker from pBluescript II KS(+) between EcoRI and PstI | 26 |
| pSUP202pol6K | Tcr (pSUP202pol4) KpnI linker into SmaI site | 69 |
| pSUP3535 | Tcr (pSUP202pol4) 3.2-kb EcoRI-DraI fragment from pME3535, transcriptional lacZ fusion vector | Laboratory collection |
| pRJ9519 | Apr [(pBluescript SK(+)] 308-bp BstXI-KpnI fragment containing the B. japonicum rrn terminator cloned into the HincII and KpnI sites | 8 |
| pRJ8870 | Apr (pRJ9519) 210-bp SacII-XbaI fragment containing a second B. japonicum rrn terminator cloned into the SacII and XbaI sites | This work |
| pRJ9601 | Apr [pBluescript SK(+)] B. japonicum rrn promoter and rrn terminator on 468-bp SacI-SmaI fragment | 8 |
| pRJ8817 | Apr (pRJ9519) fixGHIS promoter on 524-bp XbaI-EcoRI fragment | 49 |
| pRJ8860 | Apr (pRJ9519) blr6062 (cycS) promoter on 409-bp XbaI-SpeI fragment | This work |
| pRJ8861 | Apr (pRJ9519) blr6070 promoter on 455-bp BamHI-EcoRI fragment | This work |
| pRJ8862 | Apr (pRJ9519) bsr7087 promoter on 316-bp BamHI-EcoRI fragment | This work |
| pRJ8863 | Apr (pRJ9519) bll2388 promoter on 336-bp BamHI-EcoRI fragment | This work |
| pRJ8864 | Apr (pRJ9519) bll6073 promoter on 509-bp XbaI-SpeI fragment | This work |
| pRJ8865 | Apr (pRJ9519) blr4637 promoter on 407-bp BamHI-EcoRI fragment | This work |
| pRJ8867 | Apr (pRJ9519) bll3998 promoter on 641-bp BamHI-EcoRI fragment | This work |
| pRJ8869 | Apr (pRJ9519) blr4655 promoter on 428-bp BamHI-EcoRI fragment | This work |
| pRJ8871 | Apr (pRJ8870) fixK1-cycS intergenic region on 171-bp XbaI-EcoRI fragment | This work |
| pRJ8872 | Apr [(pBluescript SK(+)] 3′-flanking sequence of cycS on 554-bp HindIII-XbaI fragment | This work |
| pRJ8875 | Apr [(pBluescript SK(+)] 5′-flanking sequence of cycS on 561-bp Acc65I-HindIII fragment | This work |
| pRJ8879 | Apr (pRJ8872) 561-bp Acc65I-HindIII fragment from pRJ8875 | This work |
| pRJ8880 | Apr Kmr (pRJ8879) cycS::aphII, 1,182-bp HindIII from pBSL86 (same orientation) | This work |
| pRJ8881 | Apr Kmr (pRJ8879) cycS::aphII, 1,182-bp HindIII from pBSL86 (opposite orientation) | This work |
| pRJ8882 | Kmr Tetr (pSUP202pol6K) 2,297-bp Acc65I-XbaI fragment from pRJ8880 | This work |
| pRJ8883 | Kmr Tetr (pSUP202pol6K) 2,297-bp Acc65I-XbaI fragment from pRJ8881 | This work |
| pRJ8884 | Tcr (pSUP3535) cycS-lacZ, cycS promoter on 541-bp SmaI fragment | This work |
| pRJ8886 | Apr [(pBluescript SK(+)] 5′-flanking sequence of cycS on 741-bp SmaI-XbaI fragment | This work |
Media and growth conditions.
Escherichia coli cells were routinely grown in Luria-Bertani (LB) medium (51) at 37°C. Where appropriate, antibiotics were used at the following concentrations (in μg per ml): ampicillin, 200; kanamycin, 30; and tetracycline, 10. B. japonicum was grown aerobically and microaerobically (0.5% O2 in the gas phase) in a modified peptone-salts-yeast extract medium (59) that contained the following ingredients (per liter): KH2PO4, 300 mg; Na2HPO4, 300 mg; CaCl2·2H2O, 5 mg; MgSO4·7H2O, 100 mg; peptone, 3 g; yeast extract, 1 g; H3BO3, 10 mg; ZnSO4·7H2O, 1 mg; CuSO4·5H2O, 0.5 mg; Na2MoO4·2H2O, 0.1 mg; MnCl2·4 H2O, 0.1 mg; FeCl3, 0.19 mg; thiamine-HCl, 1 mg; biotin, 1 mg; Na-panthothenate, 1 mg; l-arabinose, 1 g. Yeast extract-mannitol medium (17) supplemented with 10 mM KNO3 was used for B. japonicum growth under anoxic conditions (N2 in the gas phase). Further details relevant for the growth of microaerobic and anaerobic cultures in transcriptome analyses have been described elsewhere (37, 56). The concentrations of antibiotics in B. japonicum cultures were as follows (in μg per ml): chloramphenicol, 20; spectinomycin, 100; kanamycin, 100; streptomycin, 50; and tetracycline, 50 (solid medium) or 25 (liquid medium).
Plant growth.
Seeds of soybean (Glycine max [L.] Merr. cv. Williams) were surface sterilized (5 min with ethanol and 15 min with 30% H2O2), rinsed several times with abundant sterile water, and incubated in darkness for 48 h on water-agar plates (1.5% agar). The inoculation and growth of the plants were carried out as described previously (34, 36). For transcriptome analyses, nodules were harvested 21 days postinoculation. They were then immediately frozen in liquid nitrogen and stored at −80°C for later RNA isolation. The in-nodule nitrogenase activity of B. japonicum strains was determined with an acetylene reduction assay (34, 36).
RNA isolation, cDNA synthesis, and microarray analysis.
B. japonicum cultures were grown to mid-exponential phase, which corresponded to an optical density at 600 nm of 0.4 to 0.5 in microoxic cultures (peptone-salts-yeast extract-arabinose medium) and an optical density at 600 nm of 0.175 to 0.225 in anoxic cultures (yeast extract-mannitol-nitrate medium). Cell harvest, isolation of total RNA, cDNA synthesis, fragmentation, labeling, and conditions for microarray hybridization were done as described recently (37, 46, 56). A description of the custom-designed B. japonicum gene chip BJAPETHa520090 (Affymetrix, Santa Clara, CA) is given elsewhere (37).
For transcriptome profiling of bacteroids, all nodules from five plants infected with either the wild type or the fixK2 or fixJ mutants were collected for each RNA extraction and hybridization experiment. The RNA was isolated by using a protocol of Pessi et al. (56). Amounts of 2.2 μg and 5.5 to 8 μg cDNA generated from RNA of culture-grown bacteria and nodules, respectively, were hybridized to the arrays. The amount of bacteroid-derived cDNA was estimated from the proportion of bacterial-to-plant rRNA in nodules (Bioanalyzer; Agilent Technologies, Palo Alto, CA). A minimum of six or three independent biological samples of each strain grown under free-living or symbiotic conditions, respectively, were analyzed. The primary data analysis was done with Affymetrix GeneChip Operating Software (GCOS) version 1.2. GeneSpring GX 7.3.1 software (Agilent Technologies) was used for comparative analyses. Only those probe sets that were called “present” or “marginal” in ≥69% of the replicates of each experiment were considered for further analysis. The details of data processing, normalization, and further analyses are described elsewhere (56). Genes were considered to be differentially expressed only when they had passed the statistical tests and when the change in expression (measured as n-fold change [FC]) was ≥2 or ≤−2 in comparisons between two strains or two different conditions.
Operon prediction and genome-wide FixK2 binding site search.
Operon prediction was done essentially by applying previously described criteria (37, 52). Genes were considered to be in an operon-like organization if they were oriented in the same direction and separated by less than 32 bp. This distance was enlarged to 100 bp if the first three letters in the gene names were identical. Additionally helpful was a tiling analysis of all probe sets within and around a gene of interest (37). For the identification of potential FixK2 binding sites, we used a position-specific frequency matrix (PSFM) consisting of experimentally verified FixK2 binding sites (see Table S1 in the supplemental material) in combination with a motif prediction algorithm (28). A similar strategy has previously been applied successfully for the identification of NifA+σ54- and RegR-dependent targets (37, 46). Putative promoter regions of 500 bp in length were searched for the PSFM motif. Sites considered to be putative FixK2 binding sites were only those that had a higher score than that of the lowest-scoring motif in the set of already validated FixK2 binding sites which had been used for the generation of the PSFM. For the identification of putative FixJ binding sites, a previously described de novo transcription factor binding site prediction was applied (29). This bioinformatics tool was applied for genes that showed decreased expression in a ΔfixJ strain (FC, ≤−2) but did not depend on FixK2.
In vitro transcription experiments.
The plasmids used as transcription templates were based on pRJ9519 and pRJ8870 (Table 1), which contain one and two B. japonicum rrn transcription terminators, respectively. Plasmid pRJ8870 was particularly useful in the analysis of a promoter region of two adjacent but divergently transcribed genes, as it harbors two transcriptional terminators located at different positions. Simultaneous transcription from divergently oriented promoters then yields transcripts differing by about 50 nucleotides. Multiple-round in vitro transcription assays were carried out at 37°C with RNA polymerase holoenzyme purified from B. japonicum at 37°C as described previously (49). Different amounts (0 to 2.5 μM dimer) of FixK2 protein purified as described earlier (49) were added to the reaction mixture. Suitable RNA size markers were prepared in vitro with T3 RNA polymerase (49). Electrophoresis of radioactive transcription products was done in a denaturing 6% polyacrylamide gel, and the reaction products were visualized with a phosphorimager. The quantification of signal intensities was performed with Bio-Rad Quantity One software, version 4.6.1 (Bio-Rad, Reinach, Switzerland).
