Abstract
The GDVII strain of Theiler's murine encephalomyelitis virus (TMEV) causes an acute fatal polioencephalomyelitis in mice. Infection of susceptible mice with the DA strain of TMEV results in an acute polioencephalomyelitis followed by chronic immune-mediated demyelination with virus persistence in the central nervous system (CNS); DA virus infection is used as an animal model for multiple sclerosis. CD1d-restricted natural killer T (NKT) cells can contribute to viral clearance and regulation of autoimmune responses. To investigate the role of CD1d in TMEV infection, we first infected CD1d-deficient mice (CD1−/−) and wild-type BALB/c mice with GDVII virus. Wild-type mice were more resistant to virus than CD1−/− mice (50% lethal dose titers: wild-type mice, 10 PFU; CD1−/− mice, 1.6 PFU). Wild-type mice had fewer viral antigen-positive cells with greater inflammation in the CNS than CD1−/− mice. Second, an analysis of DA virus infection in CD1−/− mice was conducted. Although both wild-type and CD1−/− mice had similar clinical signs during the first 2 weeks after infection, CD1−/− mice had an increase in neurological deficits over those observed in wild-type mice at 3 to 5 weeks after infection. Although wild-type mice had no demyelination, 20 and 60% of CD1−/− mice developed demyelination at 3 and 5 weeks after infection, respectively. TMEV-specific lymphoproliferative responses, interleukin-4 (IL-4) production, and IL-4/gamma interferon ratios were higher in CD1−/− mice than in wild-type mice. Thus, CD1d-restricted NKT cells may play a protective role in TMEV-induced neurological disease by alteration of the cytokine profile and virus-specific immune responses.
Theiler's murine encephalomyelitis virus (TMEV) is a member of the family Picornaviridae and is divided into two subgroups, GDVII and Theiler's original (TO), according to neurovirulence within the central nervous system (CNS) (40). The GDVII subgroup viruses, including the GDVII and FA strains, cause an acute fatal polioencephalomyelitis in mice (41). Infected mice show weight loss and encephalitic signs, including hunched back and ruffled fur, and die within 10 days, regardless of mouse strain.
The TO subgroup viruses, including the Daniels (DA) and BeAn strains, also cause acute polioencephalomyelitis 1 week postinfection (p.i.) (acute phase) but show relatively low neurovirulence. During the acute phase, most mice show only mild clinical signs, such as impairment of the righting reflex. Infected mice survive and recover around 2 weeks p.i. Thereafter, clearance or persistence of virus depends on the strain of mice. BALB/c and C57BL/6 mice clear virus from the CNS after the acute phase, develop little or no chronic disease, and are considered resistant mouse strains (22, 29). On the other hand, infection of susceptible strains of mice, such as SJL/J mice, with the TO subgroup results in virus persistence and chronic inflammatory demyelinating lesions in the white matter of the spinal cord (chronic phase), similar to multiple sclerosis (MS) (30). TMEV-induced demyelination is seen from 3 to 4 weeks after infection, and virus persistence and immune-mediated pathogenesis play important roles in the disease course (41).
CD1d is a major histocompatibility complex (MHC) class I-like molecule and is required for the development of natural killer T (NKT) cells (11, 12). NKT cells express phenotypic markers that are typical of conventional T cells and natural killer (NK) cells. Invariant T-cell receptors (TCR) encoded by Vα14 in mice and Vα24 in humans are also expressed on NKT cells (20). NKT cells can recognize glycolipids presented by CD1d (15, 49) and produce large amounts of cytokines, particularly interleukin-4 (IL-4) and/or gamma interferon (IFN-γ). This leads to modulation of a variety of immune cells; thus, NKT cells can play an important role in bridging innate and acquired immunity (6, 35).
NKT cells can play dual roles in both virus infection and immune-mediated disease. In virus infection, NKT cells play a crucial role in protective immune responses (49). For example, in lymphocytic choriomeningitis virus infection, CD1d-deficient (CD1−/−) mice, which lack NKT cells, produced increased amounts of cytokines and cleared the virus faster than wild-type mice (28). However, NKT cells can also downregulate the immune response to viruses, such as with respiratory syncytial virus (18).
In a variety of immune-mediated diseases and their models (23), NKT cells can play a regulatory (protective) role, usually via the production of Th2-type cytokines (49). However, in some instances, NKT cells appear to enhance autoimmunity via production of Th1-type cytokines (11). In MS, it has been reported that Vα24+ NKT cells are rarely observed in plaques in the CNS and are reduced in the peripheral blood (14, 48). However, others have reported no differences in the frequency of CD4− CD8− Vα24+ NKT cells between MS patients and healthy control subjects, while lower frequencies of IL-4-secreting NKT cell clones were found in relapsing-remitting MS patients, compared with NKT cells derived from progressive MS patients and controls (10). Similarly, in experimental allergic (autoimmune) encephalomyelitis (EAE), an autoimmune model for MS, contrasting roles for NKT cells have been demonstrated. NKT cells have been reported to play a protective role against demyelination, while some investigators showed no role or a detrimental role for NKT cells (9, 17, 21, 24, 32, 37). The discrepancies among reports are partly due to the fact that some glycolipid ligands, which were used for NKT cell stimulation, favor production of IFN-γ, while others preferentially induce IL-4 production by NKT cells (24, 50).
Whereas CD1d-restricted NKT cells can play a protective role in virus infection, including picornaviruses (8, 27), they can play a regulatory or deleterious role in immune-mediated diseases by production of cytokines (50). Thus, in TMEV infection, CD1d could not only contribute to clearance of virus but also modulate the immune-mediated demyelinating disease. To investigate the role of CD1d in TMEV infection, we infected BALB/c CD1−/− and wild-type mice with two strains of TMEV, GDVII and DA. In GDVII virus infection, we found that wild-type mice were more resistant to the acute disease, having a higher median lethal dose (LD50) titer than CD1−/− mice. Pathologically, wild-type mice had fewer viral antigen-positive cells with higher numbers of inflammatory cell infiltrates in the CNS than CD1−/− mice. In DA virus infection, CD1−/− mice developed demyelinating disease with more neurological deficits than wild-type mice at 3 and 5 weeks p.i. DA virus-specific lymphoproliferative responses and IL-4 production were higher in CD1−/− mice than in wild-type mice. Thus, CD1d-restricted NKT cells could play a protective role in TMEV-induced neurological diseases by alteration of the cytokine profile and antivirus immune response.
MATERIALS AND METHODS
Animal experiments.
Four-week-old CD1−/− mice on a BALB/c background, C.129S2-Cd1tm1Gru mice (34), and wild-type BALB/c mice (Jackson Laboratory, Bar Harbor, ME) were infected intracerebrally with 0.1 to 1,000 PFU of the GDVII strain or 2 × 105 PFU of the DA strain of TMEV. Mice were weighed and observed daily for up to 6 months. Clinical signs of neurological disease were evaluated by measuring an impairment in the righting reflex (47). When the proximal end of the mouse's tail is grasped and twisted to the right and then to the left, a healthy mouse resists being turned over (score of 0). If the mouse is flipped onto its back but immediately rights itself on one side or both sides, it is given a score of 1 or 1.5, respectively. If it rights itself in 1 to 5 s, the score is 2. If righting takes more than 5 s, the score is 3. The LD50 was calculated using the Reed and Muench calculation of the 50% endpoint (4).
Neuropathology.
