Abstract
Coronaviruses are positive-strand RNA viruses of extraordinary genetic complexity and diversity. In addition to a common set of genes for replicase and structural proteins, each coronavirus may carry multiple group-specific genes apparently acquired through relatively recent heterologous recombination events. Here we describe an accessory gene, ORF3, unique to canine coronavirus type I (CCoV-I) and characterize its product, glycoprotein gp3. Whereas ORF3 is conserved in CCoV-I, only remnants remain in CCoV-II and CCoV-II-derived porcine and feline coronaviruses. Our findings provide insight into the evolutionary history of coronavirus group 1a and into the dynamics of gain and loss of accessory genes.
Coronaviruses (CoVs), enveloped positive-strand RNA viruses of human clinical and veterinary relevance, are exceptional in terms of genetic complexity and variety. With genome sizes of ∼30 kb, they are the largest RNA viruses known thus far (17, 42). One major contributing factor to CoV diversity is high-frequency RNA recombination (1, 28, 33). New sero- and biotypes have arisen from homologous RNA recombination, i.e., the exchange of corresponding sequences among related CoVs (3, 21, 25, 27, 29, 43), while heterologous RNA recombination events with noncoronaviral donor RNAs have led to the acquisition of novel genes (31, 44, 56).
All CoVs have a similar genome organization with a common set of five genes arranged in a conserved order (10, 12). The polymerase gene, occupying the 5′-most 70% of the genome, encodes the replicase polyproteins from which up to 16 mature products are derived as well as an unknown number of functional processing intermediates (58). Downstream of the polymerase gene and expressed through a 3′-coterminal nested set of subgenomic (sg) mRNAs are the genes for the structural proteins S, E, M, and N (12). In addition, each CoV may possess up to seven group-specific “accessory” genes that are also expressed from sg mRNAs (34). In most cases, the functions of the accessory gene products are not known, and in general, they are not essential for replication in cultured cells (6, 36, 41, 53-55). Quite the opposite, their expression might decrease viral fitness in vitro, and mutants with inactivated accessory genes readily become selected during serial passage (22, 30, 45, 51). In field strains, however, accessory genes as a rule are maintained (13, 22, 43), and their loss—either through spontaneous mutation (37) or by design via reversed genetics—generally causes loss of virulence in the natural host (11, 20, 36).
CoVs can be divided into three main phylogenetic groups (16). Canine coronaviruses (CCoVs), common enteric pathogens of dogs (8, 47), belong to subgroup 1a together with feline coronaviruses types I and II (FCoV-I and FCoV-II, respectively) and transmissible gastroenteritis virus (TGEV) of swine (16, 18). Like FCoV, CCoVs occur in two serotypes (39), CCoV types I and II (CCoV-I and CCoV-II, respectively) sharing ∼90% sequence identity in most of their genome (A. Lorusso, N. Decaro, C. Buonavoglia, and R. J. de Groot, unpublished data). In the coding region for the S ectodomain, however, sequence identity is only 56%.
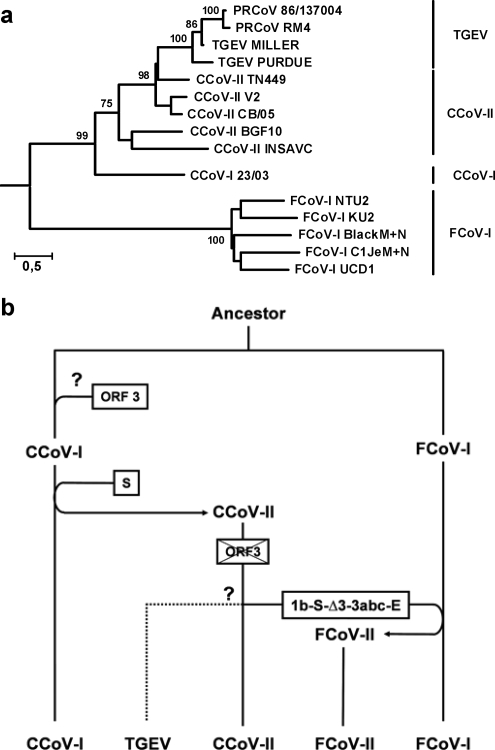
The evolutionary history of CoV group 1a is not completely understood, but it apparently entailed multiple homologous and heterologous recombination events. The available data suggest that CCoV-I and FCoV-I arose by linear descent from a common ancestor and that recombination of CCoV-I with an unknown CoV led to acquisition of a new S gene, thus giving rise to CCoV-II (Lorusso et al., unpublished). In turn, CCoV-II strains donated this S gene and flanking sequences in recombinational exchanges to FCoV-I strains, leading to the independent emergence of FCoV-II strains (21). TGEV also appears to be of CCoV-II origin; in phylogenetic analyses of the genomic region downstream of the S gene, TGEV consistently clusters with extant CCoV-II field strains (7).