Primer extension experiments.
The in vivo transcription start site of cycS was mapped in a primer extension experiment using cycS-specific oligonucleotides according to previously described protocols (5, 54). RNA was isolated as described above from the wild-type B. japonicum strain and from fixK2 mutant cells grown in microoxic or anoxic (applicable only to the wild type) conditions. Determination of the transcription start site of the in vitro-synthesized cycS transcript was carried out according to the method of Mesa et al. (49), using primer 9519-1. The extension products were analyzed on denaturing 6% polyacrylamide gels adjacent to sequencing ladders generated with the same oligonucleotides and plasmids pRJ8886 and pRJ8860 (for the in vivo and in vitro start sites, respectively).
Construction of cycS mutant strains.
B. japonicum cycS mutant strains 8882 and 8883 were constructed by marker exchange mutagenesis. To do so, 5′ and 3′ flanking regions of the cycS gene were amplified by PCR and cloned into pSUP202pol6K (Table 1). A kanamycin resistance cassette from pBSL86 was inserted in both orientations between the two B. japonicum DNA fragments. The resulting plasmids, pRJ8882 and pRJ8883, were transferred via conjugation into B. japonicum 110spc4 by using E. coli S17-1 as donor. The correct genomic structures of the cycS mutations were confirmed by PCR. The mutant strains are listed in Table 1.
Construction of a chromosomally integrated cycS-lacZ fusion, and β-galactosidase activity testing.
A transcriptional cycS-lacZ fusion was obtained by PCR amplification of a 541-bp SmaI fragment with the cycS promoter region which was then cloned into pSUP3535, yielding plasmid pRJ8884. Plasmid pRJ8884 was mobilized by conjugation into B. japonicum strains 110spc4, 9043, and 9039K2. The correct genomic integration was verified by PCR. The determination of β-galactosidase activities was carried out as described previously (27).
Cell fractionation, SDS-polyacrylamide gel electrophoresis, and cytochrome c staining.
B. japonicum cells were grown anoxically and harvested at stationary phase. Cell fractionation was carried out as indicated earlier (27). Soluble fractions were loaded without boiling onto sodium dodecyl sulfate (SDS)-18% polyacrylamide gels (42). The proteins were stained for heme-dependent peroxidase activity by using a “Supersignal West pico chemiluminescent substrate” chemiluminescence detection kit (Perbio Science, Lausanne, Switzerland). The protein concentration was estimated by using a Bio-Rad assay (Bio-Rad, Reinach, Switzerland) with bovine serum albumin as the standard.
Microarray data accession number.
The microarray data are available in the NCBI Gene Expression Omnibus database (GEO; http://www.ncbi.nlm.nih.gov/geo) under GEO Series accession number GSE12491.
RESULTS
Global assessment of genes controlled by the FixLJ-FixK2-FixK1 cascade in microoxically cultured cells.
Microarray analysis has led to a registry of B. japonicum genes that are induced (FC, ≥2) in microoxically grown cells (0.5% O2 in the gas phase) in comparison to their expression in oxically grown cells (21% O2 in the gas phase) (56). Which and how many of these genes are subject to regulation by the hierarchically organized transcription factors FixJ, FixK2, and FixK1 was assessed by comparing the transcriptomes of wild-type cells and fixJ, fixK2, and fixK1 mutants, all grown microoxically. The total number of genes that are upregulated in microoxic conditions and, at the same time, regulated exclusively by FixJ, FixK2, or FixK1 is shown in Fig. 1, and the corresponding genes are listed in Tables S2 and S3 in the supplemental material.
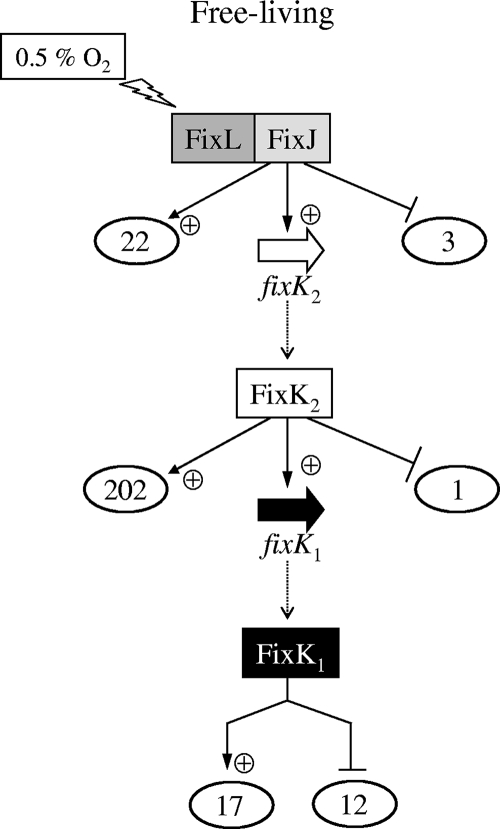
FIG. 1.
Schematic representation of microoxically induced B. japonicum genes that are regulated by FixJ, FixK2, or FixK1. The numbers of genes controlled by these regulators are circled by ovals. The fixK1 gene and its product are highlighted with a black arrow and white letters in a solid rectangle, respectively. Details are explained in the text. Note that although the model suggests a direct hierarchical organization, the existence of additional control levels in between FixLJ and FixK2 and in between FixK2 and FixK1 cannot be excluded. Therefore, the regulation of target genes by FixJ, FixK2, or FixK1 may be direct or indirect. +, positive regulation; −, negative regulation.
It becomes evident that the regulon size of FixK2 is much larger than those of FixJ and FixK1 (Fig. 1). The FixJ and FixK2 regulons consist almost solely of positively controlled genes, whereas a substantial portion of the FixK1-regulated genes are negatively controlled (12 out of 29).
Prior to this work, the only gene known to be controlled by the FixJ response regulator was fixK2 (53). An additional 25 genes have now been found to be specifically regulated by FixJ (Fig. 1). However, a more-detailed examination of these genes was not pursued for three reasons. (i) The majority have no predicted function (see Tables S2 and S3 in the supplemental material). (ii) With few exceptions, their levels of up- or downregulation are comparatively small, suggesting that they are not strongly activated or repressed by FixJ. (iii) The use of bioinformatics search tools (see Materials and Methods) has not led to the identification of a conserved nucleotide sequence motif that might serve as a FixJ binding site in the DNA regions upstream of FixJ-regulated genes. Such a putative “FixJ box” would have been helpful in a first approximation to possibly distinguish directly from indirectly controlled genes.
Our primary attention in this work was, therefore, addressed to the genes positively controlled by FixK2. Apart from previously identified FixK2-dependent genes (fixK1, rpoN1, nnrR, nirK, hemN1, and hemN2) and operons (fixNOQP, fixGHIS, and napEDABC), new genes have now been identified that must be regarded as promising candidates for being FixK2 targets (see Table S2 in the supplemental material). Comments on a few examples follow. (i) There are cytochrome genes, such as blr4955 (cytochrome b561) or blr6128 (cycB, encoding cytochrome c552), which suggests that these genes are possibly important for life under conditions of oxygen deprivation. (ii) There are genes such as bll3998, coding for a succinate-semialdehyde dehydrogenase (a tricarboxylic acid cycle bypass enzyme); blr4655, coding for a phosphoenolpyruvate synthase (gluconeogenesis enzyme); and bll6073 (phbC), coding for a poly-β-hydroxybutyrate (PHB) polymerase, that indicate an involvement of FixK2 in regulating carbon and energy metabolism. (iii) There are genes for general stress response, such as blr4635 (groEL5) and blr4653 (dnaJ), and a heat shock-related gene (blr4637), an observation that has already been made by Bobik et al. (10) when these authors examined the S. meliloti FixJ regulon. (iv) Like fixK1 and nnrR (4, 47, 53), FixK2 appears to control other regulatory genes, e.g., bll2109 and bll3466, both coding for CRP-type regulators, which could imply a further expansion of the regulatory cascade.
No FixK1-controlled gene had been identified so far. To find out how many genes are exclusively controlled by FixK1, the transcription profiling of a ΔfixK1 strain was compared with that of the wild type and the ΔfixK2 strain, all grown in microoxic conditions. A relatively small number of genes showed differential expression in the ΔfixK1 strain (17 positively and 12 negatively controlled genes) (Fig. 1; see Tables S2 and S3 in the supplemental material). Among the positively regulated genes is hemN1, whose expression was previously shown to depend on FixK2 (27). This shows that the FixK2 dependency of hemN1 expression proceeds indirectly via FixK1, although a direct contribution by FixK2 cannot be excluded. Interesting new FixK1 targets are two cytochrome genes (bll2388 [cytochrome c2] and blr6062 [cytochrome c6]), which will be the subject of further transcriptional studies (see below).
