Abstract
The role of tumor necrosis factor alpha (TNF-α) was evaluated for CXCL10-deficient (CXCL10−/−) mice which succumbed to genital herpes simplex virus type 2 (HSV-2) infection and possessed elevated levels of virus and TNF-α but not other cytokines in the central nervous system (CNS) and vaginal tissue within the first 7 days following virus exposure. Anti-TNF-α but not control antibody treatment offsets the elevated mortality rate of CXCL10−/− mice, despite increased CNS viral titers. In addition, TNF-α neutralization suppressed recruitment of leukocyte subpopulations into the CNS, which is associated with reduced CCL2 and CXCL9 expression. Collectively, the results implicate TNF-α as the principal mediator of mortality in response to genital HSV-2 infection.
Herpes simplex virus type 2 (HSV-2) is one of the most common causes of genital ulcer disease that can result in fatal central nervous system (CNS) infection in humans (4, 14, 30, 49). Nearly 600,000 to 800,000 cases are reported annually, such that as many as 40 to 60 million individuals are infected with HSV-2 in the United States (World Health Organization [http://www.who.int/vaccine_research/diseases/soa_std/en/index3.html]). During replication in the vaginal epithelium cells, the virus enters sensory nerve endings and, by retrograde transport, traffics to sacral ganglia, where it establishes a latent infection in resident neurons (8, 19). Following reactivation, the virus can traffic by anterograde transport and cause recurrent infection at the original portal of entry as well as adjacent sites (42). In the immunocompromised patient as well as newborns, the infection can be quite severe, ultimately resulting in death (20, 22, 46). Experimental evidence suggests that elevated levels of tumor necrosis factor alpha (TNF-α) may be a key factor in neuropathogenesis following viral infection (16, 48).
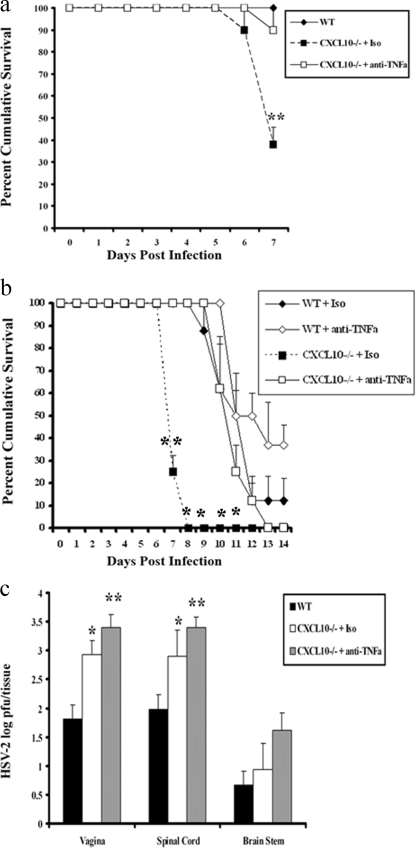
In response to CNS virus infection, TNF-α is produced by astrocytes, microglia, neurons, and infiltrating hematopoietic cells (34, 51), acting through two structurally related cell surface receptors, TNFR1 (p55) and TNFR2 (p75), constitutively expressed on neurons and glial cells (35, 48). TNF-α regulates leukocyte trafficking by inducing a number of factors, including cell adhesion molecules (ICAM-1 and VCAM-1), selectins (E and P selectins), and chemokine expression (17, 18, 31, 47). In addition, it regulates differentiation of NK cells (24), a cell population critical in the control of genital HSV-2 infection (44). Relative to acute HSV-1 infection, TNF-α suppresses virus replication and dissemination into the CNS by means which appear to be independent of either TNFR1 or TNFR2 (25, 41). At the cellular level, TNF-α synergizes with gamma interferon in the induction of nitric oxide (36), a molecule that also has potent anti-HSV action (6, 29). Although TNF-α has many positive antiviral attributes, as noted above, it can also be detrimental to brain function, possibly through the induction of high-mobility group box 1 protein expression (11, 21, 38). Recently, it was found that CXCL10-deficient (CXCL10−/−) mice are highly susceptible to genital HSV-2 infection, based on increased mortality and virus titer, which are associated with elevated TNF-α (45) but not