To date, the FCoVs and CCoVs described share the same complement of accessory genes, three of which (the “ORF3abc cluster”) are located between the S and E genes (10, 14, 24, 46, 50, 52). The two remaining ones, ORF7a and ORF7b, are located at the 3′ end of the genome (9, 24, 50) (Fig. 1a). In TGEV and related porcine CoVs, ORF3b is inactivated and ORF7b is lacking. Here we describe a novel functional accessory gene unique to CCoV-I and discuss the implications of our findings for our understanding of the evolutionary history of group 1a CoVs.
FIG. 1.
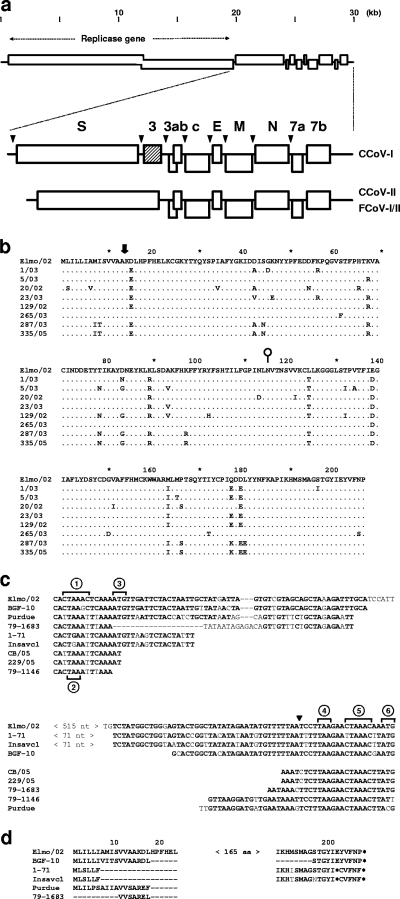
Comparative sequence analysis of CCoV-1 ORF3 and its product. (a) Schematic representation of the CCoV-I genome organization in comparison to those of CCoV-II, FCoV-I, and FCoV-II. Open boxes indicate the genes for the replicase polyproteins, structural proteins (S, E, M, and N) and accessory proteins (ORF3abc, ORF7a, and ORF7b proteins). CCoV-I ORF3 is indicated by a hatched box. (b) Conservation of ORF3 among CCoV-I field variants. An alignment is shown of ORF3 protein sequences from eight CCoV-I isolates. A conserved potential signalase cleavage site between A14 and K15 (http://www.cbs.dtu.dk/services/NetNGlyc/) is indicated by a thick black arrow; a potential N-glycosylation site at N116 is indicated by a lollipop. The corresponding ORF3 nucleotide sequences were deposited in GenBank (accession numbers AY528745 through AY528751, AY426983, and AY426984). (c) The IGRs between the S and ORF3a genes in CCoV-II and derivative viruses contain ORF3 remnants. Shown is a nucleotide sequence alignment of the 5′ and 3′ ends of Elmo/02 open reading frame and flanking IGRs to the S-ORF3a IGRs of CCoV-II strains BGF-10 (GenBank accession number AY342160), 1-71 (EF056487), Insavc1 (D13096), CB/05 (DQ112226), and 229/05 (unpublished data), TGEV strain Purdue (DQ811789), and FCoV-II strains 79-1683 (Y13921) and 79-1146 (AY994055). Of the Insavc1 and 1-71 IGRs, an internal region of 71 nt could not be aligned with certainty and was omitted from the comparison. Sequences are indicated by circled numbers as follows: 1, ORF3 TRS; 2, termination codon of the S gene; 3, ORF3 initiation codon; 4, ORF3 termination codon; 5, ORF3a TRS; 6, ORF3a initiation codon. Note that in CCoV-II strains 1-71, Insavc1, and BGF10, there is conservation both of the 5′ and 3′ ends of ORF3 and of the IGRs. In CCoV-II strains CB/05 and 229/05 as well as in the FCoV-II strains and TGEV, sequence conservation at the 3′ end is limited to the ORF3/3a IGR and the 3′-most 5 nucleotides of ORF3 (black arrowhead). Further upstream, the latter viruses can be readily aligned with each other, but not with ORF3. (d) Amino acid sequence alignment of the N and C termini of the Elmo/02 ORF3 protein with translated IGR sequences of CCoV-II strains BGF-10, 1-71, and Insavc1, TGEV strain Purdue, and FCoV-II strain 79-1683. Numbers indicate amino acid positions in the CCoV-I ORF3 protein; termination codons are indicated by asterisks. aa, amino acids.