When we looked at the genes that are negatively regulated by FixK1, an intriguing observation was made. In addition to the 12 genes shown in Fig. 1, we noticed another 34 genes whose expression was not at the same time increased in the ΔfixK2 mutant. These are listed at the bottom of Table S3 in the supplemental material. This makes a total of 46 genes that appear to be repressed, directly or indirectly, by FixK1. Surprisingly, almost all of these negatively regulated genes (45 out of 46) are known to be under positive control by the transcriptional activator NifA (37). Similarly, when we compared the transcription profile of ΔfixK1 cells with that of wild-type cells, both grown under anoxic conditions (nitrate respiration), a substantial number of genes overlapped with the NifA regulon (32 out of 44) (data not shown). Taken together, these results indicate that a hitherto unrecognized regulatory interference might exist between the FixLJ-FixK2-FixK1 cascade and the RegSR-NifA cascade (see Discussion).
Transcription profiling of the B. japonicum fixJ and fixK2 mutants in symbiosis.
A regulatory pattern that was similar though not completely identical to that found in microoxically cultivated cells was seen for genes expressed in endosymbiotic bacteroids (Fig. 2; see Tables S4 and S5 in the supplemental material). In this case, we analyzed the transcriptomes of the wild type and of ΔfixJ and ΔfixK2 mutant bacteroids, but not of ΔfixK1 bacteroids, because in contrast to fixJ and fixK2, the fixK1 gene is not essential for symbiotic nitrogen fixation (4, 53).
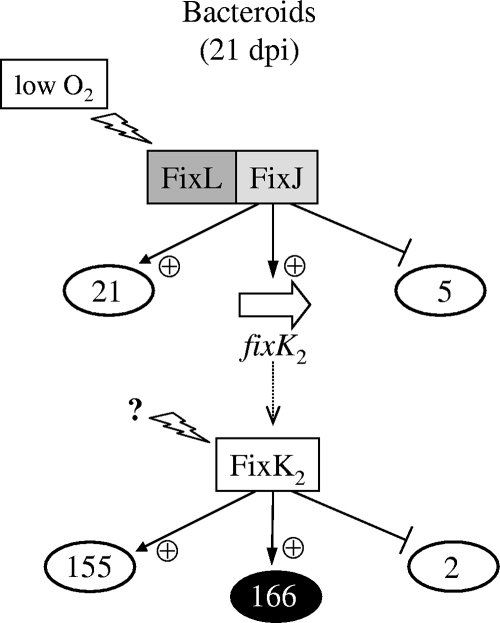
FIG. 2.
Schematic representation of soybean bacteroid-induced genes that are controlled by FixJ or FixK2. For details, see the text and the Fig. 1 legend. A set of 166 FixK2-activated genes is not at the same time dependent on FixJ (indicated in white letters on a black background). An unknown regulatory signal might be sensed at the level of FixK2 (directly or indirectly). dpi, days postinoculation; +, positively regulated; −, negatively regulated.
In a previous investigation, Pessi et al. (56) reported 692 B. japonicum genes to be induced in soybean bacteroids (21 days postinoculation). In this work, we noticed that a substantial proportion of these belong to the regulons of the FixLJ-FixK2 cascade (i.e., 183 genes). The expression of the majority (155 genes) is decreased at the same time in ΔfixK2 bacteroids and in ΔfixJ bacteroids, which demonstrates again that FixJ is the hierarchically superimposed regulator of fixK2 (Fig. 2; see Table S4 in the supplemental material). The small number of negatively controlled genes is listed in Table S5 in the supplemental material.
Unexpectedly, bacteroids were found to express 166 FixK2-activated genes (Fig. 2) which are not dependent at the same time on FixJ. A possible implication of this finding is that another type of regulatory signal or protein, uncoupled from FixJ control, acts at the level of the FixK2 protein. Interestingly, among this set of 166 genes are putative regulator and sigma factor genes. Examples are blr1880 (LuxR-like) and blr3042 (ECF-type sigma factor).
Identification of direct FixK2 targets.
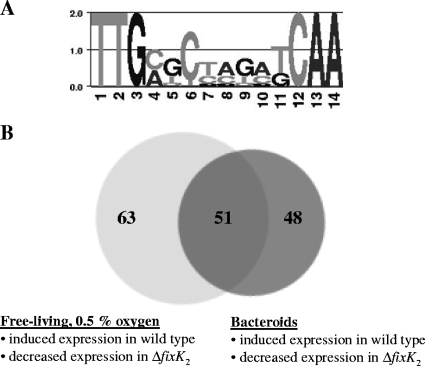
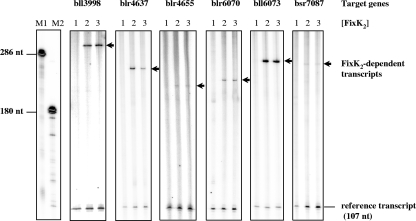
In order to find out which genes are directly controlled by FixK2, we first used a bioinformatics approach and subsequently a biochemical approach. The purpose of the bioinformatics approach was to identify genes that carry a putative FixK2 binding site (FixK2 box) (49) in their promoter regions (see Materials and Methods and Fig. 3A) (28). Relevant for this analysis were the 220 FixK2-dependent genes induced in free-living bacteria, as shown in Fig. 1 (i.e., 202 + fixK1 + 17), and the 321 FixK2-dependent genes induced in bacteroids, as shown in Fig. 2 (i.e., 155 + 166). These two sets respectively contained 114 and 99 putative FixK2 boxes in promoter regions upstream of genes organized in mono-, di-, or polycistronic transcription units (Fig. 3B). The overlap resulted in 51 FixK2 box-associated transcription units (Fig. 3B and Table 2). These 51 cases provided the basis for the selection of seven promoter regions that were tested for direct FixK2-dependent activation of transcription in vitro. These are bll2388, bll3998, blr4637, bll6061 (fixK1), blr6070, bll6073 (phbC), and bsr7087. Included in this study also were two genes induced in free-living bacteria, but not in bacteroids, in a FixK2-dependent manner (blr4655 [ppsA] and blr6062 [cytochrome c6]). All nine genes were used as templates for transcription activation in vitro with purified RNA polymerase and purified FixK2 protein from B. japonicum. One of them (bll2388) did not result in the synthesis of a detectable transcript. The results depicted in Fig. 4 show that six examples yielded clearly identifiable transcripts of meaningful sizes (i.e., their transcription start sites were within the canonical distance downstream of the putative FixK2 box). Although transcript formation was weak in two cases (blr4655 and bsr7087), the transcripts were synthesized only when FixK2 protein was present in the assay. These six genes are now considered to be new direct targets of FixK2. The transcription of the other two genes (bll6061 and blr6062) will be described in the next paragraph.
FIG. 3.
Strategies for the identification of direct FixK2 targets. (A) Sequence logo for the FixK2 binding site created with “WebLogo” (16). The consensus motif is based on the sequences listed in Table S1 in the supplemental material (see also Materials and Methods). (B) Venn diagram representing FixK2-dependent mono-, di-, or polycistronic transcription units which contain putative FixK2 boxes in their upstream promoter regions. The left circle contains 114 transcription units induced in free-living, microoxic culture, whereas the right circle contains 99 transcription units induced in bacteroids. For further details, see the text and Table 2.
TABLE 2.