interleukin-6 (data not shown). It was hypothesized that the increase in HSV-2-mediated mortality of CXCL10−/− mice was due to excessive expression of TNF-α as opposed to an increase in virus titer within the CNS. In order to test this hypothesis, anti-TNF-α antibody (Ab) was administered to HSV-2-infected CXCL10−/− mice. Sixty percent of isotype control Ab-treated CXCL10−/− mice succumbed to infection on day 7 postinfection, which was significantly (P < 0.01) higher than levels for anti-TNF-α Ab-treated CXCL10−/− or nontreated wild-type (WT) mice (Fig. 1a). To determine whether survival lasted throughout the acute infection, mice were monitored for 14 days after infection. In this case, late mortality among anti-TNF-α Ab-treated CXCL10−/− mice was observed (Fig. 1b). The mortality of anti-TNF-α Ab-treated CXCL10−/− mice was delayed to day 12 postinfection, compared to isotype control Ab-treated CXCL10−/− mice, in which all mice succumbed by day 8 postinfection (Fig. 1b). Similarly, to CXCL10−/− mice, HSV-2 WT mice benefitted by anti-TNF-α Ab treatment, in comparison to isotype control Ab-treated WT mice (Fig. 1b). To determine what impact the administration of anti-TNF-α Ab had on virus replication, anti-TNF-α Ab-treated CXCL10−/− mice were compared to isotypic control Ab-treated and nontreated WT mice, and levels of virus recovered in the vaginal tissue, spinal cord, and brain stem were measured. The administration of anti-TNF-α Ab did not modify the amount of infectious virus recovered in the vaginal tissue, spinal cord, or brain stem of CXCL10−/− mice in comparison to that for isotypic control Ab-treated, HSV-2-infected CXCL10−/− mice (Fig. 1c). In fact, a trend for an increase in virus disseminated to all tissues surveyed was observed, consistent with the notion that TNF-α Ab suppresses HSV replication (25, 41). Both treated groups of CXCL10−/− mice possessed significantly more infectious virus than WT mice.
FIG. 1.
Anti-TNF-α treatment offsets the elevated mortality rate of CXCL10−/− mice. (a) WT and CXCL10−/− mice (9) (n = 18 mice/group) were rendered susceptible to genital HSV-2 by using Depo-Provera (37) and infected with HSV-2 (2000 PFU/vagina). On day 5 postinfection, 100 μg of anti-mouse TNF-α or isotypic (Iso) control Ab was administered retro-orbitally into HSV-2-infected CXCL10−/− mice. The mice were monitored and recorded for survival up to day 7 postinfection. The results are shown as means ± standard errors of the means (SEM) of results from six experiments. (b) The survival study was repeated, including WT mice with anti-mouse TNF-α or isotypic control Ab treatment, and mice were monitored and recorded for survival up to day 14 postinfection. The results are shown as means ± SEM of results from three experiments (n = 8 mice/group). (c) Mice were exsanguinated, and vaginal tissue, spinal cords, and brain stems were removed, processed, and assayed for viral titer by a standard plaque assay (15). The viral titers are expressed as mean log numbers of PFU ± SEM summarized from three experiments (n = 9 mice/group). **, P values of <0.01; *, P values of <0.05 for comparison of WT to anti-TNF-α Ab-treated CXCL10−/− mice or WT to isotypic control Ab-treated CXCL10−/− mice, as determined by analysis of variance (ANOVA) and Tukey's post hoc t test.
