Abstract
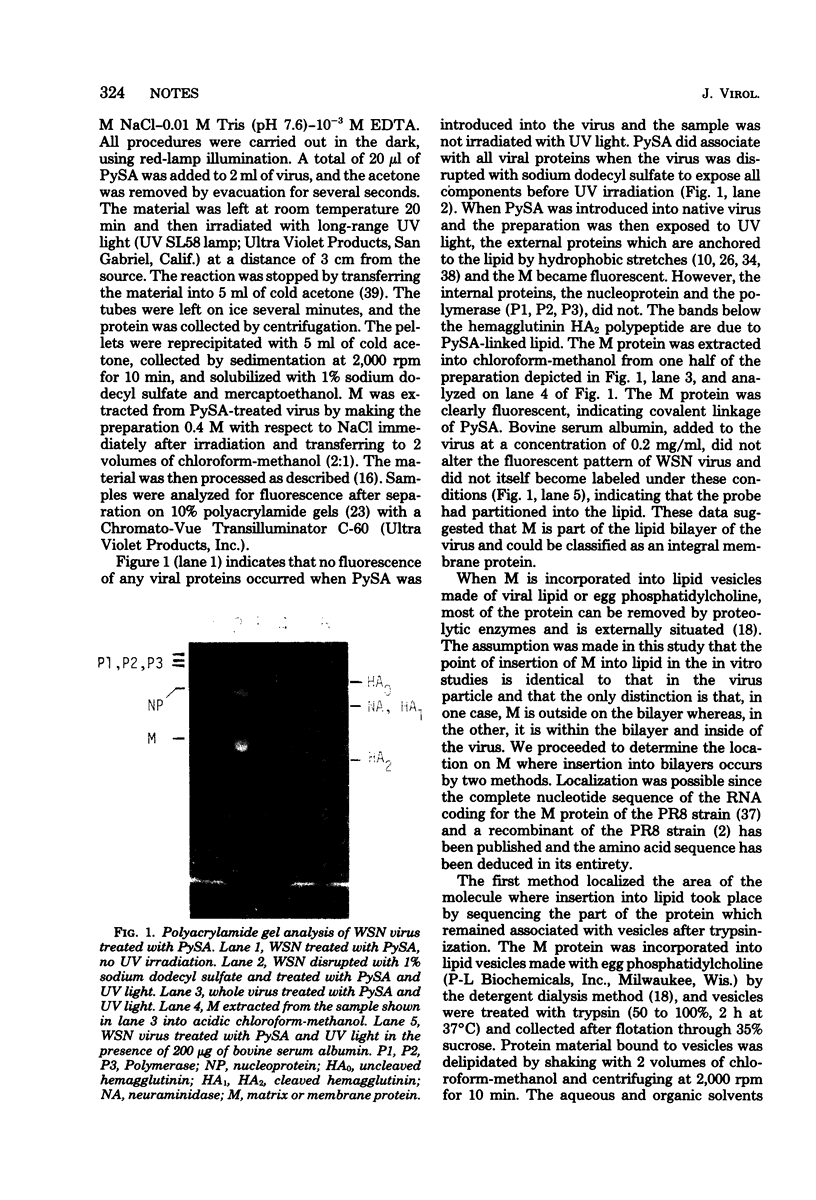
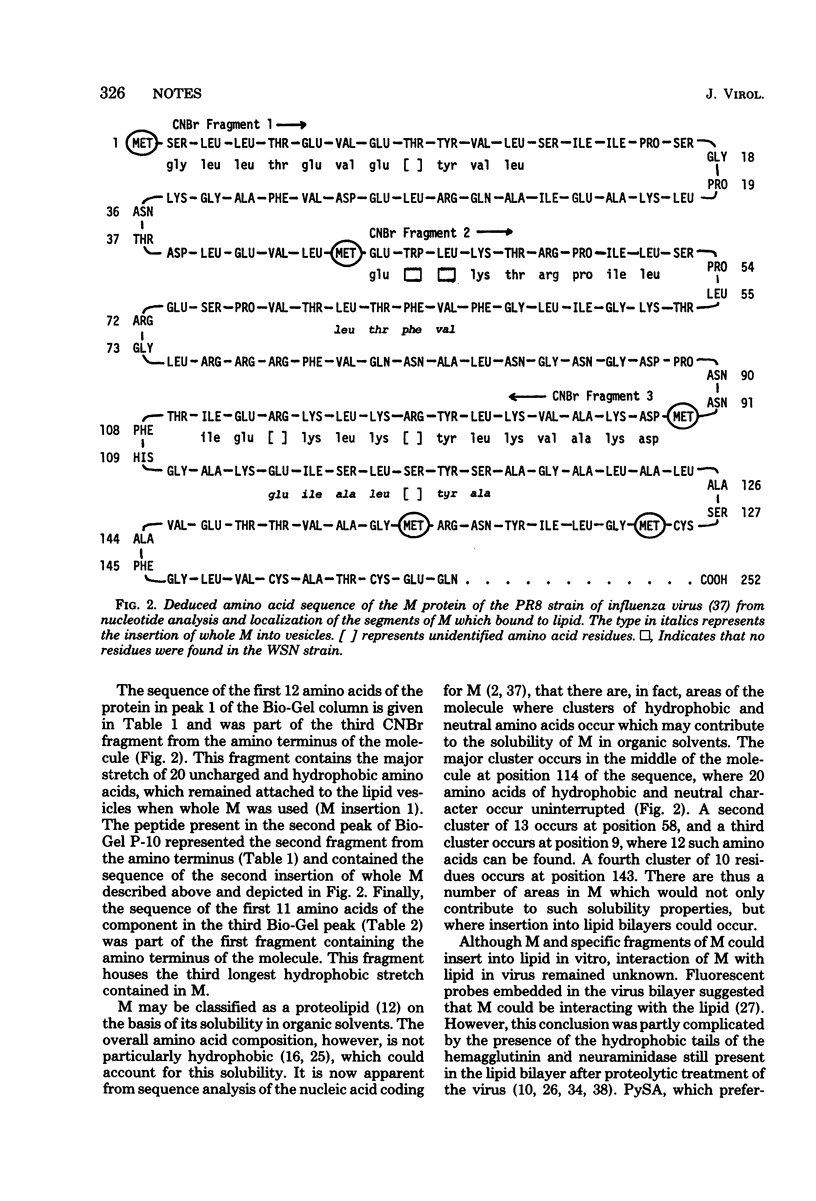
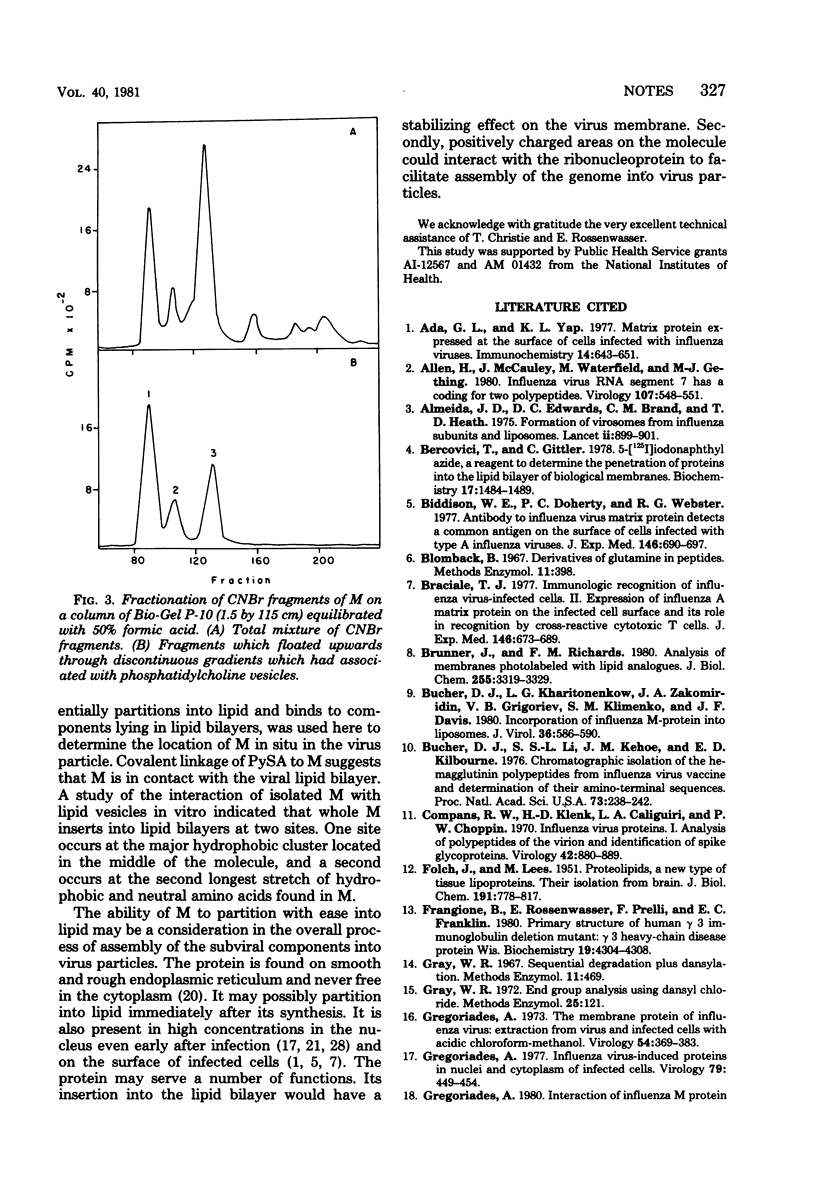
Recent studies with isolated M protein from influenza virus have shown that the protein has a high affinity for lipid. The ability of M to partition into lipid vesicles merely by shaking vesicles and M together is suggestive evidence that the protein could be interacting with the lipid in the virus particle. A more direct analysis was carried our here to determine whether M is in contact with the viral lipid in situ, by using the photoactivatable hydrophobic probe, pyrenesulfonyl azide. Covalent linkage of this probe to M indicated that a segment of M residues with in the virus membrane in contact with the lipid bilayer. M inserted into lipid vesicles at two locations on the molecule. A major insertion into lipid occurred in the middle of the molecule where a large cluster of 20 hydrophobic and neutral amino acids occurs. A second insertion occurred approximately one fourth in from the amino terminus, where a smaller segment of 13 uncharged amino acids is found. Confirmation that M inserted into lipid at these locations came also from results with cyanogen bromide fragments of M. Of the 12 to 13 fragments produced, 3 specifically bound to lipid vesicles. These were the first, second, and third contiguous segments beginnings at the amino terminus and containing the two hydrophobic areas noted above.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen H., McCauley J., Waterfield M., Gething M. J. Influenza virus RNA segment 7 has the coding capacity for two polypeptides. Virology. 1980 Dec;107(2):548–551. doi: 10.1016/0042-6822(80)90324-4. [DOI] [PubMed] [Google Scholar]
- Almeida J. D., Edwards D. C., Brand C. M., Heath T. D. Formation of virosomes from influenza subunits and liposomes. Lancet. 1975 Nov 8;2(7941):899–901. doi: 10.1016/s0140-6736(75)92130-3. [DOI] [PubMed] [Google Scholar]
