Abstract
The vaccinia virus WR53.5L/F14.5L gene encodes a small conserved protein that was not detected previously. However, additional proteomic analyses of different vaccinia virus isolates and strains revealed that the WR53.5 protein was incorporated into intracellular mature virus (IMV). The WR53.5 protein contains a putative N-terminal transmembrane region and a short C-terminal region. Protease digestion removed the C terminus of WR53.5 protein from IMV particles, suggesting a similar topology to that of the IMV type II transmembrane protein. We generated a recombinant vaccinia virus, vi53.5L, that expressed WR53.5 protein under isopropyl-β-d-thiogalactopyranoside (IPTG) regulation and found that the vaccinia virus life cycle proceeded normally with or without IPTG, suggesting that WR53.5 protein is not essential for vaccinia virus growth in cell cultures. Interestingly, the C-terminal region of WR53.5 protein was exposed on the cell surface of infected cells and mediated calcium-independent cell adhesion. Finally, viruses with inactivated WR53.5L gene expression exhibited reduced virulence in mice when animals were inoculated intranasally, demonstrating that WR53.5 protein was required for virus virulence in vivo. In summary, we identified a new vaccinia IMV envelope protein, WR53.5, that mediates cell adhesion and is important for virus virulence in vivo.
The Poxviridae are a family of large enveloped DNA viruses that replicate in the cytoplasm of the host cells (5). The best known member of this family is variola virus, the causative agent of smallpox disease in humans (3). Other poxvirus members also cause diseases in humans. For example, monkeypox virus produces a smallpox-like disease, and molluscum contagiosum virus causes relatively benign wart-like lesions (7, 25).
Vaccinia virus, the prototype of the Orthopoxvirus genus of the family Poxviridae, infects many vertebrate cell lines and animals. Vaccinia virus replicates in the cytoplasm of host cells and encodes more than 200 open reading frames (ORFs) in a 190-kb double-stranded DNA genome (8). Vaccinia virus contains early, intermediate, and late classes of viral genes that encode viral transcription factors to activate viral gene expression in a cascade-regulated manner. Virion morphogenesis occurs in the cytoplasm and produces several forms of infectious particles, namely, intracellular mature virus (IMV), intracellular enveloped virus (IEV), cell-associated enveloped virus, and extracellular enveloped virus (EEV) (reference 5 and the references therein) particles. The IMV is the most abundant particle, with a single membrane in cells; however, the origin of the membrane is unknown (13, 14). Some IMVs are wrapped with Golgi apparatus-derived membranes to form IEVs, which are transported through microtubules to the cell periphery to become cell-associated enveloped virus particles and EEVs (5).
Recently, we along with others reported proteomic studies of vaccinia IMV, revealing that vaccinia IMV contains more than 70 viral proteins (4, 24, 31, 35). In this study, we identified a new IMV protein WR53.5/F14.5 that was previously undetected in all three studies. We also investigated the role of this new virion protein in the vaccinia virus life cycle.
(Roza Izmailyan, also known as Ruzan A. Izmailyan in a previous publication [19], conducted this research in partial fulfillment of the requirements for a Ph.D. from Academia Sinica, Taiwan, Republic of China.)
MATERIALS AND METHODS
Reagents, cells, and viruses.
Mycophenolic acid, hypoxanthine, and xanthine (Sigma) were dissolved at a concentration of 10 mg/ml in 0.1 N NaOH and stored at −20°C. Cytosine β-d-arabinofuranoside (AraC) was purchased from Sigma. BSC40, BSC1, and RK13 cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2% penicillin and streptomycin from Gibco. Alexa Fluor 647-phalloidin (200 U/ml) was obtained from Molecular Probes Inc. and used at a dilution of 1:125. Two wild-type WR strain vaccinia viruses were used in this study: the original WR strain obtained from S. Pennathur (designated WR-1) and another wild-type WR strain obtained from G. L. Smith (WR-2). VT7LacOI was obtained from B. Moss (33). IMV particles were purified on a 36% sucrose cushion, followed by 25 to 40% sucrose gradient centrifugation, as described previously (20), and stored at −70°C. Since sucrose gradient centrifugation resulted in mostly IMV particles with little IEV/EEV contamination (6), further purification using a CsCl gradient to separate the IMV preparation from IEV or EEV was not performed. Rabbit antibodies (Abs) recognizing the vaccinia virus proteins H3L and D8L were described previously (15, 23). An anti-core protein Ab was provided by J. Krijnse Locker (27). Rabbit anti-53.5 Ab was generated using a synthetic peptide, EENNEEDARIKEEQELLLLY, derived from the WR53.5 peptide sequences (QCB Inc.) and used at a 1:1,000 dilution in all the experiments.
Generation of vi53.5L virus. (i) Plasmid construction.