Mice were perfused with phosphate-buffered saline, followed by 4% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). The brain, coronally divided into five slabs, and the spinal cord, transversely divided into 12 segments, were embedded in paraffin. Four-micrometer-thick sections were stained with Luxol fast blue for myelin visualization. Histological scoring was performed as previously described (47). Brain sections were scored for meningitis (0, no meningitis; 1, mild cellular infiltrates; 2, moderate cellular infiltrates; 3, severe cellular infiltrates), perivascular cuffing (0, no cuffing; 1, 1 to 10 lesions; 2, 11 to 20 lesions; 3, 21 to 30 lesions; 4, 31 to 40 lesions; 5, over 40 lesions), and demyelination (0, no demyelination; 1, mild demyelination; 2, moderate demyelination; 3, severe demyelination). Each score from the brain was combined for a maximum score of 11 per mouse. For scoring of spinal cord sections, each spinal cord segment was divided into four quadrants: the ventral funiculus, the dorsal funiculus, and each lateral funiculus. Any quadrant containing meningitis, perivascular cuffing, or demyelination was given a score of 1 in that pathological class. The total number of positive quadrants for each pathological class was determined and then divided by the total number of quadrants present on the slide and multiplied by 100 to give the percent involvement for each pathological class. An overall pathological score was also determined by giving a positive score if any pathology was present in the quadrant. This was also presented as the percent involvement.
Viral antigen-positive cells were visualized by immunohistochemistry with TMEV antiserum, using the avidin-biotin peroxidase complex technique (Vector, Burlingame, CA) with 3,3′-diaminobenzidine tetrahydrochloride (Sigma-Aldrich) as chromogen (45). Enumeration of viral antigen-positive cells in the CNS was performed with a light microscope, at a magnification of ×200, using five coronal brain sections and 12 transverse spinal cord sections per mouse, as described previously (46). All antigen-positive cells were counted in the five brain and 12 spinal cord sections in all mice. We used five wild-type mice and five CD1−/− mice in GDVII virus infection and four to eight mice per group at each time point in DA virus infection.
Lymphoproliferation.
Spleens were removed from TMEV-infected mice at 1, 3, and 5 weeks and 2 and 6 months p.i., and mononuclear cells (MNCs) were isolated using Histopaque 1083 (Sigma-Aldrich). A volume of 100 μl of 2 × 105 MNCs in RPMI 1640 (Mediatech, Inc., Herndon, VA) supplemented with 1% glutamine (Mediatech), 1% antibiotics (Mediatech), 50 μmol/liter 2-mercaptoethanol (Sigma-Aldrich), and 10% fetal bovine serum (Invitrogen, Carlsbad, CA) was added to each well of 96-well plates. This was incubated with 100 μl of solution containing 2 × 105 DA antigen presenting cells (DA-APCs) or 2 × 105 sham-infected APCs (nAPCs). DA-APCs were made from whole spleen cells infected in vitro with DA virus at a multiplicity of infection of 1 and irradiated with 2,000 rads using a 137Cs irradiator (46), while nAPCs were prepared from sham-infected spleen cells. The cells were cultured for 4 days, after which time each well was pulsed with 1 μCi of tritiated thymidine (Perkin-Elmer Life Sciences, Boston, MA). Then, 18 to 24 h later, the cells were harvested onto a FilterMAT (Skatron Instrument Inc., Sterling, VA) using a multiwell cell harvester (Molecular Devices, Sunnyvale, CA), and 3H incorporation was determined using an LS 6500 multipurpose scintillation counter (Beckman Coulter, Inc., Fullerton, CA). All cultures were performed in triplicate. A stimulation index was calculated using the following formula: (cpm of MNCs incubated with TMEV-APCs)/(cpm of MNCs incubated with nAPCs).
Cytokine assays.
Spleen MNCs were harvested 1, 3, and 5 weeks and 2 and 6 months p.i. and cultured at 2 × 106 cells/ml in six-well plates (Corning Inc., Corning, NY) in the presence or absence of concanavalin A (5 μg/ml) or DA virus at a multiplicity of infection of 1. Culture supernatants were harvested 48 h after stimulation. IFN-γ and IL-4 were measured using the enzyme-linked immunosorbent assay system OptEIA set (BD PharMingen, San Jose, CA), according to the manufacturer's instructions (44).
RESULTS
Protective role of CD1d in GDVII virus infection.
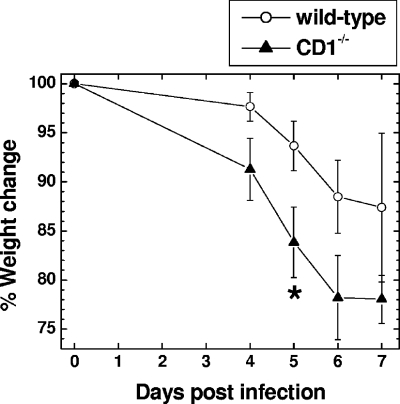
We first investigated the role of CD1d in mice infected with a neurovirulent strain of TMEV, GDVII. GDVII virus causes acute fatal polioencephalitis in mice, regardless of the strain. Most infected mice die within 10 days. In the CNS, GDVII virus infection results in massive apoptosis of neurons and substantial viral replication. This is associated with a failure in both the cellular and humoral immune response against virus (41, 43). We inoculated 0.1 to 1,000 PFU of GDVII virus intracerebrally in wild-type and CD1−/− mice. Between days 4 and 5 p.i., most mice infected with GDVII virus started to show signs of encephalitis, such as weight loss, ruffled fur, and a hunched posture. Most infected mice died around day 7 p.i. (Table 1). All mice that had clinical signs died, and no mice with clinical signs recovered. The day of onset of clinical signs and survival period were similar between the wild-type and CD1−/− mice infected with 10 to 1,000 PFU of GDVII virus, while CD1−/− mice showed more rapid weight loss than wild-type mice (Fig. 1). At low infectious doses, more wild-type mice survived than CD1−/− mice; the LD50 titer for wild-type mice was 10 PFU and that for CD1−/− mice was 1.6 PFU.
TABLE 1.
Mortality of wild-type and CD1−/− mice infected with GDVII virusa
| PFUb | Wild type
|
CD1−/−
|
||
|---|---|---|---|---|
| Mortalityc | Survival to dayd: | Mortality | Survival to day: | |
| 1,000 | 5/5 | 4-6 (4.8 ± 0.4) | 8/8 | 5-7 (6.3 ± 0.3) |
| 100 | 9/10 | 5-11 (6.7 ± 0.6) | 8/8 | 5-8 (6.0 ± 0.4) |
| 10 | 5/10 | 6-7 (6.2 ± 0.2) | 5/5 | 6-8 (6.6 ± 0.4) |
| 1 | 1/5 | 8 | 3/8 | 6-13 (9.0 ± 2.1) |
| 0.1 | 0/4 | NAe | ||
Mice were infected intracerebrally with the GDVII strain of TMEV.
PFU of virus inoculated.
Number of dead mice/total number of mice inoculated with virus.
Survival day following inoculation of virus (mean survival day ± standard error of the mean).
NA, not applicable.
FIG. 1.
Weight change in wild-type and CD1−/− mice infected intracerebrally with 100 PFU of the neurovirulent GDVII strain of TMEV. CD1−/− mice lost more weight than wild-type mice 5 days p.i. *, P < 0.05, t test. Shown are means ± standard errors of the means of percent changes in original weight (on day zero) of nine wild-type mice and eight CD1−/− mice, all of which died of encephalitis.
CD1−/− mice infected with GDVII virus have more infected cells with less inflammation.