ORF3, a functional accessory gene unique to CCoV-I.
During sequence analysis of CCoV-I variant Elmo/02 (39), we discovered, immediately downstream of the S gene, a 624-nucleotide (nt) open reading frame (ORF3) that is absent in all other group 1a CoVs studied so far. ORF3 is preceded by a canonical group 1a transcription-regulating sequence (TRS) (9, 24, 26, 48), which suggests that in infected cells it is expressed through a separate dedicated sg mRNA species. The encoded protein, 207 residues in length with a predicted N-terminal signal peptide and a single potential N-glycosylation site at Asn116, bears no significant sequence identity to any viral or cellular protein in the NCBI database (Fig. 1a and b).
To study whether ORF3 is conserved among CCoV-I strains, viral RNA was extracted from fecal samples taken from eight dogs with natural CCoV-I infection, and reverse transcription-PCR was performed with primers designed after regions in the S gene and ORF3a. Comparative sequence analysis of the resulting amplicons showed that ORF3 was present and intact in every CCoV-I strain tested, with nucleotide and amino acid sequence identities of 91.7 to 99.7% and of 90 to 100%, respectively (Fig. 1b). Apparently, under field conditions, CCoV-I strains maintain ORF3. Most nucleotide changes are synonymous; the ratio of the rate of nonsynonymous substitutions to the rate of synonymous substitutions (5) ranges between 0 and 0.25, which is indicative of purifying selection. We interpret the combined observations to suggest that the ORF3 product is functional during natural CCoV-I infection and that amino acid changes that interfere with its function are selected against.
CCoV-I ORF3 encodes a glycoprotein.
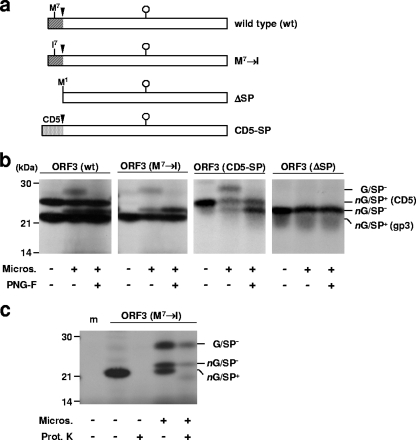
CCoV-I cannot be propagated in tissue culture (38), impeding analysis of viral mRNAs and proteins in the context of the infected cell. To study the biochemical properties of the CCoV-I ORF3 product experimentally, we performed in vitro translation in the TNT coupled rabbit reticulocyte lysates in the presence and absence of dog pancreas microsomes (Promega). The assays were carried out with a series of pTUG-31-based expression plasmids containing ORF3 of CCoV Elmo/02 and derivatives thereof, cloned downstream of the T7 RNA polymerase promoter (Fig. 2a). Translation of the wild-type gene in the absence of microsomes yielded not one but two products with molecular weights of 22,000 (22K) and 25K [Fig. 2b, panel ORF3 (wt), leftmost lane]. Scrutiny of the Elmo/02 ORF3 sequence revealed an AUG at codon position seven (Fig. 2a). Arguing that internal initiation of translation at this site might give rise to an additional, smaller product, we replaced Met7 by Ile (note that in many naturally occurring CCoV-I strains, Ile is found at this position [Fig. 1b]). Upon expression of this mutant in the absence of microsomes, only a single protein species was found. Surprisingly, however, it was not the 25K product but the faster migrating 22K product [Fig. 2b, panel ORF3 (M7→I), leftmost lane]. Apparently, the intact signal peptide of the ORF3 protein causes aberrant migration in sodium dodecyl sulfate (SDS)-polyacrylamide gels. Indeed, expression of a mutant with the ORF3 signal peptide replaced by that of CD5 yielded a single protein species of 25K [Fig. 2b, panel ORF3 (CD5-SP), leftmost lane], in accordance with its calculated molecular mass (25.7 kDa).