To construct pMITEO-53.5 containing an inducible copy of the WR53.5L gene, the full-length WR53.5L ORF was generated by PCR using the primers 5′-AAACCATGGTCATCGGTCTAGTCATA-3′ and 5′-CCCGGATCCTCAATATAGCAACAGTAGTTC-3′ (NcoI and BamHI restriction sites, respectively, are underlined) and the genomic DNA of vT7LacOI as the template (16). The PCR product was digested with NcoI and BamHI and cloned into pMITEOlac.20/3 to produce pMITEO-i53.5L. Three DNA fragments were used to replace the endogenous WR53.5L gene with a xanthine-guanine phosphoribosyltransferase ORF expression cassette (gpt). The 560-bp 5′ flanking fragment containing the WR54L promoter and coding sequences was generated by PCR using the primers 5′-TATAGACTAAAAAAGAAACGT-3′ and 5′-GCGGCCGCGTCTCTAGCTTTCACTTAA-3′ (NotI site underlined) and vT7lacOI genomic DNA as the template. The 580-bp 3′ flanking fragment containing the WR53L promoter and the coding sequences was generated by PCR using the primers 5′-GCGGCCGCGTACATAATTGAAAATCTA-3′ and 5′-CTCGAGGACTTTGTAGCTCTCCCA-3′ (NotI and XhoI sites, respectively, are underlined) and vT7lacOI genomic DNA as the template. The 5′ and 3′ flanking DNA fragments were cloned into the pCRII-Topo vector (Invitrogen) to create pCRII-Topo-54/53-52. The 1.95-kb i53.5L expression cassette was purified from NotI-digested pMITEO-i53.5 and cloned into pCRII-Topo-54/53 to obtain pCRII-Topo-54/i53.5/53. The sequences of the PCR fragments were confirmed by DNA sequencing.
(ii) Construction of the recombinant vi53.5L virus.
The recombinant vi53.5L virus was constructed following previously established protocols (19). In brief, 3 × 105 BSC1 cells were infected with vT7LacOI at a multiplicity of infection (MOI) of 1 PFU per cell and subsequently transfected with 1 μg of pCRII-54/i53.5L/53 with Lipofectamine (Invitrogen). After removal of the transfection mixture, DMEM containing 10% FBS and 100 μM isopropyl-β-d-thiogalactopyranoside (IPTG) was added to cells, and lysates were prepared at 30 h postinfection (p.i.) and used to select for plaques formed by vi53.5L, which expresses xanthine-guanine phosphoribosyltransferase (Gpt) and WR53.5 protein. Pure recombinant vi53.5L viruses were obtained after three rounds of plaque purification. The insertion of Gpt and the inducible WR53.5L gene into the endogenous WR53.5L locus was confirmed by PCR.
Construction of the recombinant WR Δ53.5 virus.
A deletion mutant virus deleting the endogenous WR53.5L ORF was constructed as described below. The plasmid pCRII-Topo-54/53-52 containing the 5′ and 3′ flanking DNA fragments was created as described above, and a gpt cassette was inserted. The resulting plasmid, pCRII-Topo-54/gpt/53, was transfected into the cells infected with wild-type WR vaccinia virus (WR-2), and the Gpt-positive clones were purified clonally as described above.
Virus growth curves.
In brief, BSC40 cells were infected with vi53.5L at an MOI of 5 PFU per cell for 1 h at 37°C, washed, and incubated in complete DMEM containing 10% FBS with or without 100 μM IPTG; cells were then harvested at various times after infection for virus titer determination on BSC40 cells in the presence of 100 μM IPTG. The experiments were repeated three times, and the averages are presented.
Membrane protein extraction from IMV.
Vaccinia IMVs were extracted with 1% NP-40 with or without 50 mM dithiothreitol (DTT) and separated into membrane and core fractions, essentially as described previously (34). Proteins in the pellet and supernatant were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to immunoblot analyses.
Protease treatment of IMV.
Purified IMV particles were treated with trypsin as described previously (18, 26). In brief, 1 × 108 particles/sample were incubated with buffer containing 1 mM CaCl2 (pH 7.6)-50 mM Tris-HCl (pH 7.8) in the presence or absence of 25, 50, and 100 μg/ml trypsin (Promega). After 1 h at 37°C, virus was centrifuged, and the samples were subjected to immunoblot analyses.
Extracellular Ca2+ depletion assay.
Extracellular Ca2+ depletion assays were performed as previously described (1, 28). BSC40 cells were either mock infected or infected with vi53.5 virus at an MOI of 5 PFU per cell, cultured in medium with or without 100 μM IPTG for 2 days, and washed three times with phosphate-buffered saline (PBS). Cell morphology was recorded under microscopy from seven random areas immediately before and after treatment with 1 mM EGTA at 37°C for 20 min.
Cell surface and total protein staining assays. (i) Confocal immunofluorescence microscopy.