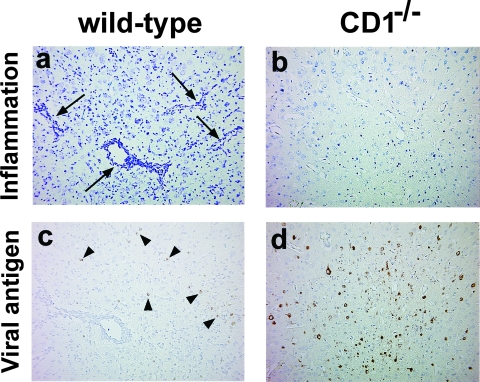
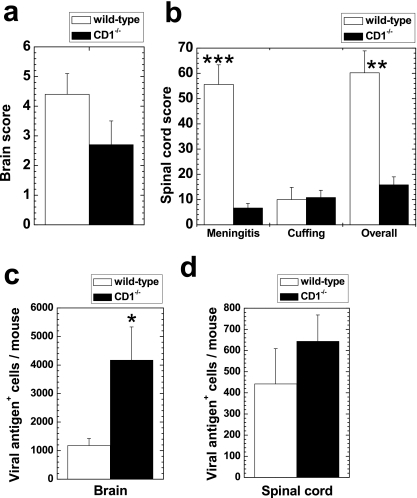
To investigate a possible protective role of CD1d in GDVII virus infection, we compared the extent of neuropathology between wild-type and CD1−/− mice infected with GDVII virus. In the brain, wild-type mice had a moderate amount of perivascular MNC inflammation in the gray matter of the cerebral cortex, the hippocampus, and the thalamus (Fig. 2a). In contrast, CD1−/− mice had much fewer perivascular cuffs in the brain parenchyma (Fig. 2b). In the spinal cord, wild-type mice had a moderate level of meningitis, while CD1−/− mice had less meningeal cell infiltration. Perivascular cuffing was minimal in the spinal cord in both wild-type and CD1−/− mice. Quantification of inflammatory scores showed that wild-type mice had a significantly higher meningitis score (P < 0.001, t test) and overall inflammation score (P < 0.01) than CD1−/− mice (Fig. 3b). Wild-type mice also had higher brain inflammation scores than CD1−/− mice, although the difference did not reach statistical significance (Fig. 3a) (P = 0.15).
FIG. 2.
Neuropathology of wild-type (a and c) and CD1−/− mice (b and d) infected with 1,000 PFU of GDVII virus, 6 days p.i. In the gray matter of the frontal cortex, moderate perivascular inflammation was seen in wild-type mice (a; arrow), but no inflammation was seen in CD1−/− mice (b). In contrast, consecutive sections of the frontal cortex had a low number of neurons weakly positive for viral antigen in wild-type mice (c; arrowhead), while CD1−/− mice had a high number of neurons strongly positive for viral antigen (d). (a and b) Luxol fast blue staining; (c and d) immunohistochemisty against viral antigen. Magnification, ×77.
FIG. 3.
Inflammation scores (a and b) and numbers of viral antigen-positive cells (c and d) of wild-type and CD1−/− mice infected with 1,000 PFU of GDVII virus. (a and b) Wild-type mice had higher inflammation scores than CD1−/− mice both in the brain and the spinal cord. **, P < 0.01; ***, P < 0.001 (t test). (c and d) In contrast, fewer viral antigen-positive cells were observed in the CNS from wild-type mice than in CD1−/− mice. *, P < 0.05. Results are means + standard errors of the means of neuropathology scores from five mice per group.
We also compared numbers of viral antigen-positive cells between the wild-type and CD1−/− mice, using consecutive sections to those used for the neuropathology. Interestingly, we found greater numbers of viral antigen-positive cells in CD1−/− mice (Fig. 2d) than in wild-type mice (Fig. 2c). Quantification of viral antigen-positive cells showed that CD1−/− mice had significantly higher numbers of viral antigen-positive cells than wild-type mice in the brain (Fig. 3c) (P < 0.05, t test). Similarly, in the spinal cord, CD1−/− mice had higher numbers of viral antigen-positive cells than wild-type mice, although this difference did not reach statistical significance (Fig. 3d) (P = 0.38). These results suggest that the CD1d molecule plays a protective role by induction of an inflammatory response, resulting in fewer viral antigen-positive cells in the CNS.
CD1−/− mice infected with DA virus develop mild clinical signs around 3 weeks p.i.
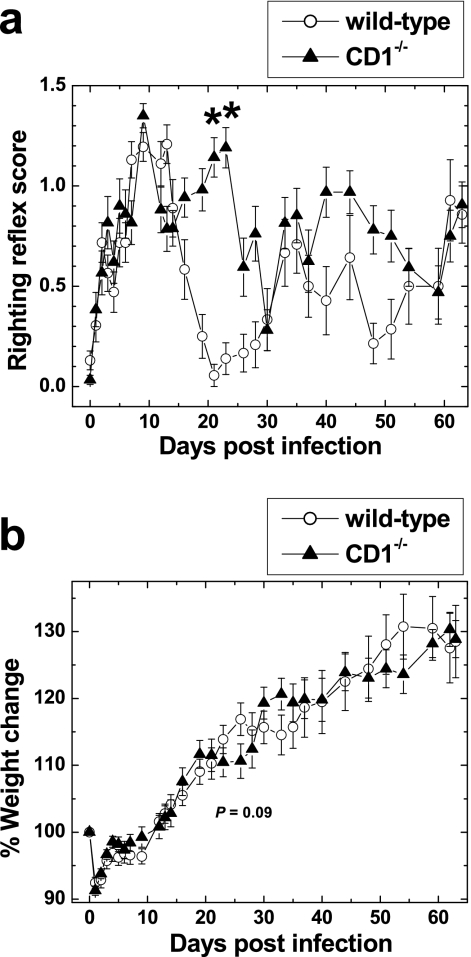
We next tested whether CD1−/− mice infected with a virus from the TO subgroup of TMEV would develop clinical signs similar to wild-type BALB/c mice. BALB/c mice are known to be resistant to chronic TMEV-induced demyelinating disease (22, 29). Viruses from the TO strain infect neurons in the CNS, 1 week p.i., but thereafter virus is cleared from the CNS without causing demyelination. We infected wild-type and CD1−/− mice with DA virus of the TO subgroup of TMEV and observed the mice for clinical signs. During the acute phase, 1 to 2 weeks after infection, we observed similar levels of impairment of the righting reflex in both wild-type and CD1−/− mice (Fig. 4a). The peak of impaired righting reflex scores was seen around 7 to 10 days p.i., and infected mice recovered during the following 1 week. However, 3 to 4 weeks p.i., 81% of CD1−/− mice developed mild impairment of the righting reflex, while most wild-type mice showed no impairment; there were significant differences in clinical signs between the two groups of mice (P < 0.05) (Fig. 4a). At 3 to 4 weeks p.i., CD1−/− mice also gained less weight, although this did not reach statistical significance, compared with wild-type mice (Fig. 4b) (P = 0.09). The exacerbation of the righting reflexes in most CD1−/− mice subsided in the following 2 weeks. Thereafter, no differences were noted in righting reflex scores between wild-type and CD1−/− mice during a 6-month observation period.
FIG. 4.
Alteration in clinical signs of CD1−/− mice infected with the DA strain of TMEV. TMEV was injected on day zero. (a) Both wild-type and CD1−/− mice developed an impaired righting reflex during the acute phase, 1 and 2 weeks p.i., to a similar extent. At 3 weeks p.i., CD1−/− mice still had impairment of the righting reflex, but wild-type mice showed complete recovery. There were significant differences in righting reflex scores between the two groups. *, P < 0.05. (b) At 3 to 4 weeks p.i., CD1−/− mice had less weight gain than wild-type mice, although it did not reach statistical significance (P = 0.09). Shown are means ± standard errors of the means of righting reflex scores and percent changes in original weight (on day zero) of 17 wild-type mice and 16 CD1−/− mice. Results are representative of two experiments.
CD1−/− mice infected with DA virus develop a demyelinating disease.
We compared the neuropathology of wild-type versus CD1−/− mice 1, 3, and 5 weeks and 2 and 6 months after DA virus infection. In the brain of wild-type mice, mild meningitis and perivascular cuffing were observed in the gray matter, only at 1 week p.i. (Fig. 5a). After 3 weeks p.i., we did not see abnormalities in brain pathology, except for a few cells present in the meninges in a few mice; mean brain pathology scores were close to 0 at 3 and 5 weeks p.i. (Fig. 6a) and 2 and 6 months p.i. (data not shown). In CD1−/− mice, we found similar pathology in the brain, compared with that of wild-type mice, at all time points (Fig. 5b and 6a).