FIG. 2.
ORF3 encodes a glycoprotein with a cleavable N-terminal signal sequence. (a) Linear representation of the wild-type ORF3 protein and derivatives expressed from pTUG-31-based expression plasmids. The signal sequences of the ORF3 product and of CD5 are indicated by shading. Signalase cleavage sites (black arrowheads) and potential N-glycosylation sites (white lollipops) are indicated. (b) In vitro translation of wild-type (wt) ORF3 and derivatives. Translations were performed either in the absence (−) or presence (+) of dog pancreas microsomes (Micros.). Prior to SDS-polyacrylamide gel electrophoresis (PAGE) analysis, translation products were treated with PNGase F (PNG-F) (+) or left untreated (−). The positions and masses (in kilodaltons) of proteins from the molecular size markers are shown at the left. The positions of the various products are shown at the right as follows: G/SP−, glycosylated product without signal peptide; nG/SP+ (CD5), nonglycosylated product with CD5 signal peptide; nG/SP−, nonglycosylated product without signal peptide; nG/SP+ (gp3), nonglycosylated product with gp3 signal peptide. (c) Proteinase K protection assay. ORF3 derivative M7→I was translated either in the absence (−) or presence (+) of microsomes (Micros.) and treated with proteinase K (Prot. K) (+) or mock treated (−) prior to SDS-PAGE analysis. The positions of the various products are shown on the right as described above for panel b; the positions of molecular size standards and their masses (in kilodaltons) are shown on the left. A sample from a translation reaction supplemented with water instead of expression plasmid was included as a negative control (m).
Translation of wild-type ORF3 and derivatives in the presence of microsomes consistently yielded two additional products of 28K and 23K [Fig. 2b, panels ORF3 (wt), ORF3 (M7→I), and ORF3 (CD5-SP), middle lanes) that were fully protected from digestion by proteinase K (Fig. 2c) and hence appeared to be contained within the microsomal lumen. The 28K product is N glycosylated; treatment of translation products with endoglycosidase PNGase (Promega) resulted in loss of this protein species with a concomitant increase in the amount of the 23K product; the latter comigrated with mutant ΔSP that lacks a signal peptide [Fig. 2b, panel ORF3 (ΔSP)]. The combined findings conclusively show that CCoV-I ORF3 codes for a 28K glycoprotein (gp3) with a cleavable N-terminal signal sequence; hydrophobicity plots did not reveal additional transmembrane regions (data not shown), indicating that gp3 is either a secretory or peripheral membrane protein.
ORF3 remnants in CCoV-II and in CCoV-II-related viruses.
Comparative sequence analyses suggest that the horizontal gene transfer that resulted in the CCoV-I/II split-up was restricted to the coding sequences for the signal peptide and ectodomain of S and should have left ORF3 intact (not shown). Under the assumption that CCoV-I represents the parental biotype and CCoV-II its recombinant offspring, the latter must have lost ORF3 subsequently. Indeed, close inspection of the intergenic regions (IGRs) separating the S and ORF3a genes in CCoV-II variants (4, 7, 24, 32, 40), in FCoV-II strains 79-1146 (NCBI accession number AY994055) and 79-1683 (NCBI accession number Y13921) (the S gene and downstream sequences are of CCoV-II origin in this strain; 21), and in TGEV (57) revealed remnants of ORF3 and/or its preceding IGR (Fig. 1c and d). In FCoV- I strains C1Je and Black (14, 46), however, there is no trace of ORF3, and the S gene and ORF3a are separated by a very short 11-nt IGR that except for the TRS bears little similarity to the IGRs preceding ORF3 or ORF3a in CCoV. Presumably, ORF3 was acquired after type I FCoV and CCoV diverged, although the possibility that ORF3 was already present in their common ancestor and then lost completely in the FCoV lineage cannot be excluded (Fig. 3). In any case, our findings suggest that while gp3 is important during CCoV-I infection, it became obsolete in CCoV-II.