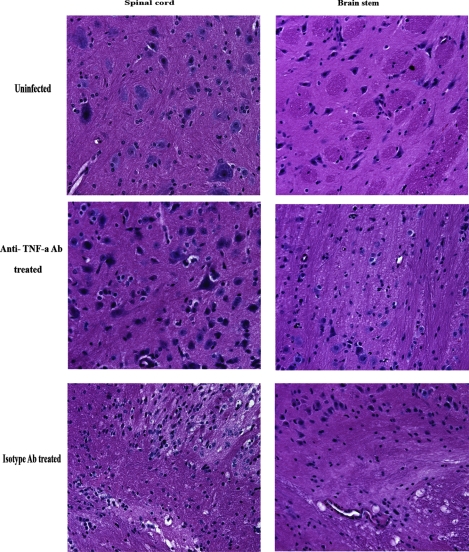
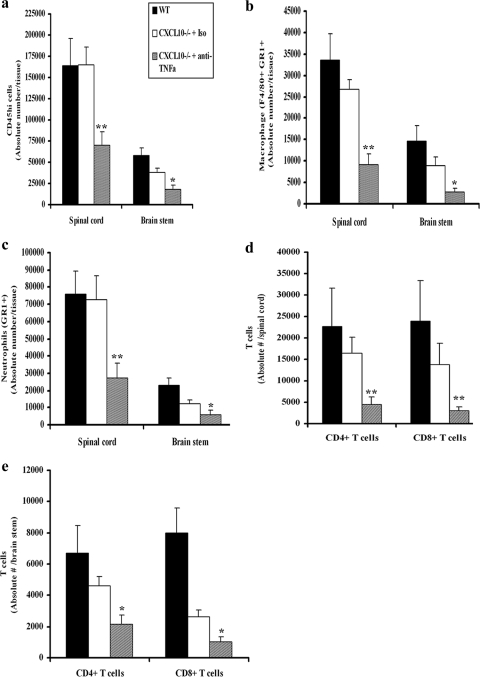
Adhesion molecules (e.g., selectins, ICAM-1, and VCAM-1) which facilitate trafficking of leukocytes into inflamed tissue and cause leakiness of endothelial lining are induced or upregulated by TNF-α (1, 5). Since neutrophils, macrophages, and T cells are thought to contribute to immune surveillance of HSV-2 (32, 33, 36) as well as serve as a source of inflammatory mediators that can harm the nervous system, it was reasoned that the application of anti-TNF-α Ab to HSV-2-infected mice would prevent or suppress the extravasation of leukocytes into the CNS. In support of this idea, significantly reduced neuropathology, as evidenced by a reduced level of infiltrating cells, degeneration of tissue, and loss of neurons, was observed in the brain stems and spinal cords of anti-TNF-α Ab-treated CXCL10−/− mice, compared to levels for isotype Ab-treated CXCL10−/− mice (Fig. 2). Consistent with this observation, there were significant reductions in the absolute numbers of CD45[sup]hi leukocytes, activated macrophages (defined as F4/80+ Gr1+), and neutrophils (F4/80− Gr1+) residing in the CNSs of anti-TNF-α Ab-treated mice, compared to levels for isotype control Ab-treated CXCL10−/− or nontreated WT mice on day 7 postinfection (Fig. 3a to c). Similarly, the absolute numbers of CD4+ and CD8+ T cells were also significantly reduced in the CNSs of anti-TNF-α Ab-treated, HSV-2-infected CXCL10−/− mice (Fig. 3d and e). No significant difference was found in the numbers of leukocytes residing in the CNSs of WT and isotype control Ab-treated CXCL10−/− mice, except for CD8+ T cells, which were significantly reduced in the brain stems of the HSV-2-infected, isotype control Ab-treated CXCL10−/− group (Fig. 3b). These results are consistent with other findings showing that abrogation of TNF-α or TNFR1 expression significantly reduces leukocyte infiltration (13, 43).
FIG. 2.
Reduced inflammatory pathology and infiltrates in the CNSs of anti-TNF-α treated CXCL10−/− mice. Following Depo-Provera treatment, CXCL10−/− mice (n = 3/group) were infected with HSV-2 (2,000 PFU/vagina). On day 5 postinfection, 100 μg of anti-mouse TNF-α or isotypic control Ab was administered retro-orbitally into HSV-2-infected CXCL10−/− mice. On day 7 postinfection, mice were exsanguinated and the brain stems and spinal cords were removed from each mouse and processed for histological analysis following hematoxylin and eosin staining. Tissues from uninfected CXCL10−/− mice were used as a control. Magnification, ×400.