- Bercovici T., Gitler C. 5-[125I]Iodonaphthyl azide, a reagent to determine the penetration of proteins into the lipid bilayer of biological membranes. Biochemistry. 1978 Apr 18;17(8):1484–1489. doi: 10.1021/bi00601a020. [DOI] [PubMed] [Google Scholar]
- Biddison W. E., Doherty P. C., Webster R. G. Antibody to influenza virus matrix protein detects a common antigen on the surface of cells infected with type A influenza viruses. J Exp Med. 1977 Sep 1;146(3):690–697. doi: 10.1084/jem.146.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J. Immunologic recognition of influenza virus-infected cells. II. Expression of influenza A matrix protein on the infected cell surface and its role in recognition by cross-reactive cytotoxic T cells. J Exp Med. 1977 Sep 1;146(3):673–689. doi: 10.1084/jem.146.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner J., Richards F. M. Analysis of membranes photolabeled with lipid analogues. Reaction of phospholipids containing a disulfide group and a nitrene or carbene precursor with lipids and with gramicidin A. J Biol Chem. 1980 Apr 25;255(8):3319–3329. [PubMed] [Google Scholar]
- Bucher D. J., Kharitonenkov I. G., Zakomirdin J. A., Grigoriev V. B., Klimenko S. M., Davis J. F. Incorporation of influenza virus M-protein into liposomes. J Virol. 1980 Nov;36(2):586–590. doi: 10.1128/jvi.36.2.586-590.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucher D. J., Li S. S., Kehoe J. M., Kilbourne E. D. Chromatographic isolation of the hemagglutinin polypeptides from influenza virus vaccine and determination of their amino-terminal sequences. Proc Natl Acad Sci U S A. 1976 Jan;73(1):238–242. doi: 10.1073/pnas.73.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compans R. W., Klenk H. D., Caliguiri L. A., Choppin P. W. Influenza virus proteins. I. Analysis of polypeptides of the virion and identification of spike glycoproteins. Virology. 1970 Dec;42(4):880–889. doi: 10.1016/0042-6822(70)90337-5. [DOI] [PubMed] [Google Scholar]