FIG. 3.
Hypothetical scenario for the evolution of CoV cluster 1a. (a) Rooted neighbor-joining tree inferred from multiple amino acid sequence alignments of the M and N proteins, illustrating the evolutionary relationships between members of phylogroup 1a. Human CoV 229e served as an outgroup. Support from bifurcations from 100 bootstraps is indicated. PRCoV, porcine respiratory CoV. (b) CCoV-I and FCoV-I apparently arose from a common ancestor by linear descent. As these viruses diverged, several distinct RNA recombination events led to the emergence of CCoV-II, FCoV-II, and TGEV. Details are explained in the text. Question marks indicate steps that are not yet completely understood.
Is gp3 advantageous only in combination with CCoV-I S protein?
The function of gp3 is not known, but based upon its biochemical properties, it may act either in the infected cell within the compartments of the exocytotic route or in the extracellular milieu. Given that apart from ORF3 the main difference between CCoV-I and CCoV-II strains lies in the type of S proteins they carry, it would seem that the function of gp3 is in some way connected to the function of S, i.e., gp3 apparently provides an advantage only in combination with a type I spike protein. Conceivably, gp3 may be involved in the biogenesis of CCoV-I S or required for S-mediated attachment or fusion during entry. There is the alternative possibility, however, that gp3 is advantageous to the virus only in certain types of host cells or tissues correlating with the cell tropism conferred by S. Accumulating evidence suggests that FCoV-I and -II, and hence by extension CCoV-I and -II, recognize different receptors, which may well translate to a difference in host cell preference (2, 15, 23).
ORF3 would not be the sole example of an accessory gene lost after a tropism change. ORF3c is conserved among group 1 CoVs, yet it is inactivated in FCoV variants that cause feline infectious peritonitis; loss of expression seemingly correlates with a shift from enteric to systemic infection and in host cell tropism from enterocytes to monocytes (49). In severe acute respiratory syndrome CoV, loss of nsP8 may have been the consequence of cross-species transmission and adaptation to the human host (19, 35). In the case of TGEV, adaptation of CCoV-II to swine apparently was accompanied by inactivation of ORF3b and loss of ORF7b. Clearly, further studies of CoV accessory proteins are warranted, as these studies will not only broaden our understanding of coronavirus host adaptation and speciation but may also open new avenues to antiviral intervention.
Acknowledgments
We are grateful to Martijn A. Langereis and Arno L. W. van Vliet for advice and to Jolanda de Groot-Mijnes for critically reading the manuscript.
This work was supported by grants from the Italian Ministry of University: PRIN 2005 (N.D.), project “Il coronavirus del cane: aspetti molecolari e patogenetici”; and PRIN 2006 (C.B.), project “Infezione del cane da coronavirus pantropico: aspetti epidemiologici, patogenetici e molecolari.”
