Abstract
Pseudorabies virus encodes a membrane protein (Us9) that is essential for the axonal sorting of virus particles within neurons and anterograde spread in the mammalian nervous system. Enhanced green fluorescent protein (GFP)-tagged Us9 mimicked the trafficking properties of the wild-type protein in nonneuronal cells. We constructed a pseudorabies virus strain that expressed Us9-GFP and tested its spread capabilities in the rat visual system and in primary neuronal cultures. We report that Us9-EGFP does not promote anterograde spread of infection and may disrupt packing of viral membrane proteins in lipid rafts, an essential step for Us9-mediated axonal sorting.
Pseudorabies virus (PRV) is an alphaherpesvirus that establishes a reactivatable, latent infection in the peripheral nervous system of adult swine. In most natural infections, virus replication rarely proceeds beyond the peripheral nervous system. However, in nonnatural hosts, infection continues to spread to second-order and third-order neurons in the central nervous system (CNS). It is this facet of PRV infection that makes it a good tracer of mammalian neuronal circuitry (10).
PRV Us9, a small type II membrane protein, is essential for the targeting of herpesvirus structural proteins to the axon of infected neurons, a key step to anterograde transmission of the virus (i.e., spread from presynaptic to postsynaptic neurons) (14, 18). Us9 is a tail-anchored membrane protein that lacks an obvious signal sequence and has a 68-amino-acid cytosolic domain, a 26-amino-acid transmembrane domain, and virtually no ectodomain (3 amino acids).
Our lab has examined the trafficking patterns of Us9 in pig kidney (PK15) epithelial cells (2, 4, 5). Brideau et al. fused enhanced green fluorescent protein (EGFP) to the short ectodomain of Us9, a region that is topologically outside the cell and unlikely to interfere with the function of the cytosolic domain (2). Us9-EGFP was efficiently incorporated into purified virions and localized to a perinuclear region in or near the Golgi compartment of PK15 cells, indistinguishable from the localization of wild-type Us9 in transfected and infected cells (2). Us9-EGFP was also actively retrieved from the cell surface, and this phenotype was used to map endocytic motifs within the Us9 cytoplasmic domain (4). Taken together, these data suggested that Us9-EGFP functionally mimicked the wild-type Us9 protein.
One caveat to this suggestion is that a Us9-null mutant does not have an observable phenotype in PK15 or MDBK cells, i.e., no defect in replication or cell-to-cell spread. An anterograde sorting defect was observed only within primary neurons (3, 18). Thus, it was unclear if Us9-EGFP was a functional surrogate for Us9 in the context of neuronal infection. We constructed a PRV strain having the wild-type Us9 coding region deleted (3) that ectopically expressed Us9-GFP in the nonessential gG locus of the viral genome (Fig. 1A). Previous insertions of GFP or β-galactosidase reporter genes in gG did not impact the spread of PRV in the nervous system (1, 16). We then assessed the ability of this PRV strain (PRV 164) to undergo transneuronal spread in the rat visual system.
FIG. 1.
Analysis of Us9-EGFP mediated anterograde spread in the rat visual system. (A) Schematic representation of the genome of PRV 164. A CMV Us9-GFP expression cassette was inserted into the gG locus (striped box upstream of gD), and the endogenous Us9 open reading frame was deleted (black box between gE and Us2). IR, inverted repeat. (B) Approximately 2.5 × 105 PFU of either PRV Becker or PRV 164 was injected into the vitreous humor of Sprague-Dawley male rats. At the time of imminent death, the animals were sacrificed and the brains were fixed and sliced into 35-μm-thick coronal sections with a freezing microtome. Infected tissue was stained for PRV antigen or Us9-EGFP (with Rb133 or Us9 rabbit polyvalent antiserum, respectively). Areas reached by anterograde, transneuronal spread are highlighted in red. D, dorsal; V, ventral.
The rat eye model is a powerful tool for identifying herpesvirus genes required for the transmission of infection from the peripheral nervous system to the CNS (i.e., neuroinvasion) (7, 12, 15). Upon injection of virus into the vitreous humor of the eye, the infection spreads transsynaptically to second-order neurons in the CNS, via both anterograde and retrograde neural circuits (17). Replication initiates in retinal ganglion cells and proceeds to secondary neurons of the CNS that are in direct synaptic contact with the optic nerve (retinorecipient areas of the brain). Wild-type PRV spreads to visual centers in the brain, including the lateral geniculate nucleus (LGN), the dorsal and ventral aspects (dLGN and vLGN), and the intergeniculate leaflet (IGL), as well as the superior colliculus (SC). Retinorecipient circadian rhythm centers are also infected, including the suprachiasmatic nucleus (SCN) (6, 19). Virus spread from the retina to the dLGN, vLGN, and SC requires anterograde, transsynaptic movement; infection of the SCN and IGL occurs via a retrograde, multisynaptic route in sympathetic neurons (16). Therefore, Us9-null mutants traffic efficiently to the SCN and IGL but do not infect the dLGN, the vLGN, or the SC (3).