FIG. 3.
Macrophage, neutrophil, and T-cell infiltration into the CNS. Depo-Provera-treated WT and CXCL10−/− mice (n = 9/group) were infected with HSV-2 (2,000 PFU/vagina). On day 5 postinfection, 100 μg of anti-mouse TNF-α or isotypic control Ab (Iso) was administered intravenously (retro-orbitally) into HSV-2-infected CXCL10−/− mice. On day 7 postinfection, the mice were exsanguinated and the brain stem and spinal cord were removed from each mouse, processed, and analyzed for total infiltrating leukocytes (i.e., CD45HI) (a), activated macrophages (i.e., F4/80+ Gr1+) (b), neutrophil (c), CD3+ CD4+ T-cell or CD3+ CD8+ T-cell (spinal cord) (d), and CD3+ CD4+ T-cell or CD3+ CD8+ T-cell (brain stem) (e) content by flow cytometry (44). Bars represent the means ± SEM from three experiments. **, P values of <0.01; *, P values of <0.05 for comparison of the anti-TNF-α Ab-treated CXCL10−/− mice to the other two groups, as determined by ANOVA and Tukey's post hoc t test.
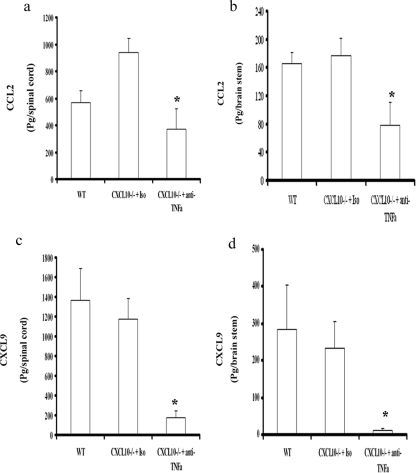
TNF-α can induce CCL2 expression and mobilize monocytes/macrophages and T cells through the G protein-coupled, seven-transmembrane-domain chemokine receptor CCR2 (2, 3, 26, 27). Likewise, neutrophils are highly responsive to another TNF-α-induced chemokine, CXCL1 (23, 40), and CXCL9 and CXCL10 are potent chemoattractant molecules for T cells (12). Since neutrophils and macrophages, along with T cells, were reduced in the brain stems and spinal cords of HSV-2-infected CXCL10−/− mice treated with anti-TNF-α Ab, expression of CCL2, CXCL1, and CXCL9 in the CNSs of the infected mice was assessed as a means to identify a possible scenario responsible for the dramatic change in leukocyte influx. CCL2 expression in the spinal cords and brain stems of anti-TNF-α Ab-treated, HSV-2-infected CXCL10−/− mice was reduced in comparison to that for the isotypic control Ab-treated, HSV-2-infected animals, similar to the level found in infected WT mice (Fig. 4a and b). There was no significant difference in the expression levels of CXCL1 in the brain stems or spinal cords between the anti-TNF-α Ab-treated group and either the isotypic control or WT mice (data not shown). CXCL9 levels were also reduced in the anti-TNF-α Ab-treated, HSV-2-infected mice in comparison to those in HSV-2-infected WT mice (Fig. 4c and d).
FIG. 4.
CCL2 and CXCL9 levels in the CNSs of mice. Depo-Provera-treated WT and CXCL10−/− mice (n = 12/group) were infected with HSV-2 (2,000 PFU/vagina). On day 5 postinfection, 100 μg of anti-mouse TNF-α or isotypic control Ab was administered intravenously (retro-orbitally) into HSV-2-infected CXCL10−/− mice. On day 7 postinfection, the mice were exsanguinated and the spinal cord and brain stem were removed from each mouse, processed, and analyzed for CCL2 (spinal cord) (a), CCL2 (brain stem) (b), CXCL9 (spinal cord) (c), and CXCL9 (brain stem) (d) content by an enzyme-linked immunosorbent assay. Samples were run in duplicate and analyzed along with a known standard provided for each analyte by the manufacturer, with the unknown amount extrapolated from the standard curve generated for each assay. The bars represent the means ± SEM of results from three experiments for the indicated chemokine. *, P values of <0.05 for comparison of the isotypic control Ab-treated CXCL10−/− mice to the other two groups (A) or for comparison of the anti-TNF-α Ab-treated CXCL10−/− mice to the WT mice (B), as determined by ANOVA and Tukey's post hoc t test.