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Banner, L. R., and M. M. Lai. 1991. Random nature of coronavirus RNA recombination in the absence of selection pressure. Virology 185441-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benbacer, L., E. Kut, L. Besnardeau, H. Laude, and B. Delmas. 1997. Interspecies aminopeptidase-N chimeras reveal species-specific receptor recognition by canine coronavirus, feline infectious peritonitis virus, and transmissible gastroenteritis virus. J. Virol. 71734-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brian, D. A., and W. Spaan. 1997. Recombination and coronavirus defective interfering RNAs. Semin. Virol. 8101-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buonavoglia, C., N. Decaro, V. Martella, G. Elia, M. Campolo, C. Desario, M. Castagnaro, and M. Tempesta. 2006. Canine coronavirus highly pathogenic for dogs. Emerg. Infect. Dis. 12492-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Comeron, J. M. 1995. A method for estimating the numbers of synonymous and nonsynonymous substitutions per site. J. Mol. Evol. 411152-1159. [DOI] [PubMed] [Google Scholar]
- 6.Curtis, K. M., B. Yount, and R. S. Baric. 2002. Heterologous gene expression from transmissible gastroenteritis virus replicon particles. J. Virol. 761422-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decaro, N., V. Martella, G. Elia, M. Campolo, C. Desario, F. Cirone, M. Tempesta, and C. Buonavoglia. 2007. Molecular characterisation of the virulent canine coronavirus CB/05 strain. Virus Res. 12554-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decaro, N., V. Martella, D. Ricci, G. Elia, C. Desario, M. Campolo, N. Cavaliere, L. Di Trani, M. Tempesta, and C. Buonavoglia. 2005. Genotype-specific fluorogenic RT-PCR assays for the detection and quantitation of canine coronavirus type I and type II RNA in faecal samples of dogs. J. Virol. Methods 13072-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Groot, R. J., A. C. Andeweg, M. C. Horzinek, and W. J. M. Spaan. 1988. Sequence analysis of the 3′ end of the feline coronavirus FIPV 79-1146 genome: comparison with the genome of porcine coronavirus TGEV reveals large insertions. Virology 167370-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Groot, R. J., R. J. ter Haar, M. C. Horzinek, and B. A. van der Zeijst. 1987. Intracellular RNAs of the feline infectious peritonitis coronavirus strain 79-1146. J. Gen. Virol. 68995-1002. [DOI] [PubMed] [Google Scholar]
- 11.de Haan, C. A. M., P. S. Masters, X. Shen, S. Weiss, and P. J. M. Rottier. 2002. The group-specific murine coronavirus genes are not essential, but their deletion, by reverse genetics, is attenuating in the natural host. Virology 296177-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Vries, A. A. F., M. C. Horzinek, P. J. M. Rottier, and R. J. de Groot. 1997. The genome organization of the Nidovirales: similarities and differences between arteri-, toro-, and coronaviruses. Semin. Virol. 833-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dijkman, R., M. F. Jebbink, B. Wilbrink, K. Pyrc, H. L. Zaaijer, P. D. Minor, S. Franklin, B. Berkhout, V. Thiel, and L. van der Hoek. 2006. Human coronavirus 229E encodes a single ORF4 protein between the spike and the envelope genes. Virol. J. 3106-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dye, C., and S. G. Siddell. 2007. Genomic RNA sequence of feline coronavirus strain FCoV C1Je. J. Feline Med. Surg. 9202-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dye, C., N. Temperton, and S. G. Siddell. 2007. Type I feline coronavirus spike glycoprotein fails to recognize aminopeptidase N as a functional receptor on feline cell lines. J. Gen. Virol. 881753-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorbalenya, A. E. 2008. Genomics and evolution of the Nidovirales, p. 15-28. In S. Perlman, T. Gallagher, and E. J. Snijder (ed.), Nidoviruses. ASM Press, Washington, DC.
- 17.Gorbalenya, A. E., L. Enjuanes, J. Ziebuhr, and E. J. Snijder. 2006. Nidovirales: evolving the largest RNA virus genome. Virus Res. 11717-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gorbalenya, A. E., E. J. Snijder, and W. J. Spaan. 2004. Severe acute respiratory syndrome coronavirus phylogeny: toward consensus. J. Virol. 787863-7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Zhang, Y. J. Guan, K. M. Butt, K. L. Wong, K. W. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302276-278. [DOI] [PubMed] [Google Scholar]