The left eye of male Sprague-Dawley rats was infected with 2.5 × 105 PFU of either PRV Becker or PRV 164. At the time of imminent death (∼72 h postinfection [hpi] for PRV Becker and ∼86 hpi for PRV 164), animals were sacrificed and brains were fixed and sliced into 35-μm-thick coronal sections. Infected tissue was stained for PRV antigen or Us9-EGFP (with Rb133 or Us9 rabbit polyvalent antiserum, respectively). PRV Becker spread to the SCN, LGN, and SC as predicted. By contrast, PRV 164 spread only to the SCN and IGL via retrograde circuits and was indistinguishable from the spread phenotype of a Us9-null mutant (Fig. 1B) (3). Control staining with anti-Us9 antibody confirmed that the Us9-GFP fusion was expressed in infected tissue. This finding suggests that Us9-GFP does not function in anterograde spread of infection and does not mimic wild-type Us9 in this capacity.
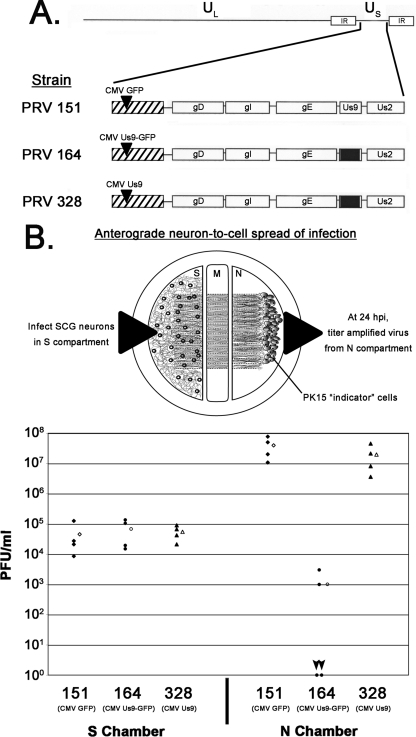
To rule out the possibility that overexpression from the cytomegalovirus (CMV) promoter or insertion into the gG locus was causing the defect, we introduced a stop codon between the Us9 and EGFP open reading frames, thereby creating a virus that expressed wild-type Us9 under the CMV promoter in the gG locus (designated PRV 328). Using a trichamber culturing system (9, 13), we compared the neuron-to-cell spread capabilities of PRV 151 (CMV-GFP in gG), PRV 164 (CMV-Us9-GFP in gG), and PRV 328 (CMV Us9 in gG). Figure 2A shows virus schematics. Dissociated superior cervical ganglion (SCG) neurons from embryonic rats were plated in the soma (S) compartment and allowed to mature for 2 weeks (Fig. 2B, top panel). During this period, axons were guided between a series of grooves across the methocellulose compartment to the neurite (N) compartment. PK15 cells were then plated on the neurites in the N compartment. Cell bodies in the S compartment were infected, and virus particles were sorted into axons in a Us9-dependent manner. The primary infection was confined to the S compartment via silicone vacuum grease and a methocellulose barrier. Thus, infection spread to the N compartment in the anterograde direction exclusively through axons that emanated from neuronal cell bodies and contacted PK15 cells (9, 13).
FIG. 2.
Dropping the EGFP tag from Us9 restores anterograde, neuron-to-cell spread in vitro. (A) Schematic of the PRV strains 151, 164, and 328. IR, inverted repeat. (B) SCG neurons were plated in the S compartment and allowed to extend neurites into the N compartment for 2 weeks. Axons were guided by a series of grooves scratched into the surface of the tissue culture dish. After 2 weeks, a monolayer of indicator PK15 cells was plated on top of the axon termini in the N compartment. Cell bodies in the S compartment were then infected at an MOI of 10 with PRV 151, 164, or 328. Four chambers were used for each type of infection (closed symbols). At 24 hpi, medium and infected cells were harvested together from either the S or the N compartment. Total PFU/ml were determined for each chamber. The mean value for the four samples is denoted by the offset open symbol. Black arrowheads denote the two plates infected with PRV 164 that showed no anterograde, neuron-to-cell spread. M, methocellulose compartment.