- FOLCH J., LEES M. Proteolipides, a new type of tissue lipoproteins; their isolation from brain. J Biol Chem. 1951 Aug;191(2):807–817. [PubMed] [Google Scholar]
- Frangione B., Rosenwasser E., Prelli F., Franklin E. C. Primary structure of human gamma 3 immunoglobulin deletion mutant: gamma 3 heavy-chain disease protein Wis. Biochemistry. 1980 Sep 2;19(18):4304–4308. doi: 10.1021/bi00559a024. [DOI] [PubMed] [Google Scholar]
- Gregoriades A., Hirst G. K. Mechanism of influenza recombination. III. Biochemical studies of temperature-sensitive mutants belonging to different recombination groups. Virology. 1976 Jan;69(1):81–92. doi: 10.1016/0042-6822(76)90196-3. [DOI] [PubMed] [Google Scholar]
- Gregoriades A. Influenza virus-induced proteins in nuclei and cytoplasm of infected cells. Virology. 1977 Jun 15;79(2):449–454. doi: 10.1016/0042-6822(77)90372-5. [DOI] [PubMed] [Google Scholar]
- Gregoriades A. Interaction of influenza M protein with viral lipid and phosphatidylcholine vesicles. J Virol. 1980 Nov;36(2):470–479. doi: 10.1128/jvi.36.2.470-479.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoriades A. The membrane protein of influenza virus: extraction from virus and infected cell with acidic chloroform-methanol. Virology. 1973 Aug;54(2):369–383. doi: 10.1016/0042-6822(73)90150-5. [DOI] [PubMed] [Google Scholar]
- Hay A. J. Studies on the formation of the influenza virus envelope. Virology. 1974 Aug;60(2):398–418. doi: 10.1016/0042-6822(74)90335-3. [DOI] [PubMed] [Google Scholar]