Of the chemokines induced by TNF-α, CXCL9 operates through CXCR3 and has been found to be instrumental in chemotaxis of CD8+ T cells to the CNS following genital HSV-2 infection (45), whereas CCL2 selectively attracts immune cells, including macrophages, monocytes, polymorphonuclear leukocytes, and T cells that bear the receptor CCR2 (39). The association of CCL2 and CCR2 in neuropathogenesis has also been demonstrated for other primate and rodent models of neuronal injury or virus infection (10, 28, 50). We also find strong expression of CCL2 in both the spinal cords and the brain stems of HSV-2-infected CXCL10−/− mice, which is associated with an increase in TNF-α (45). It is tempting to speculate that a delay in the mortality of HSV-2 infected CXCL10−/− mice treated with anti-TNF-α Ab is due to a reduction in infiltrating leukocytes as a result of reduced CCL2 expression, including macrophages that secrete a variety of soluble factors that can result in neuropathology (including TNF-α and inducible nitric oxide synthase), consistent with a recent observation for an experimental autoimmune encephalomyelitis model (7). Therefore, the absence of CXCL10 appears to be overcompensated for by the ensuing immune response, with TNF-α having a lead role in the neuropathology, as opposed to a direct effect of virus within the CNS.
Acknowledgments
We acknowledge Andrew Luster for the CXCL10−/− mice and are thankful to Todd Wuest and Gabriel Nyugen for their excellent technical help.
This work was supported by grant AI067309 and Core Grant EY12190 from the National Institutes of Health.
Footnotes
Published ahead of print on 6 August 2008.
REFERENCES
- 1.Bevilacqua, M. P. 1993. Endothelial-leukocyte adhesion molecules. Annu. Rev. Immunol. 11767-804. [DOI] [PubMed] [Google Scholar]
- 2.Boring, L., J. Gosling, F. S. Monteclaro, A. J. Lusis, C. L. Tsou, and I. F. Charo. 1996. Molecular cloning and functional expression of murine JE (monocyte chemoattractant protein 1) and murine macrophage inflammatory protein 1alpha receptors: evidence for two closely linked C-C chemokine receptors on chromosome 9. J. Biol. Chem. 2717551-7558. [DOI] [PubMed] [Google Scholar]
- 3.Campbell, S. J., V. H. Perry, F. J. Pitossi, A. G. Butchart, M. Chertoff, S. Waters, R. Dempster, and D. C. Anthony. 2005. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am. J. Pathol. 1661487-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carr, D. J. J., and L. Tomanek. 2006. Herpes simplex virus and the chemokines that mediate the inflammation. Curr. Top. Microbiol. Immunol. 30347-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi, J., D. R. Enis, K. P. Koh, S. L. Shiao, and J. S. Pober. 2004. T lymphocyte-endothelial cell interactions. Annu. Rev. Immunol. 22683-709. [DOI] [PubMed] [Google Scholar]
- 6.Croen, K. D. 1993. Evidence for an antiviral effect of nitric oxide. Inhibition of herpes simplex virus type 1 replication. J. Clin. Investig. 912446-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dogan, R. E., A. Elhofy, and W. J. Karpus. 2008. Production of CCL2 by central nervous system cells regulates development of murine experimental autoimmune encephalomyelitis through the recruitment of TNF- and iNOS-expressing macrophages and myeloid dendritic cells. J. Immunol. 1807376-7384. [DOI] [PubMed] [Google Scholar]
- 8.Duerst, R. J., and L. A. Morrison. 2003. Innate immunity to herpes simplex virus type 2. Viral Immunol. 16475-490. [DOI] [PubMed] [Google Scholar]