For confocal immunofluorescence microscopy, BSC40 cells were seeded on coverslips in 12-well plates and infected with WR-2 or vi53.5L virus at an MOI of 5 PFU per cell at 37°C for 60 min. Cells were cultured in medium with or without 100 μM IPTG for 1 and 2 days, washed three times with PBS, and fixed with 4% paraformaldehyde for 15 min at room temperature. These infected cells were stained with anti-53.5 Ab (1:1,000) without permeabilization (nonpermeabilized), followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit Abs (Sigma) (1:1,000) in PBS-0.2% bovine serum albumin to visualize cell surface staining of WR53.5. These surface-stained cells were subsequently permeabilized in PBS-0.2% saponin and stained with anti-53.5 Ab (1:1,000) again, followed by tetramethyl-rhodamine goat anti-rabbit immunoglobulin G (1:1,000) (Molecular Probes) to visualize total WR53.5 protein in cells. Intracellular actin cytoskeleton was stained with Alexa Fluor 647-phalloidin (1:150) (Molecular probes). Intracellular DNA was visualized by staining with 0.5 μg/ml of 4′,6′-diamidino-2-phenylindole(DAPI; Molecular Probes). Cell images were collected with an LSM510 META confocal laser scanning microscope (Carl Zeiss, Germany) using a 63× objective lens and the confocal microscopy software Release, version 2.8 (Carl Zeiss).
(ii) Flow cytometry.
For flow cytometry, BSC40 cells (4.5 × 105) in a 35-mm dish were either mock infected or infected with WR-2 or vi53.5 virus at an MOI of 5 PFU/cell at 37°C for 60 min, washed twice with PBS, and incubated in appropriate medium (WR-2 in normal medium; vi53.5 in medium with or without 100 μM IPTG) for another 24 h. Cells were subsequently detached by the addition of 5 mM EDTA and stained with rabbit anti-53.5 Ab (1:1,000) at 4°C for 1 h, followed by FITC-conjugated goat anti-rabbit Ab for fluorescence-activated cell sorter (FACS) analyses. Besides mock-infected cells which were included as background calibration in FACS analysis, two additional controls were included in the experiments: (a) anti-53.5 Ab staining of cells infected with vi53.5L and grown in the absence of IPTG and (ii) secondary FITC-conjugated goat anti-rabbit Ab staining of cells infected with vi53.5L and grown in the presence of IPTG.
Measurement of WRΔ53.5 virus virulence in mice.
Groups of five male BALB/c mice between 7 to 8 weeks old were anesthetized and inoculated intranasally with either PBS (mock-infected control), 1 × 105, 1 × 106, or 1 × 107 PFU/mouse of sucrose-purified IMVs of wild-type vaccinia virus WR (WR-2) or WRΔ53.5 in a 10-μl volume. A portion of the virus inoculum was titrated on BSC40 cells again to ensure that the inoculated titers were accurate. Mice were weighed daily and recorded as described previously (23). All the mice were housed and treated in accordance with Academia Sinica animal care guidelines.
RESULTS
The conserved vaccinia virus WR53.5/F14.5 ORF encodes a late viral envelope protein in IMV particles.
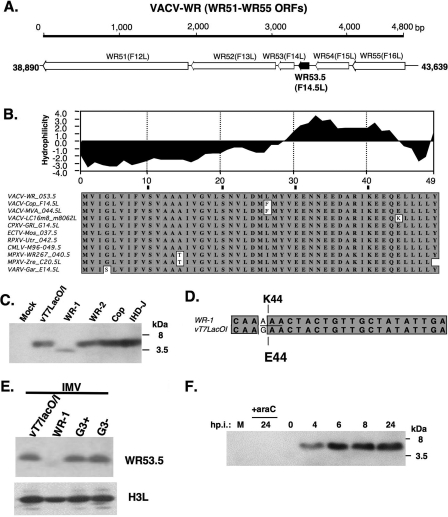
Our recent mass spectrometry analysis of newly prepared vT7lacOI IMV particles revealed two tryptic peptides that had not been detected before. These two peptides, YVEENNEEDAR and IKEEQELLLLY, matched a small ORF WR53.5 protein in the vaccinia virus WR strain genome (Fig. 1A), encoding a conserved polypeptide of 49 amino acids with a predicted molecular mass of 5.5 kDa (http://www.poxvirus.org/). Hydropathy analysis predicted that the WR53.5 protein has two hydrophobic domains at the N and C termini (Fig. 1B). Alignment of the amino acid sequences of vaccinia virus WR53.5L and its orthologues present in the Orthopoxvirus genus revealed a high level of homology among these proteins (∼98% conserved residues) including the F14.5L ORF in vaccinia virus Copenhagen strain (11). Rabbits were immunized with a synthetic peptide derived from the C-terminal region of WR53.5 protein amino acid sequences (see Materials and Methods), and the antiserum thus produced, anti-53.5, was tested in immunoblot analyses of lysates prepared from virus-infected cells (Fig. 1C). The anti-53.5 antiserum recognized a small 3.5-kDa viral protein in cells infected with our original WR strain vaccinia virus (WR-1) and a 5.5-kDa protein in cells infected with other vaccinia viruses including two WR strains (vT7lacO/I and WR-2), Copenhagen, and IHD-J strains. Although WR and Copenhagen strains have been sequenced before, we resequenced the WR53.5L gene locus in the above virus genomes and found that the WR53.5L ORF in WR-1 specifies a K at residue 44, encoded by AAA, whereas an E encoded by GAA was found in other vaccinia virus genomes such as those of the vT7LacO/I, WR-2, Copenhagen, and IHD-J strains (Fig. 1D). This suggested to us that a nonconserved G-to-A mutation occurred in the WR-1 genome, resulting in a glutamic acid-to-lysine change at position 44 and a faster electrophoretic mobility on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels. Since E44 was more frequently found than K44 in the different WR53.5 orthologues (Fig. 1B), we tentatively designated the WR53.5 carrying E44 (WR53.5E44) in WR-2 the wild-type WR53.5 protein. Wild-type WR53.5 protein was detected in IMV particles prepared from vT7lacO/I and viG3L virus (19), a recombinant virus derived from vT7LacO/I; however, the WR53.5K44 protein was barely detected in purified WR-1 IMVs (Fig. 1E). Expression of the wild-type WR53.5 protein was monitored in cells infected with vaccinia virus WR-2, and a 5.5-kDa protein was detected at 4 h p.i., which increased in abundance until 24 h p.i. (Fig. 1F). Expression of the 5.5-kDa protein was blocked by AraC, which inhibits viral DNA replication, suggesting that WR53.5L is a late gene. Indeed, there is a canonical later promoter TAAATG (32) overlapping the initiation codon of ORF WR53.5 in the vaccinia virus genome.