List of the 51 FixK2 box-associated promoter regions and genes whose expression is decreased in the fixK2 mutant in microoxic free-living conditions and in bacteroids compared to their expression in the wild type
| Locus taga | Geneb | FC value in microoxic conditionsc | FC value for bacteroidsd | Descriptione | Predicted operon structuref | Positiong | Motifh |
|---|---|---|---|---|---|---|---|
| blr0497 | −27.4 | −21.6 | Hypothetical protein | −31 | TTGATCCAGCGCAA | ||
| bll0818 | −9.3 | −7.5 | Unknown protein | −66 | TTGATCCCGGTCAA | ||
| blr1289 | −23.1 | −2.2 | Hypothetical protein | −37 | TTGATCCAGCGCAA | ||
| blr1311 | −69.9 | −10.0 | Outer membrane protein | −60 | TTGATCGGCGTCAA | ||
| bll1766 | −4.9 | −3.8 | Outer membrane protein | −228 | TTGATTGGTATCAA | ||
| blr1883 | rpoN1 | −3.5 | −3.7 | RNA polymerase σ54 subunit | −81 | TTGCGCGACATCAA | |
| bll2007 | hemN1 | −16.8 | −15.3 | Coproporphyrinogen III dehydrogenase | −138 | TTGACATAACGCAA | |
| bll2109 | −2.5 | −4.8 | Transcriptional regulatory protein CRP family | −38 | TTGCGTCACCTCAA | ||
| bll2330 | −18.9 | −4.6 | Hypothetical protein | bll2330-bsl2328 | −73 | TTGATCCAGATCAA | |
| bll2388 | cy2 | −5.3 | −10.4 | Cytochrome c2 | −140 | TTGATGCAGGACAA | |
| bll2471 | −35.5 | −10.1 | Hypothetical protein | −81 | TTGATCTAGCGCAA | ||
| blr2659 | −16.3 | −4.0 | Hypothetical protein | −79 | TTGCCTGGCATCAA | ||
| bll2662 | −14.6 | −5.2 | Hypothetical protein | −27 | TTGATCTGCATCAA | ||
| bsr2670 | −5.6 | −14.5 | Unknown protein | −141 | TTGAAGGAGGTCAA | ||
| blr2761 | orf277 | −26.7 | −12.7 | Hypothetical protein | −71 | TTGATCTATCTCAT | |
| blr2763 | fixN | −101.9 | −18.8 | Cytochrome-c oxidase | fixNOQP | −70 | TTGATTTCAATCAA |
| blr2767 | fixG | −63.3 | −53.2 | Iron-sulfur cluster-binding protein | fixGHIS | −71 | TTGAGCTGGATCAA |
| blr2987 | −10.1 | −9.7 | Hypothetical protein | −77 | TTGATTTGCGTCAA | ||
| bsr3073 | −10.0 | −32.5 | Hypothetical protein | −101 | TTGACGCGGATCAA | ||
| bll3835 | −8.7 | −8.3 | Hypothetical protein | −93 | TTGCTGCAAATCAA | ||
| bll3998 | −56.5 | −10.9 | Probable succinate-semialdehyde dehydrogenase | −120 | TTGACCTGTCTCAA | ||
| blr4114 | −62.5 | −25.6 | Hypothetical protein | blr4114-blr4115 | −113 | TTGATCTGGATCAT | |
| blr4174 | −46.7 | −6.5 | Hypothetical protein | −71 | TTGATCGAGCGCAA | ||
| bsr4175 | −5.0 | −5.7 | Hypothetical protein | −324 | CTGCGCCAGCTCAA | ||
| blr4637 | −111.5 | −20.7 | Probable HspC2 heat shock protein | −86 | TTGAGCAAAATCAA | ||
| bsl4650 | −20.9 | −6.6 | Unknown protein | −165 | CTGATCTAGCGCAA | ||
| bll4651 | −31.3 | −7.1 | Hypothetical protein | −98 | TTGATGTCGATCAA | ||
| blr4652 | −95.2 | −6.9 | Hypothetical protein | blr4652-blr4654 | −48 | TTGATCGACATCAA | |
| blr4955 | −16.1 | −8.1 | Putative cytochrome b561 | −161 | ATGAGGTGGATCAA | ||
| bsl5002 | −5.5 | −5.6 | Unknown protein | −31 | TTGATCTGCATCAT | ||
| bsr5273 | −102.6 | −68.5 | Unknown protein | −59 | TTGCGGTGCATCAA | ||
| bll5315 | −34.4 | −10.2 | Hypothetical protein | −49 | TTGATCCTGCGCAA | ||
| bsr5316 | −22.5 | −9.7 | Hypothetical protein | −48 | CTGATCTAGATCAA | ||
| bll5655 | −36.2 | −3.6 | Alcohol dehydrogenase | −69 | TTGACTCCAATCAA | ||
| bll6061 | fixK1 | −19.1 | −3.9 | Transcriptional regulatory protein CRP family | −100 | TTGATCTGGGTCAA | |
| bll6069 | −29.4 | −14.6 | Hypothetical protein | −72 | TTGACCTCCCTCAA | ||
| blr6070 | −7.3 | −2.4 | Putative alcohol dehydrogenase | blr6070-blr6072 | −78 | TTGAGGGAGGTCAA | |
| bll6073 | phbC | −27.9 | −6.0 | Probable poly-β-hydroxybutyrate polymerase | −81 | TTGATGCAGCTCAA | |
| blr6074 | −90.9 | −5.6 | Hypothetical protein | −143 | TTGAGCTGCATCAA | ||
| blr6128 | cycB | −16.0 | −13.7 | Cytochrome c552 | −266 | TTGCGGCAGATCAA | |
| bll6987 | −2.0 | −4.0 | Hypothetical signal peptide protein | −466 | TTGACATCGATCAA | ||
| bsr7036 | napE | −64.1 | −6.8 | Periplasmic nitrate reductase protein | napEDABC | −101 | TTGATCCAGATCAA |
| bll7086 | hemN2 | −97.1 | −4.4 | Anaerobic coproporphyrinogen III oxidase | −140 | TTGCGCGAGCGCAA | |
| bsr7087 | −53.8 | −30.7 | Unknown protein | bsr7087-blr7088 | −115 | TTGCGCTCGCGCAA | |
| blr7345 | −16.8 | −4.0 | Unknown protein | −76 | TTGATCCGCATCAA | ||
| bsl7372 | −35.1 | −2.9 | Hypothetical protein | −67 | TTGACGGAGATCAA | ||
| bll7553 | −11.4 | −4.3 | Unknown protein | −73 | TTGATATGCGTCAA | ||
| bsl7781 | −3.0 | −4.2 | Unknown protein | −102 | TTGATTCGGCGCAA | ||
| bll7787 | −19.1 | −15.3 | Unknown protein | −118 | TTGACCCAGATCAA | ||
| blr7961 | −41.7 | −28.3 | Probable HspC2 heat shock protein | −82 | TTGAGACAAATCAA | ||
| bll7982 | −13.5 | −8.3 | Hypothetical protein | bll7982-bll7981 | −96 | TTGATCTGAAACAA |
Nomenclature according to Kaneko and coworkers (38).
Gene name as indicated in the EMBL-EBI database.
Values from a comparison of ΔfixK2 cells with wild-type cells grown in microoxic conditions. Note that the expression of these genes under these conditions is induced in the wild-type strain (compared to the expression in free-living oxic cells [56]).
Values from a comparison of ΔfixK2 bacteroids with wild-type bacteroids. Note that the expression of these genes under these conditions is induced in the wild-type strain (compared to the expression in free-living oxic cells [56]).
Protein description according to Kaneko and coworkers (38).
Operons were predicted as described in Materials and Methods.
Position of the 5′-end nucleotide of the motif relative to the annotated translational start site of the associated gene.
Predicted FixK2 binding site.
FIG. 4.
In vitro transcription activation mediated by purified FixK2. Supercoiled template plasmids comprising the promoter regions of target genes (shown at top) and a strong transcriptional terminator were used for multiple-round in vitro transcription assays with FixK2 protein and RNA polymerase from B. japonicum cells. FixK2 dimer concentrations were as follows: no protein (lane 1), 1.25 μM (lane 2), and 2.5 μM (lane 3). Transcripts synthesized in vitro in the presence of [α-32P]UTP were separated on a 6% denaturing polyacrylamide gel and visualized by phosphorimager analysis of the dried gel. RNA size markers (M1 and M2) were generated as described earlier (49). The positions of the FixK2-dependent transcripts are marked by arrows. Also shown is a FixK2-independent reference transcript that is encoded on the vector portion of the template plasmids. nt, nucleotides.
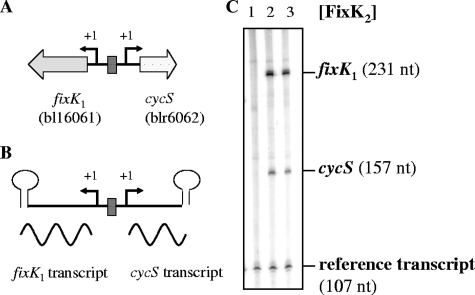
Divergent transcription of fixK1 and cycS.
A comparison between the FixK2 and FixK1 regulons in cells grown under microoxic conditions revealed 17 genes whose expression specifically depended on FixK1 (see Table S2 in the supplemental material). Among those we found blr6062, which had been annotated as a putative cytochrome c gene (cytochrome c6-like) (2, 38). Therefore, blr6062 will be named cycS hereafter. Incidentally, cycS is located directly adjacent but in the opposite orientation to the fixK1 gene (bll6061) (Fig. 5A). Only one FixK2 box was identified between the two genes, suggesting that it serves for the transcription activation not only of fixK1 but also of cycS. This inference was tested by inducing FixK2-dependent transcription activation in vitro, using as DNA template the fixK1-cycS-spanning fragment illustrated in Fig. 5B. Indeed, two transcripts were detected, one representing fixK1 mRNA and the other cycS mRNA (Fig. 5C).
FIG. 5.
FixK2-dependent transcription from the divergently oriented fixK1 and cycS promoters. Shown are a simplified map of the B. japonicum fixK1 and cycS genes (A), a schematic of the relevant template (B), and the results of its use for in vitro transcription (C). The single FixK2 binding site is symbolized by a dark box. The transcription start sites of fixK1 and cycS are marked as “+1.” The stem-loops symbolize the transcription terminators. Transcripts from the template plasmid pRJ8871 were generated by multiple-round in vitro transcription using B. japonicum RNA polymerase and purified FixK2 (no protein, lane 1; 1.25 μM, lane 2; and 2.5 μM, lane 3). The positions and sizes of the fixK1 transcript, the cycS transcript, and the vector-encoded reference transcript are indicated. nt, nucleotides.
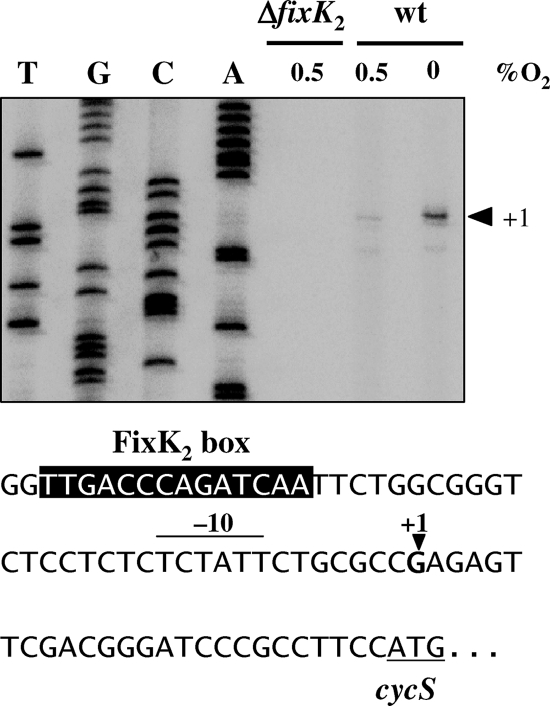
In order to determine the 5′ end of the cycS RNA, primer extension experiments were performed by using RNA isolated from the wild-type B. japonicum strain and from the ΔfixK2 strain, grown under different conditions (Fig. 6). The results of reverse transcription revealed a cycS transcription start point at a G located 25 nucleotides upstream of the annotated cycS start codon (Fig. 6). The same transcription start site was identified when the in vitro-synthesized cycS mRNA was used for primer extension (data not shown). The results of densitometric analysis presented in Fig. 6 showed that the amount of cDNA derived from RNA in anoxically grown cells (conditions of nitrate respiration) was sixfold higher than the amount from microoxically grown cells. That the transcription of cycS depends on FixK2 was confirmed, as deduced from the absence of the corresponding extension product in the microoxically grown fixK2 mutant. Taken together, the results of these experiments allowed us to precisely locate the axis of symmetry of the FixK2 box at position −39.5 (TTGACCCAGATCAA) upstream of the cycS transcription start site and the reverse complementary box (TTGATCTGGGTCAA) at position −40.5 in the fixK1 promoter region (53).