We infected SCG neurons in the S compartment with PRV 151, PRV 164, or PRV 328 and harvested amplified virus from the S and N compartments 24 hpi. Comparable amounts of infectious virus were harvested from the S compartment for all three strains (Fig. 2B, bottom panel). However, anterograde spread to second-order PK15 cells was dramatically different. PRV 151 produced a mean titer of ∼4 × 107 PFU/ml in the N compartment, similar to what has been reported for wild-type strain Becker (13). PRV 164 was dramatically lower, with a mean titer of ∼1.0 × 103. Two plates showed no infectious particles in the N compartment (Fig. 2B, bottom panel, arrowheads), and two plates had 103 PFU/ml, which are likely single spread events (13). PRV 164 behaved indistinguishably from a Us9-null mutant in this system (13), consistent with what we observed for anterograde transneuronal spread of PRV 164 in vivo (Fig. 1). Importantly, removing the GFP tag from Us9 restored wild-type neuron-to-cell spread kinetics (PRV 328, N compartment), supporting the notion that EGFP fused to Us9 disrupts its function in anterograde sorting and spread.
We recently reported that PRV Us9 is highly enriched in detergent-insoluble lipid rafts and that this enrichment is necessary for anterograde sorting of virus structural proteins (13). We hypothesized that Us9-EGFP may not partition into lipid rafts efficiently, thereby reducing its ability to function in anterograde sorting. We tested this idea in differentiated PC12 cells, a rat pheochromocytoma cell line that responds to nerve growth factor and acquires many of the characteristics of sympathetic neurons (11). These cells are ideal for biochemical analyses as they recapitulate the Us9 sorting phenotypes observed in primary rat SCG neurons (13) and can be cultured and differentiated in large quantities (total protein amounts from primary cultures are meager). Differentiated PC12 cells were cultured as described previously (8, 13), infected with PRV 328 (CMV Us9) or PRV 164 (CMV Us9-EGFP) at a multiplicity of infection (MOI) of 10, incubated for 12 h, and subjected to a well-described lipid raft flotation assay (13). The ganglioside GM1 (the prototypic raft marker) and transferrin receptor (nonraft marker) were used as controls for proper solubilization and separation of detergent-soluble and detergent-insoluble fractions. Us9-EGFP was abundant in the raft fraction of the flotation gradient, though not as enriched as wild-type Us9 (Fig. 3). We also noted that the raft partitioning of certain viral glycoproteins was aberrant in the presence of Us9-EGFP in undifferentiated PC12 cells (data not shown). Thus, it is unclear if the Us9-null phenotype exhibited by PRV 164 is due to a decrease of raft partitioning of Us9-EGFP and/or a more global disruption of raft structure due to the bulky EGFP moiety attached to the C-terminal ectodomain of Us9 (i.e., wild-type Us9 is 98 amino acids in length with virtually no ectodomain, whereas Us9-EGFP is 352 amino acids in length with a large ectodomain).
FIG. 3.
Targeting of Us9-EGFP to detergent-resistant microdomains. Differentiated PC12 cells were infected with PRV 328 (A) or PRV 164 (B) for 12 h and then lysed with cold 1% Triton X-100. Lysates were separated on a discontinuous Optiprep density gradient (5%, 30%, and 40%) by ultracentrifugation at 4°C for 20 h (13). Detergent-insoluble complexes (i.e., lipid rafts) floated to the 5%/30% interface, while detergent-soluble proteins remained at the bottom of the gradient. Fractions were collected from the top to the bottom of the tube (1 ml each). Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blotting analysis was performed using biotinylated cholera toxin B subunit (for GM1) and antiserum to PRV Us9 and transferrin receptor (TfR). Numbers at right of each panel are molecular masses in kilodaltons.
The trafficking of Us9-EGFP in nonneuronal cells mimics that of wild-type Us9 and was used to map endocytic motifs within the Us9 cytosolic domain (2, 4, 5). However, Us9-EGFP does not function in anterograde spread of infection in neurons, though retrograde spread remains intact (Fig. 1). These findings highlight the difference between Us9-dependent and Us9-independent egress from infected neurons. Us9-dependent egress occurs by sorting cellular vesicles (containing virus particles) into axons for long-distance transport (13, 14). This process is dependent on Us9 localization to lipid rafts (13). By contrast, local egress from neurons (and subsequent retrograde, transneuronal spread) is not dependent on localization of Us9 to lipid rafts (13) and occurs normally in the presence of Us9-EGFP (Fig. 1). Furthermore, all Us9 missense proteins that are defective in sponsoring anterograde spread are incorporated into virion envelopes that egress from the cell body in cellular vesicles (2, 4, 5, 13). Thus, there are clearly two distinct viral egress pathways from infected neurons: local (Us9 independent) and axonal (Us9 dependent).