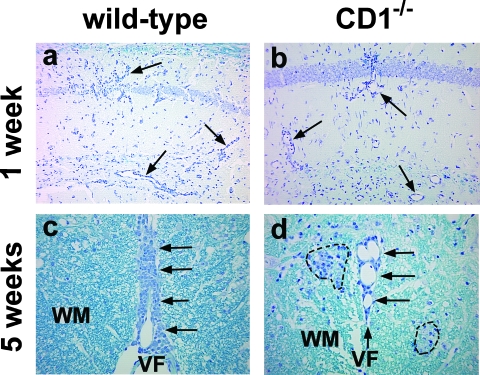
FIG. 5.
Neuropathology of wild-type (a and c) and CD1−/− mice (b and d) infected with DA virus. (a and b) At 1 week p.i. (acute phase), both wild-type and CD1−/− mice had a similar degree of perivascular inflammation (arrow) in the brain. (c and d) Spinal cord pathology, 5 weeks p.i. (chronic phase). (c) Wild-type mice had meningitis (arrow) along the ventral median fissure (VF) of the spinal cord without demyelination in the white matter (WM) of the spinal cord. (d) CD1−/− mice had meningitis (arrow) along the ventral median fissure and small demyelinating lesions (broken line) in the white matter. Shown are representative sections from the hippocampus (a and b) and the ventral funiculus of the spinal cord (c and d) from five mice per group at each time point. Luxol fast blue staining was used. Magnification, ×79 (a and b) and ×158 (c and d).
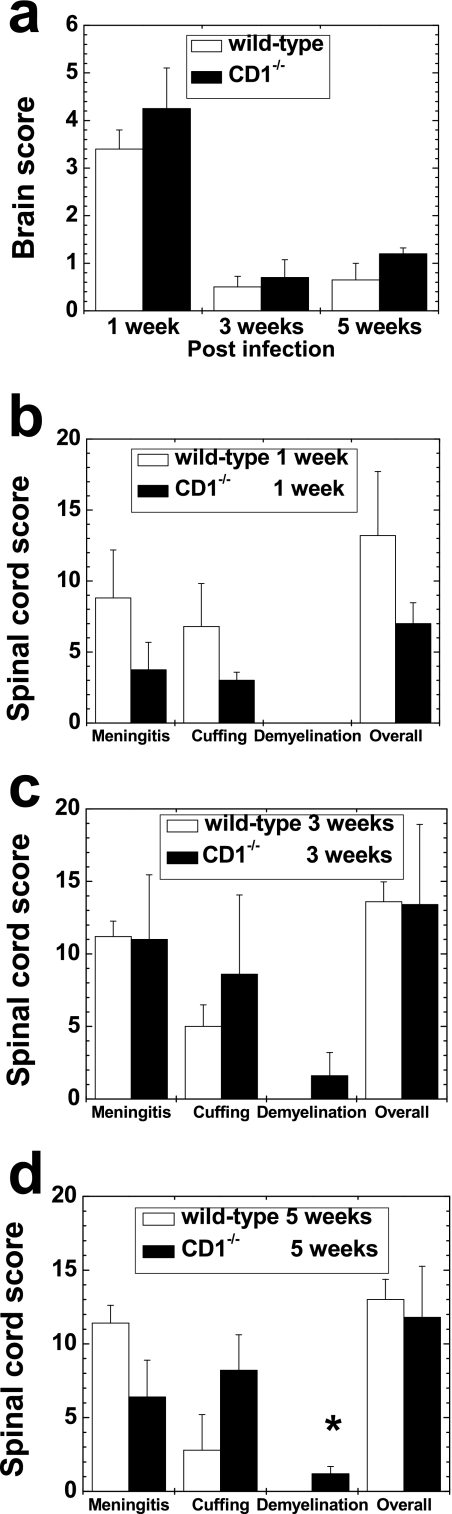
FIG. 6.
Neuropathology scores of wild-type and CD1−/− mice infected with DA virus. (a) In the brain, mild inflammation was seen in both groups of mice at 1 week p.i., and this subsided thereafter. There were no statistical differences between the two groups. (b to d) In the spinal cord, CD1−/− mice had mild demyelination at 3 and 5 weeks p.i., although wild-type mice did not have demyelination. *, P < 0.05, at 5 weeks p.i. There were no significant differences in meningitis or perivascular cuffing scores at any time points. Results are means + standard errors of the means of neuropathology scores from four to six mice per time point.
In the spinal cord of wild-type mice, mild meningitis and perivascular cuffing in the white matter were seen at 1, 3, and 5 weeks p.i. (Fig. 5c and 6b, c, and d), while no inflammation was seen at 2 and 6 months p.i. (data not shown). No demyelination was seen in the CNS of the wild-type mice at any time point. In contrast, in CD1−/− mice, we found small demyelinating lesions (Fig. 5d) in a few segments in 20% and 60% of mice at 3 and 5 weeks p.i., respectively, but not at 1 week or 2 and 6 months p.i. The extent and localization of inflammation in the spinal cord of CD1−/− mice were similar to those in wild-type mice. Using a neuropathology score, we quantified the severity of lesions. At 5 weeks p.i., CD1−/− mice had significantly higher demyelination scores than wild-type mice (P < 0.05, t test) (Fig. 6d). In the other neuropathology scores, there were no significant differences between wild-type and CD1−/− mice (Fig. 6).
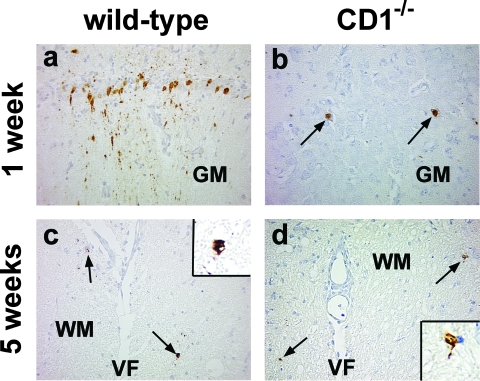
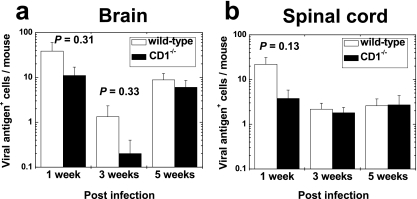
Using immunohistochemistry, we compared differences in the numbers and localization of viral antigen-positive cells between wild-type and CD1−/− mice at 1, 3, and 5 weeks and 2 and 6 months after DA virus infection. During the acute phase, 1 week p.i., viral antigen was detected in neurons around the caudoputamen (injection site), the cerebral cortex, the hippocampus, and the anterior horn cells of the spinal cord in both wild-type and CD1−/− mice (Fig. 7a and b). The numbers of viral antigen-positive cells in the CNS were much lower with DA virus infection (Fig. 8) than with GDVII virus infection (Fig. 3). Although wild-type mice tended to have more viral antigen-positive neurons than CD1−/− mice at 1 week p.i. (Fig. 8), there was no statistical difference in viral antigen-positive cells between the two groups (t test; brain, P = 0.31; spinal cord, P = 0.13). At 3 and 5 weeks p.i., only a low number of viral antigen-positive glial cells were detected in the white matter of the brainstem and the spinal cord in both mouse groups (Fig. 7c and d and 8b). Only a few mice from both groups of mice had viral antigen-positive cells in the brain or the spinal cord at 2 months p.i. and no viral antigen-positive cells were detected at 6 months p.i. Therefore, only low numbers of viral antigen-positive cells were detected in both groups (Fig. 8) and there was no significant difference between the groups at any time point (P > 0.05, t test). Localization and cell types of viral antigen-positive cells were also similar between wild-type and CD1−/− mice. Thus, there was no correlation between the numbers of viral antigen-positive cells and clinical signs.