FIG. 6.
Mapping of the transcription start site of cycS. Total RNA was isolated from microoxic (0.5% O2) or anoxic (nitrate respiring) cells of the wild-type (wt) and the ΔfixK2 strain and used for primer extension experiments with two cycS-specific primers (results are shown for only one of the primers). The sequencing ladder on the left was generated with plasmid pRJ8886 and the same primer used for transcript mapping. The relevant nucleotide sequence of the cycS promoter is shown at the bottom. The putative −10 element is overscored, the FixK2 box is highlighted by white letters on a black background, the transcription start site is marked by “+1,” and the start codon of the cycS gene is underlined.
Despite the aforementioned activation of cycS by FixK2, our microarray experiments had initially classified cycS as a member of the FixK1 regulon. In fact, among the 17 positively controlled FixK1 regulon members, the cycS gene was the one whose expression depended most strongly on FixK1 (FC, −24.9) (see Table S2 in the supplemental material). From this observation and from the fact that FixK1 is a FixK2-homologous transcription factor, we reasoned that, apart from FixK2, FixK1 might also have the capacity to activate transcription from the unique cycS-fixK1 intergenic FixK2 box. Therefore, we tested the microaerobic expression of a transcriptional cycS-lacZ fusion in a B. japonicum fixJ-fixK2 double mutant in which the fixK1 gene was constitutively expressed from a foreign promoter (aphII promoter in strain 8884JK2) (Table 1). The controls showed good cycS expression in the wild type (85 ± 16 [mean ± standard deviation] Miller units) and only background levels in a fixK2 null mutant (2 ± 0.9 Miller units). Up to 32% of wild-type cycS expression was restored in strain 8884JK2 (27 ± 5 Miller units). This suggests that FixK1, in concert with FixK2, contributes to maximal cycS expression.
The cycS gene codes for a soluble c-type cytochrome expressed in anaerobic, nitrate-grown cells.
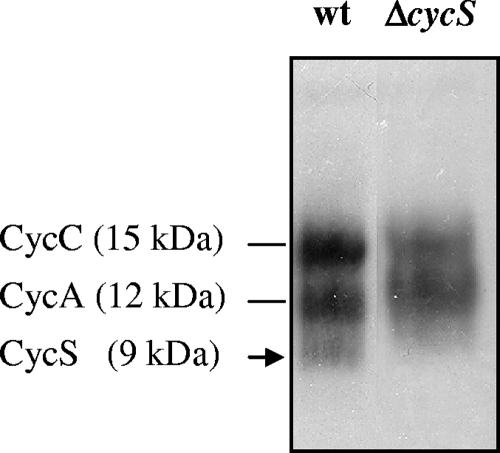
Owing to the presence of characteristic amino acid sequence motifs (signal sequence and heme-binding site [C37ARCH41]), the cycS gene product was predicted to be a soluble, periplasmic c-type cytochrome (2, 38). We sought to obtain experimental support for this assumption. Two ΔcycS mutant strains (8882 and 8883) (Table 1) were constructed by marker exchange mutagenesis. Both cycS mutants showed no differences with regard to aerobic growth and symbiotic nitrogen fixation phenotypes compared with the wild type (data not shown). In anoxic culture with nitrate as the terminal electron acceptor, however, a longer lag phase and a decreased growth rate were observed for the ΔcycS strains (see Fig. S1 in the supplemental material, showing the results with strain 8882). This was interpreted to mean that cycS is important but not essential for anaerobic nitrate respiration. Soluble and membrane proteins were extracted from the wild type and the cycS mutant (strain 8882), separated by SDS-polyacrylamide gel electrophoresis, and stained for covalently bound heme. In contrast to the membrane fraction, where no differences were found (data not shown), the soluble fraction of the mutant lacked one of the three stained bands detectable in the wild type (Fig. 7). The 15-kDa and 12-kDa proteins present in both cases had previously been identified as the B. japonicum soluble c-type cytochromes CycC and CycA (11, 66), whereas the 9-kDa cytochrome missing in strain 8882 obviously corresponds to the processed (i.e., secreted) CycS holocytochrome (predicted molecular mass of 9,825 Da, including covalently bound heme).
FIG. 7.
Heme staining of soluble proteins expressed in nitrate-respiring cells. Wild-type B. japonicum (wt) and ΔcycS strains were grown in anoxic conditions (see Materials and Methods). Proteins were separated by SDS-polyacrylamide gel electrophoresis, transferred to nitrocellulose, and stained for having covalently bound heme. Cytochromes CycC (15 kDa) and CycA (12 kDa), which were identified previously, are specified on the left. Each lane was loaded with a sample of cell extract containing 80 μg soluble proteins.
DISCUSSION
This work has led to a substantial expansion of knowledge about the regulon sizes, target genes, and regulatory ramifications associated with the hierarchically organized transcription factors in the B. japonicum FixLJ-FixK2-FixK1 cascade.
The FixJ regulon.
If one subtracts the large number of FixK2-controlled genes from the initially observed FixJ regulon, there are comparatively few genes left that might be specifically controlled solely by FixJ. A similar situation was previously noticed for S. meliloti, in which FixJ directly regulates only five genes and exerts its control via the regulatory genes nifA and fixK (10). The other three FixJ-dependent S. meliloti genes (proB2, Smc03253, and fixT3) (10) do not have orthologues in B. japonicum (National Center for Biotechnology Information [NCBI] genome comparison website, http://www.ncbi.nlm.nih.gov/sutils/geneplot.cgi). Hence, the physiological context of FixJ control—apart from that for fixK2 in B. japonicum and for nifA plus fixK in S. meliloti--remains enigmatic.
Nevertheless, we have tried to further analyze those few B. japonicum genes on which FixJ appeared to exert a marginal positive control. A biocomputing approach was applied in order to make a de novo prediction of a FixJ binding site within the promoter regions of the putative FixJ targets. This attempt, however, did not result in the identification of a consensus “FixJ box,” which made it impossible to distinguish putative direct FixJ targets from genes that are only indirectly regulated by FixJ. Likewise, the previous search for an “FixJ box” in S. meliloti had been inconclusive (23, 41).
The FixK2 regulon.
The analysis of the FixK2 regulon proved to be more straightforward, despite its large size, and was greatly aided by the genome-wide prediction of FixK2 boxes in the promoter regions of putative target genes. Not less than 51 promoter regions were identified from which FixK2-activated transcription might occur not only in free-living, microoxically grown cells but also in endosymbiotic soybean bacteroids. These and other promoter candidates allowed us to zoom in on several of them as DNA templates in cell-free transcription activation assays, using purified RNA polymerase and FixK2 protein. We thus ascertained the direct activation of transcription by FixK2 from eight novel promoter regions into their adjacent genes: bll3998, blr4637, blr4655 (ppsA), bll6061 (fixK1), blr6062 (cycS), blr6070, bll6073 (phbC), and bsr7087. This increases to 11 the total number of promoter regions for which FixK2-activated transcription was shown in vitro. Prior to this study, this feature applied to the promoters of the hemN2 gene and the fixNOQP and fixGHIS operons (49).
The newly discovered direct FixK2 targets are of interest also because the products of the respective genes might help uncover new facets in the physiology of B. japonicum in either the symbiotic or the free-living state. For example, three of these genes code for enzymes involved in carbon metabolism: bll3998, blr4655 (ppsA), and bll6073 (phbC). The bll3998 gene encodes a succinate semialdehyde dehydrogenase, implying that the tricarboxylate cycle bypass proposed by Green and collaborators (35) is under FixK2 control and operates in symbiosis. The ppsA gene codes for a phosphoenolpyruvate synthase, an anaplerotic and gluconeogenic enzyme that becomes important when C4-dicarboxylates are the carbon sources, as when they are provided by the host plant to bacteroids (20). More difficult to rationalize is why the last biosynthetic step for the carbon storage compound PHB, catalyzed by the bll6073 (phbC)-encoded PHB polymerase, is under FixK2 control. Intriguingly, while B. japonicum contains five phbC homologs (65), only this FixK2-dependent copy (bll6073) is induced in bacteroids (56). Moreover, the bll6073-encoded protein is expressed in bacteroids, suggesting that it has a function in symbiosis (62). Taken together, these are cumulative indications that hint at a hitherto unanticipated role of FixK2 in regulating certain pathways of carbon metabolism in B. japonicum which is worthy of exploration in future research.