- 20.Haijema, B. J., H. Volders, and P. J. Rottier. 2004. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis. J. Virol. 783863-3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrewegh, A. A., I. Smeenk, M. C. Horzinek, P. J. Rottier, and R. J. de Groot. 1998. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 724508-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herrewegh, A. A., H. Vennema, M. C. Horzinek, P. J. Rottier, and R. J. de Groot. 1995. The molecular genetics of feline coronaviruses: comparative sequence analysis of the ORF7a/7b transcription unit of different biotypes. Virology 212622-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hohdatsu, T., Y. Izumiya, Y. Yokoyama, K. Kida, and H. Koyama. 1998. Differences in virus receptor for type I and type II feline infectious peritonitis virus. Arch. Virol. 143839-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horsburgh, B. C., I. Brierley, and T. D. Brown. 1992. Analysis of a 9.6 kb sequence from the 3′ end of canine coronavirus genomic RNA. J. Gen. Virol. 732849-2862. [DOI] [PubMed] [Google Scholar]
- 25.Jia, W., K. Karaca, C. R. Parrish, and S. A. Naqi. 1995. A novel variant of avian infectious bronchitis virus resulting from recombination among three different strains. Arch. Virol. 140259-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapke, P. A., and D. A. Brian. 1986. Sequence analysis of the porcine transmissible gastroenteritis coronavirus nucleocapsid protein gene. Virology 15141-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kottier, S. A., D. Cavanagh, and P. Britton. 1995. Experimental evidence of recombination in coronavirus infectious bronchitis virus. Virology 213569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lai, M. M. C., R. S. Baric, S. Makino, J. G. Keck, J. Egbert, J. L. Leibowitz, and S. A. Stohlman. 1985. Recombination between nonsegmented RNA genomes of murine coronaviruses. J. Virol. 56449-456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lai, M. M. C. 1996. Recombination in large RNA viruses: coronaviruses. Semin. Virol. 7381-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lissenberg, A., M. M. Vrolijk, A. L. van Vliet, M. A. Langereis, J. D. de Groot-Mijnes, P. J. Rottier, and R. J. de Groot. 2005. Luxury at a cost? Recombinant mouse hepatitis viruses expressing the accessory hemagglutinin esterase protein display reduced fitness in vitro. J. Virol. 7915054-15063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luytjes, W., P. J. Bredenbeek, A. F. Noten, M. C. Horzinek, and W. J. Spaan. 1988. Sequence of mouse hepatitis virus A59 mRNA 2: indications for RNA recombination between coronaviruses and influenza C virus. Virology 166415-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma, G., Y. Wang, and C. Lu. 2008. Molecular characterization of the 9.36 kb C-terminal region of canine coronavirus 1-71 strain. Virus Genes 36491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Makino, S., J. G. Keck, S. A. Stohlman, and M. M. C. Lai. 1986. High-frequency RNA recombination of murine coronaviruses. J. Virol. 57729-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Narayanan, K., C. Huang, and S. Makino. 2008. Coronavirus accessory proteins, p. 235-244. In S. Perlman, T. Gallagher, and E. J. Snijder (ed.), Nidoviruses. ASM Press, Washington, DC.
- 35.Oostra, M., C. A. de Haan, and P. J. Rottier. 2007. The 29-nucleotide deletion present in human but not in animal severe acute respiratory syndrome coronaviruses disrupts the functional expression of open reading frame 8. J. Virol. 8113876-13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ortego, J., I. Sola, F. Almazan, J. E. Ceriani, C. Riquelme, M. Balasch, J. Plana, and L. Enjuanes. 2003. Transmissible gastroenteritis coronavirus gene 7 is not essential but influences in vivo virus replication and virulence. Virology 30813-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul, P. S., E. M. Vaughn, and P. G. Halbur. 1997. Pathogenicity and sequence analysis studies suggest potential role of gene 3 in virulence of swine enteric and respiratory coronaviruses. Adv. Exp. Med. Biol. 412317-321. [DOI] [PubMed] [Google Scholar]
- 38.Pratelli, A., N. Decaro, A. Tinelli, V. Martella, G. Elia, M. Tempesta, F. Cirone, and C. Buonavoglia. 2004. Two genotypes of canine coronavirus simultaneously detected in the fecal samples of dogs with diarrhea. J. Clin. Microbiol. 421797-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pratelli, A., V. Martella, N. Decaro, A. Tinelli, M. Camero, F. Cirone, G. Elia, A. Cavalli, M. Corrente, G. Greco, D. Buonavoglia, M. Gentile, M. Tempesta, and C. Buonavoglia. 2003. Genetic diversity of a canine coronavirus detected in pups with diarrhoea in Italy. J. Virol. Methods 1109-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanchez-Morgado, J. M., S. Poynter, and T. H. Morris. 2004. Molecular characterization of a virulent canine coronavirus BGF strain. Virus Res. 10427-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schwarz, B., E. Routledge, and S. G. Siddell. 1990. Murine coronavirus nonstructural protein ns2 is not essential for virus replication in transformed cells. J. Virol. 644784-4791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Siddell, S., and E. J. Snijder. 2008. An introduction to nidovirus, p. 1-13. In S. Perlman, T. Gallagher, and E. J. Snijder (ed.), Nidoviruses. ASM Press, Washington, DC.