FIG. 1.
(A) Schematic drawing of the genomic locus of the WR53.5 ORF and its neighboring ORFs (from WR51 to WR55) on the vaccinia virus WR strain genome. The length of the genomic region shown here is 4.8 kb. The numbers 38890 and 43639 are the ORF start/stop sites of the protein in the virus genome. The arrows represent each ORF and are pointed toward the direction of gene transcription. WR51/F12L is expressed early and late and encodes an IEV protein required for virus egress (37). WR52/F13L is a late gene and encodes a palmitylated EEV protein important for cell-to-cell spread (2, 17). WR53/F14L, WR54/F15L, and WR55/F16L are putative ORFs with unknown functions. (B) Hydropathy plot of WR53.5 protein and its orthologues in the Orthopoxvirus genus. The numbers at the bottom of the plot indicate the amino acid residues. Alignment of the deduced amino acid sequences for vaccinia WR53.5 and its orthologues in other poxviruses is also shown. VACV, vaccinia virus; WR, Western Reserve strain; Cop, Copenhagen strain; MVA, modified virus Ankara (strain MVA-1721); CMLV, camelpox virus (strain M96); ECTV, ectromelia virus (strains Moscow and Naval); CPXV, cowpox virus (strains Brighton Red and GRI 90); Acam, Acambis 3000 MVA; VACV-Lister (LC16m8 and LC16mO); RPXV-UTR, rabbitpox virus (strain Utrechht); VARV, variola virus (strains INDIA-1967/isolate IND3, Garcia, and Bangladesh); MPXV, monkeypox virus (strains Walter Reed and Zaire). The orthologue sequences were obtained from http://www.poxvirus.org. The boxed sequences in gray are conserved amino acid sequences. (C) Expression of WR53.5 protein in cell lysates infected with different vaccinia virus strains. BSC40 cells were infected with the indicated viruses at an MOI of 5 PFU per cell and harvested at 24 h p.i. for immunoblot analyses with anti-53.5 Abs (1:1,000). (D) Alignment of partial sequences of WR53.5 ORF of wild-type WR-1 with vT7LacOI. (E). Detection of WR53.3 protein in IMV particles of vT7LacOI, WR-1, and viG3L virus (19) grown in the presence (G3+) or absence (G3−) of IPTG. (F) WR53.5 protein is a viral late protein. BSC40 cells were infected with vT7LacOI at an MOI of 5 PFU per cell and harvested at the indicated time p.i. for immunoblot analyses using anti-53.5 Abs (1:1,000). M, mock-infected cells. AraC (40 μg/ml) was added to cells immediately after infection.
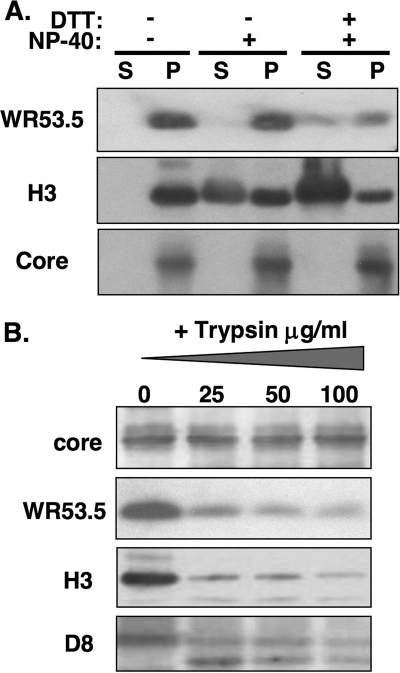
To determine whether WR53.5 protein is present in the membrane fraction of IMV, purified wild-type WR-2 IMVs were extracted with 1% NP-40 with or without 50 mM DTT, and the virion membrane proteins (supernatant) were separated from the insoluble core components (pellet) by centrifugation as previously described (34). As shown in Fig. 2A, WR53.5 protein was not extracted from vaccinia IMVs by buffer containing 1% NP-40; inclusion of 50 mM DTT during extraction did, however, result in partial release of WR53.5 protein into the supernatant, suggesting an association of WR53.5 protein with membranes. Another IMV membrane protein, H3, which served as a control, was readily extracted into the supernatant fraction. In contrast, the viral core proteins 4a/4b were resistant to detergent extraction.