FIG. 7.
Immunohistochemistry against viral antigen in wild-type (a and c) and CD1−/− mice (b and d) infected with DA virus. (a and b) At 1 week p.i., viral antigen was detected in neurons of the gray matter (GM) of the brain in wild-type mice (a) and CD1−/− mice (b). In wild-type mice, viral antigen was detectable not only in the cytoplasm of neurons but also in neuronal processes. (c and d) At 5 weeks p.i., only low amounts of viral antigen were detected in glial cells of the white matter (WM) of the spinal cord in both mouse strains. Shown are representative sections from the pyramidal cell layer of the hippocampus (a and b) and the ventral funiculus, near the ventral median fissure (VF), of the spinal cord (c and d) from five mice per group at each time point. Magnification, ×159 (a to d) and ×490 (inset).
FIG. 8.
Number of viral antigen-positive cells in the brain (a) and the spinal cord (b) of mice infected with DA virus. Both wild-type and CD1−/− mice had low numbers of viral antigen-positive cells throughout the course of infection. There were no significant differences in the numbers of viral antigen-positive cells between wild-type and CD1−/− mice at any time points. Values are mean viral antigen-positive cells per mouse brain or spinal cord + standard errors of the means for four to eight mice.
Enhanced lymphoproliferation in CD1−/− mice.
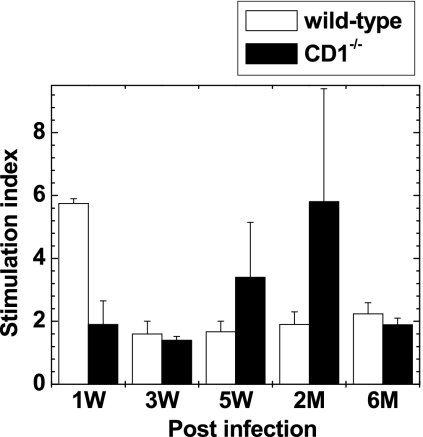
We compared virus-specific lymphoproliferative responses between wild-type and CD1−/− mice infected with DA virus. Spleen MNCs were isolated from DA virus-infected mice at different time points. Cells were stimulated for 5 days with DA-APCs, and DA virus-specific lymphoproliferative responses were measured using [3H]thymidine uptake assays. In wild-type mice, although substantial DA virus-specific responses (stimulation index > 3) were detected at 1 week p.i., thereafter DA virus-specific lymphoproliferation was low during the 6-month observation period (Fig. 9). In contrast, CD1−/− mice developed higher TMEV-specific lymphoproliferative responses at 5 weeks and 2 months p.i.
FIG. 9.
DA virus-specific lymphoproliferative responses. MNCs were isolated from wild-type and CD1−/− mice 1, 3, and 5 weeks and 2 and 6 months after DA virus infection. MNCs were cultured with DA virus antigen-presenting cells (DA-APCs) or sham-infected APCs for 5 days, and stimulation indices were calculated. Wild-type mice had substantial DA virus-specific proliferation only at 1 week p.i., while CD1−/− mice showed high proliferation at 5 weeks and 2 months p.i. Results are means + standard errors of the means of two pools of spleens from two to three mice (the total number of spleens per time point was five).
Increased IL-4 production in CD1−/− mice.
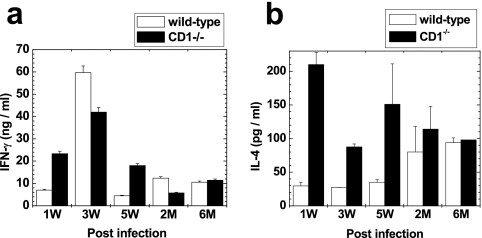
Th1/Th2 cytokines have been shown to play an important role in TMEV-induced demyelinating disease (19, 38). We compared the mitogen-induced production of IFN-γ and IL-4 by spleen MNCs between wild-type and CD1−/− mice at 1, 3, and 5 weeks and 2 and 6 months post-DA virus infection. We detected high levels (nanograms per milliliter) of IFN-γ production in both groups of mice at all time points (Fig. 10a). The levels of IFN-γ production were the highest at 3 weeks p.i. in both groups of mice. The levels of IL-4 production were higher in CD1−/− mice than in wild-type mice at all time points, particularly at 1, 3, and 5 weeks p.i. (Fig. 10b). The IFN-γ (Th1)/IL-4 (Th2) ratio was higher in wild-type mice than in CD1−/− mice during the first 2 months p.i. Cytokine production was not seen in cultures stimulated with either DA virus or medium alone at any time point (data not shown).
FIG. 10.
IFN-γ (a) and IL-4 (b) levels in DA virus-infected wild-type and CD1−/− mice at 1, 3, and 5 weeks and 2 and 6 months p.i. (a) IFN-γ was the highest at 3 weeks p.i. in both mouse strains. (b) IL-4 was higher in CD1−/− mice than in wild-type mice at all time points. Values are means + standard errors of the means of cytokine concentrations in the supernatant fluids from concanavalin A-stimulated MNCs from two pools of spleens harvested from two to three mice. All assays were performed in duplicate.
DISCUSSION
Here, we demonstrated that the neurovirulent GDVII strain of TMEV causes more rapid weight loss and higher mortality at low infectious doses in CD1−/− mice than in wild-type mice. In the CNS, CD1−/− mice had higher numbers of viral antigen-positive cells with fewer MNC infiltrates than wild-type mice. However, all GDVII virus-infected mice with clinical signs died regardless of the presence or absence of CD1d. The presence of CD1d did not protect mice from death, once infected mice showed clinical signs. This fatal outcome could be due to a failure of induction of antiviral immune responses and poor CNS infiltration of CD4+ and CD8+ T cells and B cells in GDVII virus infection, as we demonstrated previously (41). Thus, the obvious differences in viral load and inflammation in the CNS between wild-type and CD1−/− mice are likely due to the differences in innate immune responses rather than the differences in acquired antiviral immune responses. Here, CD1d-restricted cells, such as NKT cells, may play a significant but limited role in protection from GDVII virus infection, by induction of innate immune responses, leading to suppression of virus replication in the CNS. A similar protective role for NKT cells against lethal infection has been reported in BALB/c mice infected with coxsackievirus B4 (27), which also belongs to the family Picornaviridae, as does TMEV.
In contrast, during the acute phase of DA virus infection at 1 week p.i., we detected substantial levels of virus-specific lymphoproliferative responses (stimulation index, >3) only in wild-type mice, and not in CD1−/− mice, while clinical signs were similar between the wild-type and CD1−/− mice. This suggests that CD1d-restricted cells, including NKT cells, aid in the induction of virus-specific T cells, which is consistent with a role for NKT cells in bridging innate and acquired immunity as shown in other systems (36, 50). Interestingly, at 1 week p.i., wild-type mice tended to have more viral antigen-positive cells than CD1−/− mice, while there was no statistical difference between the numbers of viral antigen-positive cells in the two groups. Although we do not know why wild-type mice infected with DA virus had more virus-infected cells in the brain, higher levels of virus replication in wild-type mice compared with CD1−/− mice have also been observed in other viral infections (28).
At 3 and 5 weeks after DA virus infection, we found that CD1−/− mice, but not wild-type mice, developed clinical signs and demyelination in the CNS, with an increase in DA virus-specific lymphoproliferative responses and a decrease in the IFN-γ/IL-4 ratio. These results are in accord with findings in TMEV-induced demyelination in susceptible mouse strains, such as SJL/J mice: (i) TMEV-specific T-cell responses have been associated with demyelination (25), and (ii) blocking of IFN-γ results in exacerbation of clinical signs in TMEV infection (19). Thus, CD1d-restricted cells, such as NKT cells, could play a role in regulation of the onset of demyelinating disease by modulation of the DA virus-specific T-cell response and cytokine levels.