Acknowledgments
M.G.L. was supported by the National Institutes of Health (grants R01 33506 and R01 33063 to L.W.E. and grant F32 NS056594 to M.G.L.). D.C. and A.D.B. were supported by NIH institutional training grants (NIH 2T32-GM007388-27 and NIH 5T32GM07312, respectively).
Footnotes
Published ahead of print on 6 August 2008.
REFERENCES
- 1.Banfield, B. W., G. S. Yap, A. C. Knapp, and L. W. Enquist. 1998. A chicken embryo eye model for the analysis of alphaherpesvirus neuronal spread and virulence. J. Virol. 724580-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brideau, A. D., B. W. Banfield, and L. W. Enquist. 1998. The Us9 gene product of pseudorabies virus, an alphaherpesvirus, is a phosphorylated, tail-anchored type II membrane protein. J. Virol. 724560-4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brideau, A. D., J. P. Card, and L. W. Enquist. 2000. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. J. Virol. 74834-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brideau, A. D., T. del Rio, E. J. Wolffe, and L. W. Enquist. 1999. Intracellular trafficking and localization of the pseudorabies virus Us9 type II envelope protein to host and viral membranes. J. Virol. 734372-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brideau, A. D., M. G. Eldridge, and L. W. Enquist. 2000. Directional transneuronal infection by pseudorabies virus is dependent on an acidic internalization motif in the Us9 cytoplasmic tail. J. Virol. 744549-4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Card, J. P., M. E. Whealy, A. K. Robbins, and L. W. Enquist. 1992. Pseudorabies virus envelope glycoprotein gI influences both neurotropism and virulence during infection of the rat visual system. J. Virol. 663032-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Card, J. P., M. E. Whealy, A. K. Robbins, R. Y. Moore, and L. W. Enquist. 1991. Two alpha-herpesvirus strains are transported differentially in the rodent visual system. Neuron 6957-969. [DOI] [PubMed] [Google Scholar]
- 8.Ch'ng, T., E. A. Flood, and L. W. Enquist. 2005. Culturing primary and transformed neuronal cells for studying pseudorabies virus infection. Methods Mol. Biol. 292299-316. [DOI] [PubMed] [Google Scholar]
- 9.Ch'ng, T. H., and L. W. Enquist. 2005. Neuron-to-cell spread of pseudorabies virus in a compartmented neuronal culture system. J. Virol. 7910875-10889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Enquist, L. W., and J. P. Card. 2003. Recent advances in the use of neurotropic viruses for circuit analysis. Curr. Opin. Neurobiol. 13603-606. [DOI] [PubMed] [Google Scholar]
- 11.Greene, L. A., and A. S. Tischler. 1976. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 732424-2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kristensson, K., B. Ghetti, and H. M. Wisniewski. 1974. Study on the propagation of herpes simplex virus (type 2) into the brain after intraocular injection. Brain Res. 69189-201. [DOI] [PubMed] [Google Scholar]
- 13.Lyman, M. G., D. Curanovic, and L. W. Enquist. 2008. Targeting of pseudorabies virus structural proteins to axons requires association of the viral Us9 protein with lipid rafts. PLoS Pathog. 4e1000065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lyman, M. G., B. Feierbach, D. Curanovic, M. Bisher, and L. W. Enquist. 2007. Pseudorabies virus us9 directs axonal sorting of viral capsids. J. Virol. 8111363-11371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Margolis, T. P., J. H. LaVail, P. Y. Setzer, and C. R. Dawson. 1989. Selective spread of herpes simplex virus in the central nervous system after ocular inoculation. J. Virol. 634756-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pickard, G. E., C. A. Smeraski, C. C. Tomlinson, B. W. Banfield, J. Kaufman, C. L. Wilcox, L. W. Enquist, and P. J. Sollars. 2002. Intravitreal injection of the attenuated pseudorabies virus PRV Bartha results in infection of the hamster suprachiasmatic nucleus only by retrograde transsynaptic transport via autonomic circuits. J. Neurosci. 222701-2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pomeranz, L. E., A. E. Reynolds, and C. J. Hengartner. 2005. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiol. Mol. Biol. Rev. 69462-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tomishima, M. J., and L. W. Enquist. 2001. A conserved alpha-herpesvirus protein necessary for axonal localization of viral membrane proteins. J. Cell Biol. 154741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whealy, M. E., J. P. Card, A. K. Robbins, J. R. Dubin, H. J. Rziha, and L. W. Enquist. 1993. Specific pseudorabies virus infection of the rat visual system requires both gI and gp63 glycoproteins. J. Virol. 673786-3797. [DOI] [PMC free article] [PubMed] [Google Scholar]