- 9.Dufour, J. H., M. Dziejman, M. T. Liu, J. H. Leung, T. E. Lane, and A. D. Luster. 2002. IFN-γ-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 1683195-3204. [DOI] [PubMed] [Google Scholar]
- 10.Eugenin, E. A., K. Osiecki, L. Lopez, H. Goldstein, T. M. Caldron, and J. W. Berman. 2006. CCL2/monocyte chemoattractant protein-1 mediates enhanced transmigration of human immunodeficiency virus (HIV)-infected leukocytes across the blood-brain barrier: A potential mechanism of HIV-CNS invasion and NeuroAIDS. J. Neurosci. 261098-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faraco, G., S. Fossati, M. E. Bianchi, M. Patrone, M. Pedrazzi, B. Sparatore, F. Moroni, and A. Chiarugi. 2007. High mobility group box 1 protein is released by neural cells upon different stresses and worsens ischemic neurodegeneration in vitro and in vivo. J. Neurochem. 103590-603. [DOI] [PubMed] [Google Scholar]
- 12.Farber, J. M. 1997. Mig and IP-10: CXC chemokines that target lymphocytes. J. Leukoc. Biol. 61246-257. [PubMed] [Google Scholar]
- 13.Gimenez, M. A., J. Sim, A. S. Archambault, R. S. Klein, and J. H. Russell. 2006. A tumor necrosis factor receptor 1-dependent conversation between central nervous system-specific T cells and the central nervous system is required for inflammatory infiltration of the spinal cord. Am. J. Pathol. 1681200-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harandi, A. M., B. Svennerholm, J. Holmgren, and K. Eriksson. 2001. Differential roles of B cells and IFN-γ-secreting CD4+ T cells in innate and adaptive immune control of genital herpes simplex virus type 2 infection in mice. J. Gen. Virol. 82845-853. [DOI] [PubMed] [Google Scholar]
- 15.Harle, P., V. F. Cull, M. P. Agbaga, R. F. Silverman, B. R. Williams, C. James, and D. J. Carr. 2002. Differential effect of murine α/β interferon transgenes on antagonization of herpes simplex virus type 1 replication. J. Virol. 766558-6567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herbein, G., and W. A. O'Brien. 2000. Tumor necrosis factor (TNF)-alpha and TNF receptors in viral pathogenesis. Proc. Soc. Exp. Biol. Med. 223241-257. [DOI] [PubMed] [Google Scholar]
- 17.Horie, Y., R. P. Chevenak, R. Wolf, M. E. Gerritsen, D. C. Anderson, S. Komatsu, and N. Granger. 1997. Lymphocytes mediate TNF-α-induced endothelial cell adhesion expression. Studies on SCID and RAG-1 mutant mice. J. Immunol. 1595053-5062. [PubMed] [Google Scholar]
- 18.Hornung, F., G. Scala, and M. J. Lenardo. 2000. TNF-α-induced secretion of C-C chemokines modulates C-C chemokine receptor 5 expression on peripheral blood lymphocytes. J. Immunol. 1646180-6187. [DOI] [PubMed] [Google Scholar]
- 19.Inagaki, K., T. Daikoku, F. Goshima, and Y. Nashiyama. 2000. Impaired induction of protective immunity by highly virulent herpes simplex type 2 in a murine model of genital herpes. Arch. Virol. 1451989-2002. [DOI] [PubMed] [Google Scholar]
- 20.Kesson, A. M. 2001. Management of neonatal herpes simplex virus infection. Paediatr. Drugs 381-90. [DOI] [PubMed] [Google Scholar]
- 21.Kim, J. B., J. Choi, Y. M. Yu, K. Nam, C. S. Piao, S. W. Kim, M. H. Lee, P. L. Han, J. S. Park, and J. K. Lee. 2006. HMGB1, a novel cytokine-like mediator linking acute neuronal death and delayed neuroinflammation in the post-ischemic brain. J. Neurosci. 266413-6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimberlin, D. 2004. Herpes simplex virus, meningitis and encephalitis in neonates. Herpes 1165A-76A. [PubMed] [Google Scholar]