- 43.Smits, S. L., G. J. Gerwig, A. L. van Vliet, A. Lissenberg, P. Briza, J. P. Kamerling, R. Vlasak, and R. J. de Groot. 2005. Nidovirus sialate-O-acetylesterases: evolution and substrate specificity of coronaviral and toroviral receptor-destroying enzymes. J. Biol. Chem. 2806933-6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snijder, E. J., J. A. den Boon, M. C. Horzinek, and W. J. Spaan. 1991. Comparison of the genome organization of toro- and coronaviruses: evidence for two nonhomologous RNA recombination events during Berne virus evolution. Virology 180448-452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Song, D., J. Yang, J. Oh, J. Han, and B. Park. 2003. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF 3. Vaccine 211833-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tekes, G., R. Hofmann-Lehmann, I. Stallkamp, V. Thiel, and H. J. Thiel. 2008. Genome organization and reverse genetic analysis of a type I feline coronavirus. J. Virol. 821851-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tennant, B. J., R. M. Gaskell, R. C. Jones, and C. J. Gaskell. 1993. Studies on the epizootiology of canine coronavirus. Vet. Rec. 1327-11. [DOI] [PubMed] [Google Scholar]
- 48.Van den Born, E., and E. J. Snijder. 2008. RNA signals regulating nidovirus RNA synthesis, p. 115-131. In S. Perlman, T. Gallagher, and E. J. Snijder (ed.), Nidoviruses. ASM Press, Washington, DC.
- 49.Vennema, H., A. Poland, J. Foley, and N. C. Pedersen. 1998. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology 243150-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vennema, H., J. W. Rossen, J. Wesseling, M. C. Horzinek, and P. J. Rottier. 1992. Genomic organization and expression of the 3′ end of the canine and feline enteric coronaviruses. Virology 191134-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woods, R. 2001. Efficacy of a transmissible gastroenteritis coronavirus with an altered ORF-3 gene. Can. J. Vet. Res. 6528-32. [PMC free article] [PubMed] [Google Scholar]
- 52.Yamanaka, M., T. Crisp, R. Brown, and B. Dale. 1998. Nucleotide sequence of the inter-structural gene region of feline infectious peritonitis virus. Virus Genes 16317-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yokomori, K., L. R. Banner, and M. M. Lai. 1991. Heterogeneity of gene expression of the hemagglutinin-esterase (HE) protein of murine coronaviruses. Virology 183647-657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Youn, S., J. L. Leibowitz, and E. W. Collisson. 2005. In vitro assembled, recombinant infectious bronchitis viruses demonstrate that the 5a open reading frame is not essential for replication. Virology 332206-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yount, B., R. S. Roberts, A. C. Sims, D. Deming, M. B. Frieman, J. Sparks, M. R. Denison, N. Davis, and R. S. Baric. 2005. Severe acute respiratory syndrome coronavirus group-specific open reading frames encode nonessential functions for replication in cell cultures and mice. J. Virol. 7914909-14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeng, Q., M. A. Langereis, A. L. W. van Vliet, E. G. Huizinga, and R. J. de Groot. 2008. Structure of coronavirus hemagglutinin-esterase offers insight in corona and influenza virus evolution. Proc. Natl. Acad. Sci. USA 1059065-9069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang, X., M. Hasoksuz, D. Spiro, R. Halpin, S. Wang, S. Stollar, D. Janies, N. Hadya, Y. Tang, E. Ghedin, and L. Saif. 2007. Complete genomic sequences, a key residue in the spike protein and deletions in nonstructural protein 3b of US strains of the virulent and attenuated coronaviruses, transmissible gastroenteritis virus and porcine respiratory coronavirus. Virology 358424-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziebuhr, J. 2008. Coronavirus replicative proteins, p. 65-81. In S. Perlman, T. Gallagher, and E. J. Snijder (ed.), Nidoviruses. ASM Press, Washington, DC.