FIG. 2.
(A) Membrane extraction of vaccinia IMV WR53.5 protein. Equivalent amounts of purified wild-type (WR-2) IMV were extracted with buffer containing 1% NP-40 with or without 50 mM DTT as previously described (34) and centrifuged to separate the supernatant (S) and pellet (P) for immunoblot analyses using anti-53.5 (1:1,000), anti-H3 (1:2,000), and anti-core (1:2,000) Abs. (B) Trypsin digestion of IMV envelope proteins. Purified wild-type WR-2 IMV particles were either mock treated (50 mM Tris) or treated with the indicated concentration of trypsin at 37°C for 60 min, sedimented by centrifugation, and immunoblotted with Abs against vaccinia virus envelope WR53.5, H3, D8, and core proteins as previously described (18, 26).
It was previously shown that viral membrane proteins exposed on the surface of IMVs are sensitive to protease digestion (6, 18, 26). We then tested the protease sensitivity of WR53.5 protein in IMV particles (Fig. 2B). If the C terminus of WR53.5 protein is exposed on the IMV, trypsin treatment resulted in a loss of immunoreactivity to anti-53.5 Ab. Similar to H3 (6) and D8 proteins (26) that are sensitive to trypsin digestion, WR53.5 protein was also sensitive to trypsin treatment, in contrast to viral core proteins that were resistant to trypsin treatment. Together, these results show that WR53.5 is a late protein that is present in the membrane of vaccinia IMV with the C terminus of the protein exposed on the virions.
WR53.5 protein is not required for plaque formation and IMV/EEV production in cell culture.
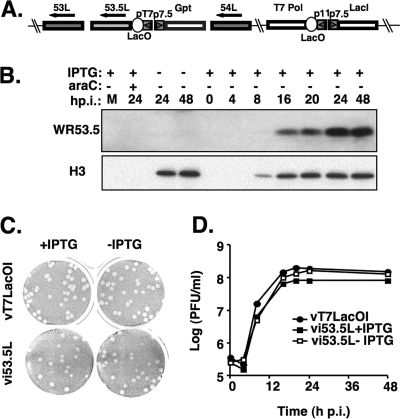
The role of WR53.5L during the vaccinia virus life cycle in cell culture was explored using a recombinant vaccinia virus, vi53.5L, that was generated from the vT7LacOI parental virus (Fig. 3A). vi53.5L contains an inducible WR53.5L/Escherichia coli gpt marker gene cassette inserted into its endogenous WR53.5L locus and was isolated in the presence of mycophenolic acid and purified after three rounds of plaque purification. Abundant WR53.5 protein was detected late in the infected cells only in the presence of 100 μM IPTG, and its production was blocked by AraC (Fig. 3B), demonstrating that expression of the WR53.5L gene was tightly regulated at the late phase by IPTG.
FIG. 3.
(A) Schematic diagram of vi53.5L virus. The WR53.5L and J2R (thymidine kinase) loci in the vi53.5L recombinant virus are indicated. The J2R locus contains T7 RNA polymerase and the lacI repressor gene as described previously (33). The inducible WR53.5L is shown as a shaded box, and the flanking WR53L/F14L and WR54L/F15L genes are shown as gray boxes. The arrows indicate the transcription direction. Abbreviations used are: T7 Pol, T7 RNA polymerase; LacO, E. coli Lac operator; lacI, E. coli lac repressor gene; p7.5 and p11, viral promoters; pT7, promoter for T7 RNA polymerase. (B) Expression of WR53.5 protein in cells infected with vi53.5L virus. BSC40 cells were infected with vi53.5L at an MOI of 5 PFU per cell, incubated with (+) or without (−) 100 μM IPTG, and harvested at the indicated time for immunoblot analyses with the anti-53.5 (1:1,000) or anti-H3 (1:2,000) Abs. M, mock-infected cells. (C) Plaque formation of vi53.5L and parental vT7LacOI virus on BSC40 cells in the presence (+) or absence (−) of IPTG. (D) One-step growth curve analysis of vi53.5L. BSC40 cells were infected with vT7LacOI or vi53.5L at an MOI of 5 PFU per cell, incubated in the presence (vi53.5L+IPTG) or absence (vT7LacO/I and vi53.5L−IPTG) of 100 μM IPTG, and harvested for plaque assays.
The role of WR53.5L in the vaccinia virus life cycle was examined in BSC40 cells infected with vi53.5L in the presence or absence of IPTG. At 3 days p.i., both the control vT7LacOI and vi53.5L virus formed similar plaques on BSC40 cells in the presence or absence of IPTG, showing that WR53.5 protein is not required for plaque formation (Fig. 3C). One-step growth analysis was performed with BSC40 cells infected with vi53.5L at an MOI of 5 PFU per cell and cultured in the presence or absence of IPTG, after which cell lysates were collected, and the IMV titers were determined. As shown in Fig. 3D, removal of IPTG did not affect growth of vi53.5L, which grew to titers similar to the vT7lacOI parental virus. Multiple-round virus growth analyses were performed with a low MOI of 0.1, and the yields of both IMV in cells and EEV in culture medium were not affected by the addition of IPTG (data not shown). We thus, concluded that the WR53.5 proteinis not required for the vaccinia virus life cycle in cell cultures.