Susceptible SJL/J mice show clinical and pathological signs of demyelination, 3 to 4 weeks after DA virus infection (after recovery from the acute phase) (42). Thus, up to this stage, DA virus infection in CD1−/− mice and susceptible SJL/J mice is similar clinically and immunologically. However, thereafter, CD1−/− mice recovered from clinical disease and no demyelination or viral persistence was observed at 2 and 6 months p.i. This is in contrast to DA virus-infected SJL/J mice, which show progressive demyelinating disease with virus persistence and high levels of virus-specific immune responses during the late chronic phase. We speculate that the differences in disease courses during the late chronic phase between CD1−/− mice and SJL/J mice could be due to differences in virus persistence in the CNS and/or virus-specific immune responses. We previously demonstrated that, during the chronic phase of DA virus infection in SJL/J mice, virus persistence was correlated with inflammation in the CNS (45). Thus, in SJL/J mice, CNS virus persistence could recruit virus-specific immune cells into the CNS, leading to inflammatory demyelination. In contrast, in CD1−/− mice, the clearance of virus should result in the lack of a driving force for the migration of virus-specific immune cells into the CNS. The low levels of virus infection in CD1−/− mice could be the reason why there were only low levels of CNS inflammation at 5 weeks and 2 months p.i., regardless of the presence of antiviral immune responses in the spleen. High levels of virus persistence can also contribute to enhancement or sustainment of virus-specific immune responses in SJL/J mice. We hypothesize that CD1−/− mice could mount virus-specific encephalitogenic immune cells due to a lack of regulatory NKT cells during the early chronic phase, although only low numbers of virus-specific immune cells were recruited into the CNS due to low levels of virus infection. Then, during the late chronic phase, virus clearance in the CNS could result in a lack of recruitment of inflammatory cells into the CNS as well as a failure in enhancement or sustainment of virus-specific immune responses.
In GDVII virus infection, CD1d-restricted cells appear to be protective. Similarly, during the acute phase of DA virus infection at 1 week p.i., wild-type mice showed greater antiviral lymphoproliferation than CD1−/− mice. Thus, CD1d-restricted cells seem to play an antiviral proinflammatory role during the acute phase of both GDVII and DA virus infections. However, during the chronic phase, wild-type mice showed lower antiviral lymphoproliferation, suggesting an immunosuppressive role for CD1d-restricted cells. We speculate that the different roles for CD1d-restricted cells could be due to (i) functional changes in the CD1d-restricted cells during the course of TMEV infection, such as cytokine production and cytotoxicity, and (ii) different roles for the cytokines produced by CD1d-restricted cells during the early versus the chronic phases of TMEV infection. A change in cytokine production by NKT cells has been shown in MS (2). The modulation of cytokines at different phases in TMEV infection has also been shown to lead to different outcomes (19). To elucidate the precise role for CD1d-restricted NKT cells in TMEV infection, further studies are required, which should include (i) analyses of the phenotypes of cell infiltrates, cytokine/chemokine profiles, and antiviral immune responses in the CNS, (ii) depletion/blocking and modulation of NKT cells and CD1d molecules by antibodies and glycolipid ligands at different time after infection, and (iii) adoptive transfer of NKT cells.
Although the role of CD1d-restricted NKT cells has not been studied in TMEV infection, the role of NK cells in TMEV infection was investigated by Paya et al. (26). In their study, DA virus-infected resistant C57BL/10 mice were treated with NK1.1 antibody or asialo GM1 antibody throughout the course of infection. Both antibody modalities resulted in exacerbation of the acute gray matter disease without an increase in viral titers in the CNS. In addition, only mice treated with asialo GM1 antibody developed demyelination. Since both asialo GM1 and NK1.1 are expressed on NK cells, NKT cells, and some other cell types (5, 33, 39), changes in pathology by asialo GM1 and NK1.1 antibody treatments in Paya et al.'s study could be attributed to depletion of not only NK cells but also NKT cells.
To address the possible regulatory role for NKT cells in TMEV-susceptible strains of mice, in other studies we have treated DA virus-infected SJL/J mice with an antibody against Vα14, an invariant TCR that is expressed on most murine NKT cells (45a). We found that depletion of NKT cells by Vα14 antibody from 3 to 5 weeks p.i. resulted in exacerbation of demyelination with higher numbers of virus-infected cells in the CNS. These results suggest that NKT cells can play a regulatory role at the onset of TMEV-induced demyelination in both resistant and susceptible strains of mice. As yet, we do not know whether the regulatory mechanisms between the two mouse strains are the same. There are differences between our current experiments and the NKT cell depletion experiments in SJL/J mice. For example, we found a difference in virus persistence in SJL/J mice between the NKT-depleted and control groups, but not in BALB/c wild-type and CD1−/− mice infected with DA virus. In addition, cytokine profiles of NKT cells in naïve mice have been shown to differ among mouse strains (31).
Cytokine profiles of NKT cells can also differ, depending on the experimental system, even in the same strain of mouse. In DA virus infection, we found that the IFN-γ/IL-4 ratio was lower in CD1−/− mice than in wild-type BALB/c mice. A similar change in the cytokine profile was reported in mice infected with coxsackievirus B3, which belongs to the family Picornaviridae, and the infected BALB/c CD1−/− mice had a lower IFN-γ/IL-4 ratio in CD4+ spleen cells than infected wild-type BALB/c mice (13). However, in an autoimmune lupus model in BALB/c mice, IL-4 production by T cells was decreased and IFN-γ levels were unchanged in CD1−/− mice compared with wild-type mice, while CD1−/− mice developed exacerbated autoimmune disease (51). Thus, the alterations of the cytokine profile in the lupus nephritis model versus our current TMEV model were different despite the fact that the immune-mediated diseases were exacerbated in CD1−/− mice in both models, suggesting a regulatory role for CD1d-restricted NKT cells.
Although we have discussed that a lack of NKT cells in CD1−/− mice could modulate different TMEV infections, it is possible that other cell types restricted by CD1d may play a role in modulating TMEV infections. Such CD1d-restricted cells, such as certain T cells and Vα14− NKT cells, have been described in other systems, including virus infection (1, 3). For example, CD1d-restricted Vγ4+ cells seem to recognize CD1d in BALB/c mice infected with coxsackievirus B3 (13). In EAE induced with myelin oligodendrocyte glycoprotein (MOG) peptide 35-55, sulfatide-specific CD1d-restricted T cells, distinct from invariant Vα14+ T cells, have been suggested to play a protective role in C57BL/6 mice (16).
It should also be noted that, in our current experiments, we did not address the role of the second subset of NKT cells that express invariant TCRα chains, Vα19-Jα33 in mice or Vα7.2-Jα33 in humans, which are restricted by the MHC class Ib molecule MR1. In EAE induced with MOG35-55 peptide, MR1-restricted Vα19 T cells can decrease clinical severity with increased production of IL-10 (7). MR1-restricted Vα19 T cells have been shown to express NKT cell markers and exist in CD1−/− mice. Thus, in our experiments, it is likely that MR1-restricted Vα19 NKT cells are present both in wild-type and CD1−/− mice, although the MR1-restricted Vα19 T cells exist at very low levels.
In summary, we have demonstrated that, in GDVII virus infection, CD1−/− mice are more susceptible to the fatal acute disease than wild-type mice. In DA virus infection, CD1−/− mice developed demyelinating disease with an increase in neurological deficits and alterations of the DA virus-specific immune response and the cytokine profile compared with wild-type mice. Therefore, CD1d-restricted NKT cells could play a protective/regulatory role in both acute encephalitis induced by GDVII virus and the demyelinating disease induced by DA virus. Our current results suggest that CD1d as well as NKT cells could be future therapeutic targets in viral encephalitis as well as immune-mediated demyelinating diseases, including MS.