- 23.Kobayashi, Y. 2008. The role of chemokines in neutrophil biology. Front. Biosci. 132400-2407. [DOI] [PubMed] [Google Scholar]
- 24.Locksley, R. M., N. Killeen, and M. J. Lenardo. 2001. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell 104487-501. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg, P., P. V. Welander, C. K. Edwards III, N. Van Rooijen, and E. Cantin. 2007. Tumor necrosis factor (TNF) protects resistant C57BL/6 mice against herpes simplex virus-induced encephalitis independently of signaling via TNF receptor 1 or 2. J. Virol. 811451-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo, Y., J. Laning, M. Hayashi, P. R. Hancock, B. Rollins, and M. E. Dorf. 1994. Serologic analysis of the mouse beta chemokine JE/monocyte chemoattractant protein-1. J. Immunol. 1533708-3716. [PubMed] [Google Scholar]
- 27.Luster, A. D., and M. E. Rothenberg. 1997. Role of the monocyte chemoattractant protein and eotaxin subfamily of chemokines in allergic inflammation. J. Leukoc. Biol. 62620-633. [DOI] [PubMed] [Google Scholar]
- 28.Ma, M., T. Wei, L. Boring, I. F. Charo, R. M. Ransohoff, and L. B. Jakeman. 2002. Monocyte recruitment and myelin removal are delayed following spinal cord injury in mice with CCR2 chemokine receptor deletion. J. Neurosci. Res. 68691-702. [DOI] [PubMed] [Google Scholar]
- 29.MacLean, A., X.-Q. Wei, F.-P. Huang, U. A. H. Al-Alem, W. Chan, and F. Y. Liew. 1998. Mice lacking inducible nitric-oxide synthase are more susceptible to herpes simplex virus infection despite enhanced Th1 cell responses. J. Gen. Virol. 79825-830. [DOI] [PubMed] [Google Scholar]
- 30.MasCasullo, V., E. Fam, M. J. Keller, and B. C. Herold. 2005. Role of mucosal immunity preventing genital herpes infection. Viral Immunol. 18595-606. [DOI] [PubMed] [Google Scholar]
- 31.Mattila, P., M. L. Majuri, P. S. Mattila, and R. Renkonen. 1992. TNF alpha-induced expression of endothelial adhesion molecules, ICAM-1 and VCAM-1, is linked to protein kinase C activation. Scand. J. Immunol. 36159-165. [DOI] [PubMed] [Google Scholar]
- 32.McDermott, M. R., C. H. Goldsmith, K. L. Rosenthal, and L. J. Brais. 1989. T lymphocytes in genital lymph nodes protect mice from intravaginal infection with herpes simplex virus type 2. J. Infect. Dis. 159460-466. [DOI] [PubMed] [Google Scholar]
- 33.Milligan, G. N. 1999. Neutrophils aid in protection of the vaginal mucosae of immune mice against challenge with herpes simplex virus type 2. J. Virol. 736380-6386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morganti-Kossman, M. C., P. M. Lenzlinger, V. Hans, P. Stahel, E. Csuka, E. Ammann, R. Stocker, O. Trentz, and T. Kossman. 1997. Production of cytokines following brain injury: beneficial and deleterious for the damaged tissue. Mol. Psychiatry 2133-136. [DOI] [PubMed] [Google Scholar]
- 35.Nadeau, S., and S. Rivest. 1999. Effects of circulating tumor necrosis factor on the neuronal activity and expression of the genes encoding the tumor necrosis factor receptors (p55 and p75) in the rat brain: a view from the blood-brain barrier. Neuroscience 931449-1464. [DOI] [PubMed] [Google Scholar]
- 36.Paludan, S. R., S. Ellermann-Eriksen, and S. C. Mogensen. 1998. NF-κB activation is responsible for the synergistic effect of herpes simplex virus type 2 infection on interferon-γ-induced nitric oxide production in macrophages. J. Gen. Virol. 792785-2793. [DOI] [PubMed] [Google Scholar]