The WR53.5 protein is expressed on the surface of virus-infected cells and regulates cell morphology and adhesion of virus-infected BSC40 cells.
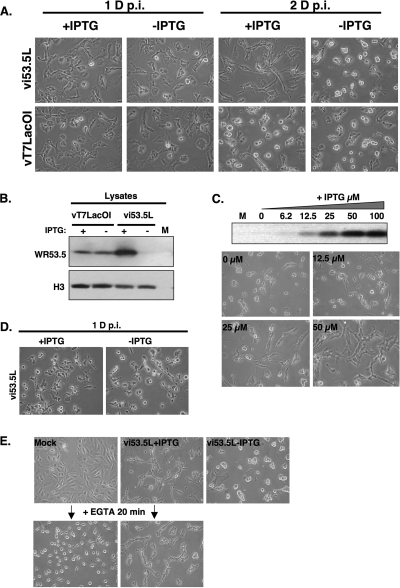
When BSC40 cells were infected with vi53.5L and maintained in medium containing 100 μM IPTG, the morphology of virus-infected cells gradually changed into an elongated shape at 1 to 2 days p.i. (Fig. 4A). In the absence of IPTG, the infected cells rounded up and became loosely attached to dishes at 2 days p.i., suggesting that expression of WR53.5 protein induced by IPTG affected cell morphology. However, the elongated morphology was not obvious in BSC40 cells infected with the parental virus vT7LacOI, which expresses WR53.5 protein from its endogenous promoter that is not regulated by IPTG (Fig. 4A). IPTG alone did not induce an elongated phenotype when tested on BSC40 cells infected with viG3L, a recombinant virus in which expression of viral G3 protein is induced by IPTG (data not shown) (19). When immunoblot analyses were performed with the above-mentioned cells, a higher level of WR53.5 protein was present in cells infected with IPTG-treated vi53.5L than with vT7LacOI (Fig. 4B). Moreover, in BSC40 cells infected with vi53.5L, increased concentrations of IPTG from 12.5 μM to 25 μM and 50 μM in the medium induced a corresponding increase of WR53.5 protein expression and the elongated morphology in the infected cells (Fig. 4C), demonstrating that a high level of WR53.5 protein expression allowed detection of its function in cell adhesion. When tested on other cell lines, the WR53.5 protein also mediated cell adhesion in RK13 (Fig. 4D) and BSC1 (data not shown) cells, though not as obviously as in BSC40 cells. To test whether WR53.5-mediated cell adhesion was dependent on calcium, we infected BSC40 cells with vi53.5L and treated the infected cells with EGTA (Fig. 4E). Mock-infected BSC40 cells adhered well to plates but rounded up after EGTA treatment for 20 min. On the other hand, cells infected with vi53.5L, which adhered well in the presence of IPTG, remained adherent even after a 20-min EGTA treatment, showing that WR53.5 protein was required for calcium-independent cell adhesion of the infected cells.
FIG. 4.
WR53.5 protein regulates cell morphology and calcium-independent cell adhesion. (A) Elongated morphology of BSC40 cells infected with vi53.5L virus in the presence of 100 μM IPTG at the late phase of infection. BSC40 cells were infected with vi53.5L or vT7LacOI at an MOI of 5 PFU per cell and cultured in medium with (+) or without (−) 100 μM IPTG for 1 and 2 days. (B) Immunoblots of WR53.5 protein expressed in the infected cells at 2 days p.i. in the presence (+) or absence (−) of 100 μM IPTG. M, mock-infected lysates. (C) WR53.5 protein mediates cell adhesion of virus-infected cells. Immunoblot analyses of WR53.5 protein induced by different IPTG concentrations at 2 days p.i. are shown in the top panel. M, mock-infected cells. BSC40 cells infected with vi53.5L, cultured with (12.5, 25, and 50 μM) or without (0 μM) IPTG for 2 days and photographed with a Nikon inverted microscope are shown in the bottom panel. (D) WR53.5 regulates cell morphology in RK13 cells. RK13 cells were infected with vi53.5L as described in panel A and cultured with or without 100 μM IPTG and photographed at 1 day p.i. with a Nikon inverted microscope. (E) WR53.5 protein mediates Ca2+-independent cell adhesion in BSC40 cells infected with vi53.5L virus. Experiments were conducted as described above. In brief, confluent monolayers of BSC40 cells were either mock infected (Mock) or infected with vi53.5L virus at an MOI of 5 PFU per cell and incubated at 37°C in the presence (vi53.5L+IPTG) or in the absence (vi53.5L−IPTG) of 100 μM IPTG. At 2 days p.i., cells were washed three times with PBS (pH 7.2) and photographed before and after 20 min EGTA treatment, as described previously (28). D, day.