- Klapper D. G., Wilde C. E., 3rd, Capra J. D. Automated amino acid sequence of small peptides utilizing Polybrene. Anal Biochem. 1978 Mar;85(1):126–131. doi: 10.1016/0003-2697(78)90282-8. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lai C. Y. Regeneration of amino acids from anilinothiazolinones. Methods Enzymol. 1977;47:369–373. doi: 10.1016/0076-6879(77)47038-1. [DOI] [PubMed] [Google Scholar]
- Laver W. G., Baker N. Amino acid composition of polypeptides from influenza virus particles. J Gen Virol. 1972 Oct;17(1):61–67. doi: 10.1099/0022-1317-17-1-61. [DOI] [PubMed] [Google Scholar]
- Lazdins I., Haslam E. A., White D. O. The polypeptides of influenza virus. VI. Composition of the neuraminidase. Virology. 1972 Sep;49(3):758–765. doi: 10.1016/0042-6822(72)90532-6. [DOI] [PubMed] [Google Scholar]
- Maeno K., Yoshii S., Yoshida T., Iinuma M., Kawamoto Y. Intracellular development of membrane protein of influenza virus. Microbiol Immunol. 1977;21(8):427–438. doi: 10.1111/j.1348-0421.1977.tb00308.x. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Wagner R. R. Glycoprotein micelles isolated from vesicular stomatitis virus spontaneously partition into sonicated phosphatidylcholine vesicles. Virology. 1980 Dec;107(2):543–547. doi: 10.1016/0042-6822(80)90323-2. [DOI] [PubMed] [Google Scholar]
- Petri W. A., Jr, Wagner R. R. Reconstitution into liposomes of the glycoprotein of vesicular stomatitis virus by detergent dialysis. J Biol Chem. 1979 Jun 10;254(11):4313–4316. [PubMed] [Google Scholar]
- Robertson B. H., Bhown A. S., Compans R. W., Bennett J. C. Structure of the membrane protein of influenza virus. I. Isolation and characterization of cyanogen bromide cleavage products. J Virol. 1979 Jun;30(3):759–766. doi: 10.1128/jvi.30.3.759-766.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sator V., Gonzalez-Ros J. M., Calvo-Fernandez P., Martinez-Carrion M. Pyrenesulfonyl azide: a marker of acetylcholine receptor subunits in contact with membrane hydrophobic environment. Biochemistry. 1979 Apr 3;18(7):1200–1206. doi: 10.1021/bi00574a013. [DOI] [PubMed] [Google Scholar]