- 37.Parr, M. B., L. Kepple, M. R. McDermott, M. D. Drew, J. J. Bozzola, and E. L. Parr. 1994. A mouse model for studies of mucosal immunity to vaginal infection by herpes simplex virus type 2. Lab. Investig. 70369-380. [PubMed] [Google Scholar]
- 38.Rendon-Mitchell, B., M. Ochani, J. Li, J. Han, H. Wang, H. Yang, S. Susarla, C. Czura, R. A. Mitchell, G. Chen, A. E. Sama, K. J. Tracey, and H. Wang. 2003. IFN-gamma induces high mobility group box 1 protein release partly through a TNF-dependent mechanism. J. Immunol. 1703890-3897. [DOI] [PubMed] [Google Scholar]
- 39.Rollins, B. J. 1996. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol. Med. Today 2198-204. [DOI] [PubMed] [Google Scholar]
- 40.Rubio, N., and F. Sanz-Rodriguez. 2007. Induction of the CXCL1 (KC) chemokine in mouse astrocytes by infection with the murine encephalomyelitis virus of Theiler. Virology 35898-108. [DOI] [PubMed] [Google Scholar]
- 41.Sergerie, Y., S. Rivest, and G. Boivin. 2007. Tumor necrosis factor-α and interleukin-1β play a critical role in the resistance against lethal herpes simplex virus encephalitis. J. Infect. Dis. 196853-860. [DOI] [PubMed] [Google Scholar]
- 42.Singh, R., A. Kumar, W. D. Creery, M. Ruben, A. Giulivi, and F. Diaz-Mitoma. 2003. Dysregulated expression of IFN-γ and IL-10 and impaired IFN-γ-mediated responses at different disease stages in patients with genital herpes simplex virus-2 infection. Clin. Exp. Immunol. 13397-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sinha, S., S. Subramanian, T. M. Proctor, L. J. Kaler, M. Grafe, R. Dahan, J. Huan, A. A. Vandenbark, G. G. Burrows, and H. Offner. 2007. A promising therapeutic approach for multiple sclerosis: recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J. Neurosci. 2712531-12539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thapa, M., W. A. Kuziel, and D. J. J. Carr. 2007. Susceptibility of CCR5-deficient mice to genital herpes simplex virus type 2 is linked to NK cell mobilization. J. Virol. 813704-3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thapa, M., R. S. Welner, R. Pelayo, and D. J. J. Carr. 2008. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J. Immunol. 1801098-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tyler, K. L. 2004. Herpes simplex virus infections of the central nervous system: encephalitis and meningitis including Mollaret's. Herpes 1157A-64A. [PubMed] [Google Scholar]
- 47.Veckman, V., P. Osterlund, R. Fagerlund, K. Melen, S. Matikeinen, and I. Julkenen. 2005. TNF-a and IFN-a enhance influenza-A-virus induced chemokine gene expression in human A549 lung epithelial cells. Virology 34596-104. [DOI] [PubMed] [Google Scholar]
- 48.Wallach, D., E. E. Varfolomeev, N. L. Malinin, Y. V. Goltsev, A. V. Kovalenko, and M. P. Boldin. 1999. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu. Rev. Immunol. 17331-367. [DOI] [PubMed] [Google Scholar]
- 49.Whitley, R. J., and R. L. Miller. 2001. Immunologic approach to herpes simplex virus. Viral Immunol. 14111-118. [DOI] [PubMed] [Google Scholar]
- 50.Zink, M. C., G. D. Coleman, J. L. Mankowski, R. J. Adams, P. M. Tarwater, K. Fox, and J. E. Clements. 2001. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J. Infect. Dis. 1841015-1021. [DOI] [PubMed] [Google Scholar]
- 51.Zou, J. Y., and F. T. Crews. 2005. TNFa potentiates glutamate neurotoxicity by inhibiting glutamate uptake in organotypic brain slice cultures: neuroprotection by NFkB inhibition. Brain Res. 103411-24. [DOI] [PubMed] [Google Scholar]