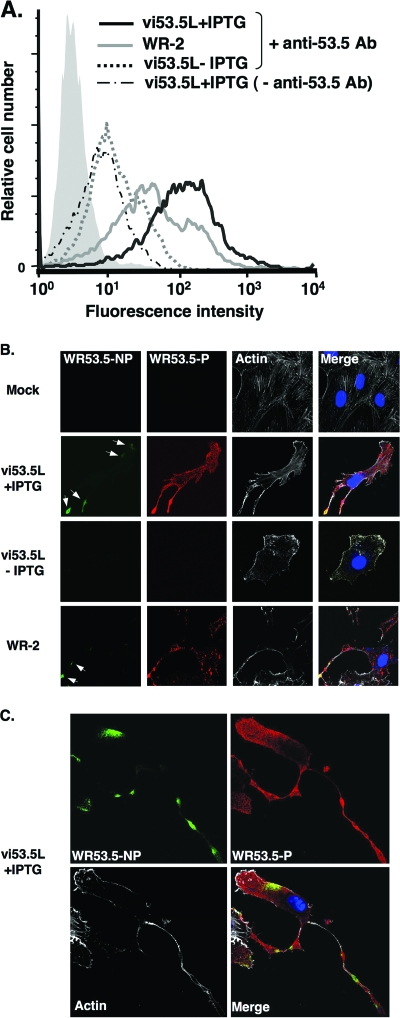
The fact that WR53.5 mediates cell adhesion suggests a possibility that it is expressed on the surface of virus-infected cells. To test this hypothesis, BSC40 cells were infected with vi53.5L, cultured in medium with or without IPTG for 1 day, and harvested for FACS analyses using anti-53.5 Abs. Wild-type WR-2 virus was also included to measure WR53.5 protein expression from its endogenous promoter. As shown in Fig. 5A, strong fluorescent staining of WR53.5 protein was detected on BSC40 cells infected with vi53.5L in the presence of IPTG. Less strong expression of WR53.5 protein was detected on cells infected with wild-type WR-2. In the absence of IPTG, anti-53.5 Ab detected a low level of fluorescence staining that was comparable to the background staining with the secondary Ab only.
FIG. 5.
Cell surface and intracellular distribution of WR53.5 protein in the infected cells. (A) Flow cytometry of WR53.5 protein expression on virus-infected cells. BSC40 cells were either mock infected or infected with WR-2 or vi53.5L, cultured in the medium with (vi53.5+IPTG) or without (vi53.5−IPTG and WR-2) IPTG for 24 h, detached by 5 mM EDTA, stained with anti-53.5 Ab and FITC-conjugated goat anti-rabbit Ab, and analyzed by FACS analysis. Mock-infected cells are shown as the shaded area. −anti-53.5 Ab, background cell staining with secondary FITC-conjugated goat anti-rabbit Ab only. (B) Immunofluorescence analyses of WR53.5 protein in the infected cells at 1 day p.i. BSC40 cells were mock infected or infected with WR-2 or vi53.5L and cultured in medium with (+) or without (−) IPTG for 24 h, fixed, and stained with anti-53.5 Ab under nonpermeable (NP) or permeable (P) conditions as described in Materials and Methods. Cell surface WR53.5 protein is shown in green (pointed by white arrows), and the total WR53.5 protein in cells is shown in red. Actin stained with Alexa Fluor 647-phalloidin is shown in white, and DNA stained with DAPI is shown in blue. Cell images were collected with an LSM510 META confocal laser scanning microscope (Carl Zeiss, Germany) using a 63× objective lens and the confocal microscopy program Release, version 2.8 (Carl Zeiss). (C) Immunofluorescence analyses of WR53.5 protein in virus-infected cells at 2 day p.i. The infected cells described in panel B were fixed at 2 days p.i. and processed to visualize cell surface WR53.5 protein (green) and total WR53.5 protein (red) in cells. Note that the cells shown in panel C were composed from two overlapping photos due to the presence of the extra-long protrusions extending from cell bodies.
To investigate the intracellular distribution of WR53.5 protein in cells, we performed immunofluorescence analyses using anti-53.5 Ab on mock- and virus-infected cells at 1 day p.i. (Fig. 5B). Under nonpermeabilized conditions, anti-53.5 Ab did not stain mock-infected cells or cells infected with vi53.5L and cultured without IPTG. With IPTG, anti-53.5 Ab stained WR53.5 protein on cells infected with vi53.5 as small patches on cell edges and at cell tips. The patch-like surface staining of WR53.5 protein was also observed in cells infected with wild type WR-2. After permeabilization, abundant intracellular staining of WR53.5 protein was specifically detected in the cytoplasm of cells infected with vi53.5L in the presence of IPTG and with wild-type WR-2. The intracellular staining of WR53.5 looked like small dots, and we believe some of these dots may represent intracellular virion particles in the cytoplasm. When cells infected with vi53.5L were allowed to grow to 2 days p.i. in the presence of IPTG (Fig. 5C), elongated cells with extremely long cell protrusions were prevalent in cultures. Cell surface staining of WR53.5 protein decorated specific patches along these cell protrusions, consistent with its role in mediating cell adherence.