Three other cases of genes directly activated by FixK2 are blr4637, which codes for a small heat shock protein (HspC2); the unknown-function gene bsr7087, which probably forms an operon together with the downstream gene blr7088 that codes for a putative periplasmic copper-binding protein (CopC) (13); and blr6070, encoding a putative Zn-containing alcohol dehydrogenase (38). For lack of functional data, speculations about the possible role of these gene products in the context of symbiotic nitrogen fixation or microoxic lifestyle, though enticing, go beyond the scope of this paper. In contrast, a little more information is available on the remaining two of the eight new direct FixK2 targets, bll6061 (fixK1) and bll6062 (cycS), which will be treated in a separate paragraph (see below).
Out of the 203 microoxically induced B. japonicum genes that exclusively depend on activation by FixK2, 60 genes have orthologs in S. meliloti, and among them, 26 genes are also controlled by FixJ and FixK in microoxically cultivated S. meliloti cells (10). Prominent examples are the fixGHIS and fixNOQP operons, responsible for the biogenesis and function of the bacteroid-specific cbb3-type respiratory cytochrome oxidase, and the napEDABC operon for the periplasmic nitrate reductase involved in denitrification. The B. japonicum-versus-S. meliloti transcriptome comparison in bacteroids was a little more complicated because only the FixJ regulon, but not the FixK regulon, was assessed in S. meliloti bacteroids (7, 10). Nevertheless, we recognized the existence of 55 S. meliloti orthologs of genes that belong to the combined FixJ-FixK2 regulon in B. japonicum bacteroids. Of these 55 genes, only 11 and 20 depended on FixJ according to the results of the studies performed by Bobik et al. (10) and Barnett et al. (7), respectively. Again, the cbb3-type cytochrome oxidase genes were among them. Yet, the differences in this respect between B. japonicum and S. meliloti were substantial, which among other reasons, might reflect differences in root nodule physiology and the smaller number of genes in the S. meliloti genomic repertoire (30, 38). In this context, it will now be of interest to compare more-closely related species, such as Bradyrhizobium sp. BTAi1, Bradyrhizobium ORS278, or even the nonsymbiotic, photosynthetic Rhodopseudomonas palustris (32, 43), once corresponding expression data have become available for these species.
A surprising result was that 166 genes were expressed in a FixK2-dependent manner in soybean bacteroids which were not concomitantly included in the list of FixJ-regulated genes. This can be interpreted to mean that a basal level of FixK2 protein may become activated in bacteroids by a new type of control (a novel regulatory protein?) which is uncoupled from FixJ control and is destined to activate a new group of genes. Also, strikingly, a large proportion of these genes does not overlap with the microoxic regulon (genes induced in microoxic conditions compared with oxic conditions) but instead belongs to the previously identified set of genes that are expressed in bacteroids only (62 genes out of 166) (56). Hence, a large number of genes activated by FixK2 in the bacteroids appear to escape from oxygen control, suggesting that a signal other than oxygen limitation is integrated at the level of FixK2.
On this occasion, we cannot help but dispute some recently published data of Chang et al. (14), who reported that not only fixK2 gene expression but also the expression of many well-known FixK2 targets, such as the fixNOQP operon, is strongly downregulated in soybean bacteroids. These data conflict with our own data on B. japonicum bacteroids (56 and this work) and those of others on S. meliloti (7, 9, 10), and they are difficult to reconcile with the essential nature of FixK2 and the cbb3-type cytochrome oxidase in symbiotic nitrogen fixation in B. japonicum, S. meliloti, and other rhizobia.
The FixK1 regulon.
The FixK2-activated fixK1 gene encodes an oxygen-sensitive FNR-like protein that is not essential for symbiotic nitrogen fixation (4, 53). Information on target genes regulated by FixK1 had been missing altogether. In the work reported here, using microoxic and nitrate-respiring, anoxic B. japonicum cells, we have identified 17 genes as being under positive control by FixK1; however, only one gene, blr6062 (cycS), was strongly activated (see below). The most-stunning result was that, contrary to data for the FixJ and FixK2 regulons, the FixK1 regulon contained a substantial number of negatively controlled genes, most of which had been known from previous work to belong to the group of genes activated by the NifA protein (37). Hence, for the first time, our work has disclosed a peculiar regulatory interaction between the FixLJ-FixK2-FixK1 cascade and the RegSR-NifA cascade, in which FixK1, directly or indirectly, exerts an antagonistic effect on genes activated by NifA. Judged by the changes in the gene regulation measured in microarrays, this effect is generally more pronounced in microoxic cultures than in anoxic cultures. In accordance with the disparate oxygen responsiveness of FixLJ and NifA in vivo (63), we propose here that a decrease in the oxygen concentration to intermediate levels (as in microoxic culture) induces the FixLJ-FixK2 cascade but still keeps some NifA-dependent genes repressed via FixK1. When the oxygen concentration drops further, cells may build up more and more of the active NifA protein, which gradually overrides the transient repression by FixK1. How this attractive fine-tuning of NifA-dependent gene expression works mechanistically has yet to be elucidated. Curiously, a reverse type of cross-pathway control may exist in S. meliloti bacteroids, because the results of transcriptome profiling have shown that a set of FixK-dependent genes were upregulated in a ΔnifA mutant (10).
The cycS gene and its product.
Particular attention was paid to the regulation of cycS (blr6062) because this gene is located adjacent but in divergent orientation to the fixK1 gene (bll6061), with only a single FixK2 box in the middle of the intergenic region. Using semisynthetic CRP-dependent promoters, El-Robh and Busby (22) had shown that CRP bound to a single DNA site could activate transcription in divergent orientations in vitro and in vivo. We show here that this works similarly for a natural constellation. FixK2 was able to activate in vitro transcription from the same DNA template not only into the fixK1 gene but also into the opposite cycS gene. While the fixK1 gene was classified by transcriptome analysis as a member of the FixK2 regulon, the activation of cycS was puzzling insofar as this gene was originally found to be a specific FixK1 regulon member. The answer to this problem could be that FixK2 in vivo first activates both fixK1 and cycS, but it is the accumulating FixK1 protein which may additionally boost cycS gene expression. In fact, we demonstrated experimentally that a constitutively expressed fixK1 gene could partially restore cycS gene expression in a B. japonicum mutant background in which the fixJ and fixK2 genes had been knocked out. Such a dual control of one target gene by two homologous transcription factors of the CRP/FNR family is not without precedent. We had previously shown that the maximal activation in vivo of the B. japonicum nitrite reductase gene nirK required both the FixK2 and NnrR proteins in cells grown anoxically with nitrate (47). Therefore, a general caveat seems to be justified: even after the demonstration of direct transcription activation in vitro, such as by FixK2 in the case of cycS, the situation in vivo may be more complex in that additional transcription factors, especially homologous ones, potentially participate in the overall control of a given target gene.
The cycS gene was annotated as a cytochrome c6 gene (38). In fact, we show in this work that the CycS protein is a hitherto unrecognized soluble, low-molecular-mass c-type cytochrome. A total of four proteins of that class have now been identified in B. japonicum, the gene products of cycA, cycB, cycC, and cycS (11, 61, 66; this work). While none of them is essential for symbiotic nitrogen fixation, the CycA protein was shown to be involved in electron transfer to the copper-containing nitrite reductase (12), the periplasmic enzyme that catalyzes the reduction of nitrite to nitric oxide. Likewise, based on the delayed denitrification phenotype of a cycS knockout mutant, we suggest a role of the CycS protein in denitrification, although it is not essential for this process. The precise biochemical function in delivering electrons to one of the N-oxide intermediates is currently not known, and the question remains unanswered as to whether one of the three other soluble c-type cytochromes can at least partly replace CycS function.
Supplementary Material
Acknowledgments
We thank Andrea Patrignani, Hubert Rehrauer, Stefan Zoller, and Ralph Schlapbach from the Functional Genomics Center Zürich (FGCZ) for help and assistance in the microarray experiments. Gabriella Pessi is gratefully acknowledged for advice in the evaluation of transcriptomics data. The expert technical assistance of Olivera Volarevic-Vogel and Sarah Wilhelm is highly appreciated. We are grateful to Dulce-Nombre Rodríguez-Navarro and Francisco Temprano (Las Torres-Tomejil, Seville, Spain) for providing soybean seeds.
This work was supported by grants from the Swiss National Foundation for Scientific Research and the ETH through Research programs for the FGCZ.
Socorro Mesa dedicates this article to the memory of her father, Francisco Mesa.