Acknowledgments
We thank Jane E. Libbey and Masaru Taniguchi for many helpful discussions and Nikki J. Kirkman, Faris Hasanovic, Andy Luu, Benjamin J. Marble, and Nathan J. Young for excellent technical assistance. We are grateful to Kathleen Borick for preparation of the manuscript.
This work was supported by the National Institutes of Health (NS34497).
Footnotes
Published ahead of print on 6 August 2008.
REFERENCES
- 1.Amano, M., N. Baumgarth, M. D. Dick, L. Brossay, M. Kronenberg, L. A. Herzenberg, and S. Strober. 1998. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: β2-microglobulin-dependent and independent forms. J. Immunol. 1611710-1717. [PubMed] [Google Scholar]
- 2.Araki, M., T. Kondo, J. E. Gumperz, M. B. Brenner, S. Miyake, and T. Yamamura. 2003. Th2 bias of CD4+ NKT cells derived from multiple sclerosis in remission. Int. Immunol. 15279-288. [DOI] [PubMed] [Google Scholar]
- 3.Baron, J. L., L. Gardiner, S. Nishimura, K. Shinkai, R. Locksley, and D. Ganem. 2002. Activation of a nonclassical NKT cell subset in a transgenic mouse model of hepatitis B virus infection. Immunity 16583-594. [DOI] [PubMed] [Google Scholar]
- 4.Burgleson, F. G., T. M. Chambers, and D. L. Wiedbrauk. 1992. Introduction to quantal virus assays, p. 53-57. In F. G. Burleson, T. M. Chambers, and D. L. Wiedbrauk (ed.), Virology: a laboratory manual. Academic Press, Inc., San Diego, CA.
- 5.Byrne, P., P. McGuirk, S. Todryk, and K. H. Mills. 2004. Depletion of NK cells results in disseminating lethal infection with Bordetella pertussis associated with a reduction of antigen-specific Th1 and enhancement of Th2, but not Tr1 cells. Eur. J. Immunol. 342579-2588. [DOI] [PubMed] [Google Scholar]
- 6.Campos, R. A., M. Szczepanik, A. Itakura, M. Akahira-Azuma, S. Sidobre, M. Kronenberg, and P. W. Askenase. 2003. Cutaneous immunization rapidly activates liver invariant Vα14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J. Exp. Med. 1981785-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Croxford, J. L., S. Miyake, Y.-Y. Huang, M. Shimamura, and T. Yamamura. 2006. Invariant Vα19i T cells regulate autoimmune inflammation. Nat. Immunol. 7987-994. [DOI] [PubMed] [Google Scholar]
- 8.Exley, M. A., N. J. Bigley, O. Cheng, S. M. A. Tahir, S. T. Smiley, Q. L. Carter, H. F. Stills, M. J. Grusby, Y. Koezuka, M. Taniguchi, and S. P. Balk. 2001. CD1d-reactive T-cell activation leads to amelioration of disease caused by diabetogenic encephalomyocarditis virus. J. Leukoc. Biol. 69713-718. (Erratum, 70:340.) [PubMed] [Google Scholar]
- 9.Furlan, R., A. Bergami, D. Cantarella, E. Brambilla, M. Taniguchi, P. Dellabona, G. Casorati, and G. Martino. 2003. Activation of invariant NKT cells by αGalCer administration protects mice from MOG35-55-induced EAE: critical roles for administration route and IFN-γ. Eur. J. Immunol. 331830-1838. [DOI] [PubMed] [Google Scholar]
- 10.Gausling, R., C. Trollmo, and D. A. Hafler. 2001. Decreases in interleukin-4 secretion by invariant CD4−CD8− Vα24JαQ T cells in peripheral blood of patients with relapsing-remitting multiple sclerosis. Clin. Immunol. 9811-17. [DOI] [PubMed] [Google Scholar]
- 11.Godfrey, D. I., and M. Kronenberg. 2004. Going both ways: immune regulation via CD1d-dependent NKT cells. J. Clin. Investig. 1141379-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grant, E. P., M. Degano, J.-P. Rosat, S. Stenger, R. L. Modlin, I. A. Wilson, S. A. Porcelli, and M. B. Brenner. 1999. Molecular recognition of lipid antigens by T cell receptors. J. Exp. Med. 189195-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huber, S., D. Sartini, and M. Exley. 2003. Role of CD1d in coxsackievirus B3-induced myocarditis. J. Immunol. 1703147-3153. [DOI] [PubMed] [Google Scholar]
- 14.Illés, Z., T. Kondo, J. Newcombe, N. Oka, T. Tabira, and T. Yamamura. 2000. Differential expression of NK T cell V α24JαQ invariant TCR chain in the lesions of multiple sclerosis and chronic inflammatory demyelinating polyneuropathy. J. Immunol. 1644375-4381. [DOI] [PubMed] [Google Scholar]
- 15.Ito, M., B. M. Blumberg, D. J. Mock, A. D. Goodman, A. B. Moser, H. W. Moser, K. D. Smith, and J. M. Powers. 2001. Potential environmental and host participants in the early white matter lesion of adreno-leukodystrophy: morphologic evidence for CD8 cytotoxic T cells, cytolysis of oligodendrocytes, and CD1-mediated lipid antigen presentation. J. Neuropathol. Exp. Neurol. 601004-1019. [DOI] [PubMed] [Google Scholar]
- 16.Jahng, A., I. Maricic, C. Aguilera, S. Cardell, R. C. Halder, and V. Kumar. 2004. Prevention of autoimmunity by targeting a distinct, noninvariant CD1d-reactive T cell population reactive to sulfatide. J. Exp. Med. 199947-957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jahng, A. W., I. Maricic, B. Pedersen, N. Burdin, O. Naidenko, M. Kronenberg, Y. Koezuka, and V. Kumar. 2001. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J. Exp. Med. 1941789-1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, T. R., S. Hong, L. Van Kaer, Y. Koezuka, and B. S. Graham. 2002. NK T cells contribute to expansion of CD8+ T cells and amplification of antiviral immune responses to respiratory syncytial virus. J. Virol. 764294-4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, B. S., A. C. Fuller, and C.-S. Koh. 2005. Cytokines, chemokines and adhesion molecules in TMEV-IDD, p. 659-671. In E. Lavi and C. S. Constantinescu (ed.), Experimental models of multiple sclerosis. Springer Science+Business Media, Inc., New York, NY.
- 20.Makino, Y., R. Kanno, T. Ito, K. Higashino, and M. Taniguchi. 1995. Predominant expression of invariant Vα14+ TCR α chain in NK1.1+ T cell populations. Int. Immunol. 71157-1161. [DOI] [PubMed] [Google Scholar]
- 21.Mars, L. T., V. Laloux, K. Goude, S. Desbois, A. Saoudi, L. Van Kaer, H. Lassmann, A. Herbelin, A. Lehuen, and R. S. Liblau. 2002. Vα14-J.α281 NKT cells naturally regulate experimental autoimmune encephalomyelitis in nonobese diabetic mice. J. Immunol. 1686007-6011. [DOI] [PubMed] [Google Scholar]
- 22.Melvold, R. W., D. M. Jokinen, R. L. Knobler, and H. L. Lipton. 1987. Variations in genetic control of susceptibility to Theiler's murine encephalomyelitis virus (TMEV)-induced demyelinating disease. I. Differences between susceptible SJL/J and resistant BALB/c strains map near the T cell β-chain constant gene on chromosome 6. J. Immunol. 1381429-1433. [PubMed] [Google Scholar]
- 23.Mieza, M. A., T. Itoh, J. Q. Cui, Y. Makino, T. Kawano, K. Tsuchida, T. Koike, T. Shirai, H. Yagita, A. Matsuzawa, H. Koseki, and M. Taniguchi. 1996. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J. Immunol. 1564035-4040. [PubMed] [Google Scholar]
- 24.Miyamoto, K., S. Miyake, and T. Yamamura. 2001. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature 413531-534. [DOI] [PubMed] [Google Scholar]
- 25.Olson, J. K., and S. D. Miller. 2005. The role of T cells and the innate immune system in the pathogenesis of Theiler's virus demyelinating disease, p. 645-657. In E. Lavi and C. S. Constantinescu (ed.), Experimental models of multiple sclerosis. Springer Science+Business Media Inc., New York, NY.