- Schulze I. T. The structure of influenza virus. I. The polypeptides of the virion. Virology. 1970 Dec;42(4):890–904. doi: 10.1016/0042-6822(70)90338-7. [DOI] [PubMed] [Google Scholar]
- Skehel J. J., Waterfield M. D. Studies on the primary structure of the influenza virus hemagglutinin. Proc Natl Acad Sci U S A. 1975 Jan;72(1):93–97. doi: 10.1073/pnas.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoffel W., Schreiber C., Scheefers H. Lipids with photosensitive groups as chemical probes for the structural analysis of biological membranes. On the localization of the G- and M-protein of vesicular stomatitis virus. Hoppe Seylers Z Physiol Chem. 1978 Aug;359(8):923–931. doi: 10.1515/bchm2.1978.359.2.923. [DOI] [PubMed] [Google Scholar]
- Tarr G. E., Beecher J. F., Bell M., McKean D. J. Polyquarternary amines prevent peptide loss from sequenators. Anal Biochem. 1978 Feb;84(2):622–7?0=ENG. doi: 10.1016/0003-2697(78)90086-6. [DOI] [PubMed] [Google Scholar]
- Winter G., Fields S. Cloning of influenza cDNA ino M13: the sequence of the RNA segment encoding the A/PR/8/34 matrix protein. Nucleic Acids Res. 1980 May 10;8(9):1965–1974. doi: 10.1093/nar/8.9.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrigley N. G., Skehel J. J., Charlwood P. A., Brand C. M. The size and shape of influenza virus neuraminidase. Virology. 1973 Feb;51(2):525–529. doi: 10.1016/0042-6822(73)90457-1. [DOI] [PubMed] [Google Scholar]
- Zakowski J. J., Wagner R. R. Localization of membrane-associated proteins in vesicular stomatitis virus by use of hydrophobic membrane probes and cross-linking reagents. J Virol. 1980 Oct;36(1):93–102. doi: 10.1128/jvi.36.1.93-102.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]