Footnotes
Published ahead of print on 8 August 2008.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Alexeyev, M. F. 1995. Three kanamycin resistance gene cassettes with different polylinkers. BioTechniques 1852-56. [PubMed] [Google Scholar]
- 2.Allen, J. W., O. Daltrop, J. M. Stevens, and S. J. Ferguson. 2003. c-type cytochromes: diverse structures and biogenesis systems pose evolutionary problems. Philos. Trans. R. Soc. Lond. B 358255-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthamatten, D., and H. Hennecke. 1991. The regulatory status of the fixL- and fixJ-like genes in Bradyrhizobium japonicum may be different from that in Rhizobium meliloti. Mol. Gen. Genet. 22538-48. [DOI] [PubMed] [Google Scholar]
- 4.Anthamatten, D., B. Scherb, and H. Hennecke. 1992. Characterization of a fixLJ-regulated Bradyrhizobium japonicum gene sharing similarity with the Escherichia coli fnr and Rhizobium meliloti fixK genes. J. Bacteriol. 1742111-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babst, M., H. Hennecke, and H. M. Fischer. 1996. Two different mechanisms are involved in the heat-shock regulation of chaperonin gene expression in Bradyrhizobium japonicum. Mol. Microbiol. 19827-839. [DOI] [PubMed] [Google Scholar]
- 6.Barnett, M. J., and R. F. Fisher. 2006. Global gene expression in the rhizobial-legume symbiosis. Symbiosis 421-24. [Google Scholar]
- 7.Barnett, M. J., C. J. Toman, R. F. Fisher, and S. R. Long. 2004. A dual-genome symbiosis chip for coordinate study of signal exchange and development in a prokaryote-host interaction. Proc. Natl. Acad. Sci. USA 10116636-16641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beck, C., R. Marty, S. Kläusli, H. Hennecke, and M. Göttfert. 1997. Dissection of the transcription machinery for housekeeping genes of Bradyrhizobium japonicum. J. Bacteriol. 179364-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Becker, A., H. Berges, E. Krol, C. Bruand, S. Rüberg, D. Capela, E. Lauber, E. Meilhoc, F. Ampe, F. J. de Bruijn, J. Fourment, A. Francez-Charlot, D. Kahn, H. Kuster, C. Liebe, A. Pühler, S. Weidner, and J. Batut. 2004. Global changes in gene expression in Sinorhizobium meliloti 1021 under microoxic and symbiotic conditions. Mol. Plant-Microbe Interact. 17292-303. [DOI] [PubMed] [Google Scholar]
- 10.Bobik, C., E. Meilhoc, and J. Batut. 2006. FixJ: a major regulator of the oxygen limitation response and late symbiotic functions of Sinorhizobium meliloti. J. Bacteriol. 1884890-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bott, M., L. Thöny-Meyer, H. Loferer, S. Rossbach, R. E. Tully, D. Keister, C. A. Appleby, and H. Hennecke. 1995. Bradyrhizobium japonicum cytochrome c550 is required for nitrate respiration but not for symbiotic nitrogen fixation. J. Bacteriol. 1772214-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bueno, E., E. J. Bedmar, D. J. Richardson, and M. J. Delgado. 2008. Role of Bradyrhizobium japonicum cytochrome c550 in nitrite and nitrate respiration. FEMS Microbiol. Lett. 279188-194. [DOI] [PubMed] [Google Scholar]
- 13.Cha, J. S., and D. A. Cooksey. 1991. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 888915-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang, W. S., W. L. Franck, E. Cytryn, S. Jeong, T. Joshi, D. W. Emerich, M. J. Sadowsky, D. Xu, and G. Stacey. 2007. An oligonucleotide microarray resource for transcriptional profiling of Bradyrhizobium japonicum. Mol. Plant-Microbe Interact. 201298-1307. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan, S., and M. R. O'Brian. 1993. Bradyrhizobium japonicum delta-aminolevulinic acid dehydratase is essential for symbiosis with soybean and contains a novel metal-binding domain. J. Bacteriol. 1757222-7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crooks, G. E., G. Hon, J. M. Chandonia, and S. E. Brenner. 2004. WebLogo: a sequence logo generator. Genome Res. 141188-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Daniel, R. M., and C. A. Appleby. 1972. Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum: effects on cytochrome P450, other haemoproteins, nitrate and nitrite reductases. Biochim. Biophys. Acta 275347-354. [DOI] [PubMed] [Google Scholar]
- 18.Delgado, M. J., N. Bonnard, A. Tresierra-Ayala, E. J. Bedmar, and P. Müller. 2003. The Bradyrhizobium japonicum napEDABC genes encoding the periplasmic nitrate reductase are essential for nitrate respiration. Microbiology 1493395-3403. [DOI] [PubMed] [Google Scholar]
- 19.Dixon, R., and D. Kahn. 2004. Genetic regulation of biological nitrogen fixation. Nat. Rev. Microbiol. 2621-631. [DOI] [PubMed] [Google Scholar]
- 20.Dunn, M. F. 1998. Tricarboxylic acid cycle and anaplerotic enzymes in rhizobia. FEMS Microbiol. Rev. 22105-123. [DOI] [PubMed] [Google Scholar]
- 21.Durmowicz, M. C., and R. J. Maier. 1998. The FixK2 protein is involved in regulation of symbiotic hydrogenase expression in Bradyrhizobium japonicum. J. Bacteriol. 1803253-3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Robh, M. S., and S. J. Busby. 2002. The Escherichia coli cAMP receptor protein bound at a single target can activate transcription initiation at divergent promoters: a systematic study that exploits new promoter probe plasmids. Biochem. J. 368835-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrieres, L., and D. Kahn. 2002. Two distinct classes of FixJ binding sites defined by in vitro selection. FEBS Lett. 517185-189. [DOI] [PubMed] [Google Scholar]
- 24.Fischer, H. M. 1996. Environmental regulation of rhizobial symbiotic nitrogen fixation genes. Trends Microbiol. 4317-320. [DOI] [PubMed] [Google Scholar]
- 25.Fischer, H. M. 1994. Genetic regulation of nitrogen fixation in rhizobia. Microbiol. Rev. 58352-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischer, H. M., M. Babst, T. Kaspar, G. Acuña, F. Arigoni, and H. Hennecke. 1993. One member of a groESL-like chaperonin multigene family in Bradyrhizobium japonicum is co-regulated with symbiotic nitrogen fixation genes. EMBO J. 122901-2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fischer, H. M., L. Velasco, M. J. Delgado, E. J. Bedmar, S. Schären, D. Zingg, M. Göttfert, and H. Hennecke. 2001. One of two hemN genes in Bradyrhizobium japonicum is functional during anaerobic growth and in symbiosis. J. Bacteriol. 1831300-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friberg, M. 2007. Algorithms for analyzing signals in DNA: applications to transcription and translation. Ph.D. thesis no. 17096. ETH, Zürich, Switzerland.
- 29.Friberg, M. T. 2007. Prediction of transcription factor binding sites using ChIP-chip and phylogenetic footprinting data. J. Bioinform. Comput. Biol. 5105-116. [DOI] [PubMed] [Google Scholar]
- 30.Galibert, F., T. M. Finan, S. R. Long, A. Pühler, P. Abola, F. Ampe, F. Barloy-Hubler, M. J. Barnett, A. Becker, P. Boistard, G. Bothe, M. Boutry, L. Bowser, J. Buhrmester, E. Cadieu, D. Capela, P. Chain, A. Cowie, R. W. Davis, S. Dreano, N. A. Federspiel, R. F. Fisher, S. Gloux, T. Godrie, A. Goffeau, B. Golding, J. Gouzy, M. Gurjal, I. Hernandez-Lucas, A. Hong, L. Huizar, R. W. Hyman, T. Jones, D. Kahn, M. L. Kahn, S. Kalman, D. H. Keating, E. Kiss, C. Komp, V. Lelaure, D. Masuy, C. Palm, M. C. Peck, T. M. Pohl, D. Portetelle, B. Purnelle, U. Ramsperger, R. Surzycki, P. Thebault, M. Vandenbol, F. J. Vorholter, S. Weidner, D. H. Wells, K. Wong, K. C. Yeh, and J. Batut. 2001. The composite genome of the legume symbiont Sinorhizobium meliloti. Science 293668-672. [DOI] [PubMed] [Google Scholar]
- 31.Gilles-Gonzalez, M. A., G. S. Ditta, and D. R. Helinski. 1991. A haemoprotein with kinase activity encoded by the oxygen sensor of Rhizobium meliloti. Nature 350170-172. [DOI] [PubMed] [Google Scholar]
- 32.Giraud, E., L. Moulin, D. Vallenet, V. Barbe, E. Cytryn, J. C. Avarre, M. Jaubert, D. Simon, F. Cartieaux, Y. Prin, G. Bena, L. Hannibal, J. Fardoux, M. Kojadinovic, L. Vuillet, A. Lajus, S. Cruveiller, Z. Rouy, S. Mangenot, B. Segurens, C. Dossat, W. L. Franck, W. S. Chang, E. Saunders, D. Bruce, P. Richardson, P. Normand, B. Dreyfus, D. Pignol, G. Stacey, D. Emerich, A. Vermeglio, C. Medigue, and M. Sadowsky. 2007. Legumes symbioses: absence of nod genes in photosynthetic bradyrhizobia. Science 3161307-1312. [DOI] [PubMed] [Google Scholar]
- 33.Gong, W., B. Hao, S. S. Mansy, G. Gonzalez, M. A. Gilles-Gonzalez, and M. K. Chan. 1998. Structure of a biological oxygen sensor: a new mechanism for heme-driven signal transduction. Proc. Natl. Acad. Sci. USA 9515177-15182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Göttfert, M., S. Hitz, and H. Hennecke. 1990. Identification of nodS and nodU, two inducible genes inserted between the Bradyrhizobium japonicum nodYABC and nodIJ genes. Mol. Plant-Microbe Interact. 3308-316. [DOI] [PubMed] [Google Scholar]