- 26.Paya, C. V., A. K. Patick, P. J. Leibson, and M. Rodriguez. 1989. Role of natural killer cells as immune effectors in encephalitis and demyelination induced by Theiler's virus. J. Immunol. 14395-102. [PubMed] [Google Scholar]
- 27.Potvin, D. M., D. W. Metzger, W. T. Lee, D. N. Collins, and A. I. Ramsingh. 2003. Exogenous interleukin-12 protects against lethal infection with coxsackievirus B4. J. Virol. 778272-8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roberts, T. J., Y. Lin, P. M. Spence, L. Van Kaer, and R. R. Brutkiewicz. 2004. CD1d1-dependent control of the magnitude of an acute antiviral immune response. J. Immunol. 1723454-3461. [DOI] [PubMed] [Google Scholar]
- 29.Rodriguez, M., J. Leibowitz, and C. S. David. 1986. Susceptibility to Theiler's virus-induced demyelination. Mapping of the gene within the H-2D region. J. Exp. Med. 163620-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roussarie, J. P., C. Ruffié, and M. Brahic. 2007. The role of myelin in Theiler's virus persistence in the central nervous system. PLoS Pathog. 3212-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Serizawa, I., Y. Koezuka, H. Amao, T. R. Saito, and K. W. Takahashi. 2000. Functional natural killer T cells in experimental mouse strains, including NK1.1− strains. Exp. Anim. 49171-180. [DOI] [PubMed] [Google Scholar]
- 32.Singh, A. K., M. T. Wilson, S. Hong, D. Olivares-Villagómez, C. Du, A. K. Stanic, S. Joyce, S. Sriram, Y. Koezuka, and L. Van Kaer. 2001. Natural killer T cell activation protects mice against experimental autoimmune encephalomyelitis. J. Exp. Med. 1941801-1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slifka, M. K., R. R. Pagarigan, and J. L. Whitton. 2000. NK markers are expressed on a high percentage of virus-specific CD8+ and CD4+ T cells. J. Immunol. 1642009-2015. [DOI] [PubMed] [Google Scholar]
- 34.Smiley, S. T., M. H. Kaplan, and M. J. Grusby. 1997. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science 275977-979. [DOI] [PubMed] [Google Scholar]
- 35.Stein-Streilein, J. 2003. Invariant NKT cells as initiators, licensors, and facilitators of the adaptive immune response. J. Exp. Med. 1981779-1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi, M., K. Seino, and T. Nakayama. 2003. The NKT cell system: bridging innate and acquired immunity. Nat. Immunol. 41164-1165. [DOI] [PubMed] [Google Scholar]
- 37.Teige, A., I. Teige, S. Lavasani, R. Bockermann, E. Mondoc, R. Holmdahl, and S. Issazadeh-Navikas. 2004. CD1-dependent regulation of chronic central nervous system inflammation in experimental autoimmune encephalomyelitis. J. Immunol. 172186-194. [DOI] [PubMed] [Google Scholar]
- 38.Theil, D. J., I. Tsunoda, J. E. Libbey, T. J. Derfuss, and R. S. Fujinami. 2000. Alterations in cytokine but not chemokine mRNA expression during three distinct Theiler's virus infections. J. Neuroimmunol. 10422-30. [DOI] [PubMed] [Google Scholar]
- 39.Trambley, J., A. W. Bingaman, A. Lin, E. T. Elwood, S.-Y. Waitze, J. Ha, M. M. Durham, M. Corbascio, S. R. Cowan, T. C. Pearson, and C. P. Larsen. 1999. Asialo GM1+ CD8+ T cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Investig. 1041715-1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsunoda, I., and R. S. Fujinami. 1999. Theiler's murine encephalomyelitis virus, p. 517-536. In R. Ahmed and I. S. Y. Chen (ed.), Persistent viral infections. John Wiley & Sons, Ltd., Chichester, West Sussex, England.
- 41.Tsunoda, I., Y. Iwasaki, H. Terunuma, K. Sako, and Y. Ohara. 1996. A comparative study of acute and chronic diseases induced by two subgroups of Theiler's murine encephalomyelitis virus. Acta Neuropathol. (Berlin) 91595-602. [DOI] [PubMed] [Google Scholar]
- 42.Tsunoda, I., L.-Q. Kuang, J. E. Libbey, and R. S. Fujinami. 2003. Axonal injury heralds virus-induced demyelination. Am. J. Pathol. 1621259-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsunoda, I., C. I. B. Kurtz, and R. S. Fujinami. 1997. Apoptosis in acute and chronic central nervous system disease induced by Theiler's murine encephalomyelitis virus. Virology 228388-393. [DOI] [PubMed] [Google Scholar]
- 44.Tsunoda, I., J. E. Libbey, L.-Q. Kuang, E. J. Terry, and R. S. Fujinami. 2005. Massive apoptosis in lymphoid organs in animal models for primary and secondary progressive multiple sclerosis. Am. J. Pathol. 1671631-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsunoda, I., T. Tanaka, Y. Saijoh, and R. S. Fujinami. 2007. Targeting inflammatory demyelinating lesions to sites of Wallerian degeneration. Am. J. Pathol. 1711563-1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45a.Tsunoda, I., T. Tanaka, M. Taniguchi, and R. S. Fujinami. Contrasting roles for Vα14+ NKT cells in a viral model for multiple sclerosis. J. Neurovirol., in press. [DOI] [PMC free article] [PubMed]
- 46.Tsunoda, I., T. Tanaka, E. J. Terry, and R. S. Fujinami. 2007. Contrasting roles for axonal degeneration in an autoimmune versus viral model for multiple sclerosis: when can axonal injury be beneficial? Am. J. Pathol. 170214-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsunoda, I., Y. Wada, J. E. Libbey, T. S. Cannon, F. G. Whitby, and R. S. Fujinami. 2001. Prolonged gray matter disease without demyelination caused by Theiler's murine encephalomyelitis virus with a mutation in VP2 puff B. J. Virol. 757494-7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Vliet, H. J. J., B. M. E. von Blomberg, N. Nishi, M. Reijm, A. E. Voskuyl, A. A. van Bodegraven, C. H. Polman, T. Rustemeyer, P. Lips, A. J. M. van den Eertwegh, G. Giaccone, R. J. Scheper, and H. M. Pinedo. 2001. Circulating Vα24+ Vβ11+ NKT cell numbers are decreased in a wide variety of diseases that are characterized by autoreactive tissue damage. Clin. Immunol. 100144-148. [DOI] [PubMed] [Google Scholar]
- 49.Van Kaer, L. 2007. NKT cells: T lymphocytes with innate effector functions. Curr. Opin. Immunol. 19354-364. [DOI] [PubMed] [Google Scholar]
- 50.Yamamura, T., K. Sakuishi, Z. Illés, and S. Miyake. 2007. Understanding the behavior of invariant NKT cells in autoimmune diseases. J. Neuroimmunol. 1918-15. [DOI] [PubMed] [Google Scholar]
- 51.Yang, J.-Q., A. K. Singh, M. T. Wilson, M. Satoh, A. K. Stanic, J.-J. Park, S. Hong, S. D. Gadola, A. Mizutani, S. R. Kakumanu, W. H. Reeves, V. Cerundolo, S. Joyce, L. Van Kaer, and R. R. Singh. 2003. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J. Immunol. 1712142-2153. [DOI] [PubMed] [Google Scholar]