- 35.Green, L. S., Y. Li, D. W. Emerich, F. J. Bergersen, and D. A. Day. 2000. Catabolism of α-ketoglutarate by a sucA mutant of Bradyrhizobium japonicum: evidence for an alternative tricarboxylic acid cycle. J. Bacteriol. 1822838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hahn, M., L. Meyer, D. Studer, B. Regensburger, and H. Hennecke. 1984. Insertion and deletion mutation within the nif region of Rhizobium japonicum. Plant Mol. Biol. 3159-168. [DOI] [PubMed] [Google Scholar]
- 37.Hauser, F., G. Pessi, M. Friberg, C. Weber, N. Rusca, A. Lindemann, H. M. Fischer, and H. Hennecke. 2007. Dissection of the Bradyrhizobium japonicum NifA+σ54 regulon, and identification of a ferredoxin gene (fdxN) for symbiotic nitrogen fixation. Mol. Genet. Genomics 278255-271. [DOI] [PubMed] [Google Scholar]
- 38.Kaneko, T., Y. Nakamura, S. Sato, K. Minamisawa, T. Uchiumi, S. Sasamoto, A. Watanabe, K. Idesawa, M. Iriguchi, K. Kawashima, M. Kohara, M. Matsumoto, S. Shimpo, H. Tsuruoka, T. Wada, M. Yamada, and S. Tabata. 2002. Complete genomic sequence of nitrogen-fixing symbiotic bacterium Bradyrhizobium japonicum USDA110. DNA Res. 9189-197. [DOI] [PubMed] [Google Scholar]
- 39.Kiley, P. J., and H. Beinert. 2003. The role of Fe-S proteins in sensing and regulation in bacteria. Curr. Opin. Microbiol. 6181-185. [DOI] [PubMed] [Google Scholar]
- 40.Körner, H., H. J. Sofia, and W. G. Zumft. 2003. Phylogeny of the bacterial superfamily of Crp-Fnr transcription regulators: exploiting the metabolic spectrum by controlling alternative gene programs. FEMS Microbiol. Rev. 27559-592. [DOI] [PubMed] [Google Scholar]
- 41.Kurashima-Ito, K., Y. Kasai, K. Hosono, K. Tamura, S. Oue, M. Isogai, Y. Ito, H. Nakamura, and Y. Shiro. 2005. Solution structure of the C-terminal transcriptional activator domain of FixJ from Sinorhizobium meliloti and its recognition of the fixK promoter. Biochemistry 4414835-14844. [DOI] [PubMed] [Google Scholar]
- 42.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 43.Larimer, F. W., P. Chain, L. Hauser, J. Lamerdin, S. Malfatti, L. Do, M. L. Land, D. A. Pelletier, J. T. Beatty, A. S. Lang, F. R. Tabita, J. L. Gibson, T. E. Hanson, C. Bobst, J. L. Torres, C. Peres, F. H. Harrison, J. Gibson, and C. S. Harwood. 2004. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat. Biotechnol. 2255-61. [DOI] [PubMed] [Google Scholar]
- 44.Lazazzera, B. A., D. M. Bates, and P. J. Kiley. 1993. The activity of the Escherichia coli transcription factor FNR is regulated by a change in oligomeric state. Genes Dev. 71993-2005. [DOI] [PubMed] [Google Scholar]
- 45.Lazazzera, B. A., H. Beinert, N. Khoroshilova, M. C. Kennedy, and P. J. Kiley. 1996. DNA binding and dimerization of the Fe-S-containing FNR protein from Escherichia coli are regulated by oxygen. J. Biol. Chem. 2712762-2768. [DOI] [PubMed] [Google Scholar]
- 46.Lindemann, A., A. Moser, G. Pessi, F. Hauser, M. Friberg, H. Hennecke, and H. M. Fischer. 2007. New target genes controlled by the Bradyrhizobium japonicum two-component regulatory system RegSR. J. Bacteriol. 1898928-8943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mesa, S., E. J. Bedmar, A. Chanfon, H. Hennecke, and H. M. Fischer. 2003. Bradyrhizobium japonicum NnrR, a denitrification regulator, expands the FixLJ-FixK2 regulatory cascade. J. Bacteriol. 1853978-3982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mesa, S., H. Hennecke, and H. M. Fischer. 2006. A multitude of CRP/FNR-like transcription proteins in Bradyrhizobium japonicum. Biochem. Soc. Trans. 34156-159. [DOI] [PubMed] [Google Scholar]
- 49.Mesa, S., Z. Ucurum, H. Hennecke, and H. M. Fischer. 2005. Transcription activation in vitro by the Bradyrhizobium japonicum regulatory protein FixK2. J. Bacteriol. 1873329-3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mesa, S., L. Velasco, M. E. Manzanera, M. J. Delgado, and E. J. Bedmar. 2002. Characterization of the norCBQD genes, encoding nitric oxide reductase, in the nitrogen fixing bacterium Bradyrhizobium japonicum. Microbiology 1483553-3560. [DOI] [PubMed] [Google Scholar]
- 51.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 52.Mwangi, M. M., and E. D. Siggia. 2003. Genome-wide identification of regulatory motifs in Bacillus subtilis. BMC Bioinformatics 418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nellen-Anthamatten, D., P. Rossi, O. Preisig, I. Kullik, M. Babst, H. M. Fischer, and H. Hennecke. 1998. Bradyrhizobium japonicum FixK2, a crucial distributor in the FixLJ-dependent regulatory cascade for control of genes inducible by low oxygen levels. J. Bacteriol. 1805251-5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nienaber, A., H. Hennecke, and H. M. Fischer. 2001. Discovery of a haem uptake system in the soil bacterium Bradyrhizobium japonicum. Mol. Microbiol. 41787-800. [DOI] [PubMed] [Google Scholar]
- 55.Page, K. M., and M. L. Guerinot. 1995. Oxygen control of the Bradyrhizobium japonicum hemA gene. J. Bacteriol. 1773979-3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pessi, G., C. H. Ahrens, H. Rehrauer, A. Lindemann, F. Hauser, H. M. Fischer, and H. Hennecke. 2007. Genome-wide transcript analysis of Bradyrhizobium japonicum bacteroids in soybean root nodules. Mol. Plant-Microbe Interact. 201353-1363. [DOI] [PubMed] [Google Scholar]
- 57.Preisig, O., D. Anthamatten, and H. Hennecke. 1993. Genes for a microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc. Natl. Acad. Sci. USA 903309-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Preisig, O., R. Zufferey, and H. Hennecke. 1996. The Bradyrhizobium japonicum fixGHIS genes are required for the formation of the high-affinity cbb3-type cytochrome oxidase. Arch. Microbiol. 165297-305. [DOI] [PubMed] [Google Scholar]
- 59.Regensburger, B., and H. Hennecke. 1983. RNA polymerase from Rhizobium japonicum. Arch. Microbiol. 135103-109. [DOI] [PubMed] [Google Scholar]
- 60.Robles, E. F., C. Sanchez, N. Bonnard, M. J. Delgado, and E. J. Bedmar. 2006. The Bradyrhizobium japonicum napEDABC genes are controlled by the FixLJ-FixK2-NnrR regulatory cascade. Biochem. Soc. Trans. 34108-110. [DOI] [PubMed] [Google Scholar]
- 61.Rossbach, S., H. Loferer, G. Acuña, C. A. Appleby, and H. Hennecke. 1991. Cloning, sequencing and mutational analysis of the cytochrome c552 gene (cycB) from Bradyrhizobium japonicum strain 110. FEMS Microbiol. Lett. 67145-152. [DOI] [PubMed] [Google Scholar]
- 62.Sarma, A. D., and D. W. Emerich. 2005. Global protein expression pattern of Bradyrhizobium japonicum bacteroids: a prelude to functional proteomics. Proteomics 54170-4184. [DOI] [PubMed] [Google Scholar]
- 63.Sciotti, M. A., A. Chanfon, H. Hennecke, and H. M. Fischer. 2003. Disparate oxygen responsiveness of two regulatory cascades that control expression of symbiotic genes in Bradyrhizobium japonicum. J. Bacteriol. 1855639-5642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Simon, R., U. Priefer, and A. Pühler. 1983. Vector plasmids for in vivo and in vitro manipulation of gram-negative bacteria, p. 96-108. In A. Pühler (ed.), Molecular genetics of the bacteria-plant interaction. Springer Verlag, Heidelberg, Germany.
- 65.Trainer, M. A., and T. C. Charles. 2006. The role of PHB metabolism in the symbiosis of rhizobia with legumes. Appl. Microbiol. Biotechnol. 71377-386. [DOI] [PubMed] [Google Scholar]
- 66.Tully, R. E., M. J. Sadowsky, and D. L. Keister. 1991. Characterization of cytochromes c550 and c555 from Bradyrhizobium japonicum: cloning, mutagenesis, and sequencing of the c555 gene (cycC). J. Bacteriol. 1737887-7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Velasco, L., S. Mesa, M. J. Delgado, and E. J. Bedmar. 2001. Characterization of the nirK gene encoding the respiratory, Cu-containing nitrite reductase of Bradyrhizobium japonicum. Biochim. Biophys. Acta 1521130-134. [DOI] [PubMed] [Google Scholar]
- 68.Velasco, L., S. Mesa, C. A. Xu, M. J. Delgado, and E. J. Bedmar. 2004. Molecular characterization of nosRZDFYLX genes coding for denitrifying nitrous oxide reductase of Bradyrhizobium japonicum. Antonie van Leeuwenhoek 85229-235. [DOI] [PubMed] [Google Scholar]
- 69.Zufferey, R., O. Preisig, H. Hennecke, and L. Thöny-Meyer. 1996. Assembly and function of the cytochrome cbb3 oxidase subunits in Bradyrhizobium japonicum. J. Biol. Chem. 2719114-9119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.