The WR53.5 protein is important for vaccinia virus virulence in mice.
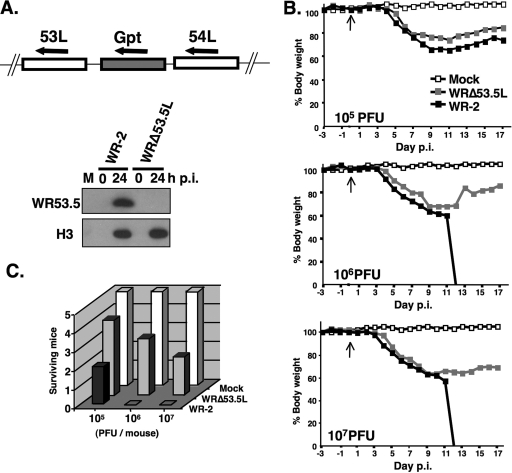
Although WR53.5 expression was tightly regulated by IPTG in vi53.5L virus, it is not suited for in vivo studies because its thymidine kinase locus was inactivated by inserting a T7 RNA pol cassette. To address whether WR53.5 protein is important for virus virulence in vivo, we generated a deletion virus, WRΔ53.5L, that inactivated the WR53.5L ORF from wild-type WR-2 vaccinia virus (Fig. 6A). The WRΔ53.5L virus grew well in cell cultures, and no differences in titers and plaque morphology were observed compared with the wild-type WR-2 virus. BALB/c mice were inoculated intranasally with 105, 106, and 107 PFU of wild-type WR-2 or WRΔ53.5L mutant virus per mouse, and the body weights of individual mice were measured every day for a period of 18 days. As shown in Fig. 6B, all the mice infected by wild-type WR-2 virus or WRΔ53.5L virus started losing weight at 4 days p.i. and continued weight loss for another 5 to 7 days. Some infected mice were severely ill and died at day 11 whereas others slowly recovered. The difference in weight loss between mice infected by wild-type WR-2 virus or WRΔ53.5 virus was small in all three dosages (Fig. 6B). However, all the mice infected with 106 and 107 PFU of wild-type WR-2 virus died by 12 days p.i. whereas 60% and 40% of mice infected by WRΔ53.5L mutant virus survived, respectively (Fig. 6C). We thus, concluded that WR53.5 protein contributes to vaccinia virus virulence in vivo.
FIG. 6.
WR53.5 protein contributes to vaccinia virus virulence in mice. (A) Generation of WRΔ53.5L mutant virus from wild-type vaccinia virus WR-2. The top panel shows a schematic representation of the WR53.5 locus that is interrupted in WRΔ53.5L by Gpt substitution. Immunoblots of cell lysates from BSC40 cells infected with wild-type WR-2 or WRΔ53.5L using anti-53.5 (1:1,000) or anti-H3 (1:2,000) Abs are shown in the bottom panel. M, mock-infected cells. (B) Body weight changes of mice infected with wild-type WR-2 and WRΔ53.5L viruses. Groups of 7- to 8-week-old male BALB/c mice (n = 5 per group) were either mock infected or infected with WR-2 or WRΔ53.5L virus intranasally at a dosage of 105, 106, or 107 PFU per mouse. The arrows indicate the time of virus inoculation. These mice were weighed daily for a period of 17 days. (C) Death of mice inoculated with wild-type WR-2 and WRΔ53.5L virus in each group.
DISCUSSION
The vaccinia virus WR53.5L/F14.5 ORF and its orthologues are present in the Orthopoxvirus genus within the Poxviridae (11). It encodes a conserved protein of 49 amino acid residues with a putative transmembrane region at the N-terminal region. Our original vaccinia virus WR strain, WR-1, encodes WR53.5/F14.5 protein with an E-to-K mutation at position 44 (WR53.544K), which was detected in lysates at a reduced level and barely detected in IMV particles by immunoblot analysis; this explains why none of the tryptic peptides derived from WR53.5 protein was detected in all previous mass spectrometry analyses. In contrast, two tryptic peptides, YVEENNEEDAR and IKEEQELLLLY, from WR53.5 protein were repeatedly detected in our new proteomic data obtained by vaccinia virus vT7lacO/I or other strains such as Copenhagen. It could be that the E44K mutation generated a cryptic trypsin cleavage site within the peptide IKEEQKLLLLY, resulting in small fragments, IKEEQK and LLLLY, that were too small to be detected by mass spectrometry analysis. Or it could be that the WR53.544K protein was less stable than the WR53.544E protein and was not packaged well in IMV, consistent with the experiment shown in Fig. 1E. Wild-type WR53.5 protein was also not reported in proteomic analyses of vaccinia IMV reported by others (4, 24, 31, 35). Here, we demonstrated that the WR53.5 protein is incorporated into vaccinia IMV, with the C-terminal region exposed to the outside of the virions. We also showed that the WR53.5 protein was expressed abundantly on the cell surface and mediated calcium-independent cell adhesion. In all our assays WR53.5 exerts the strongest adhesion phenotype on BSC40 cells, which was much easier to detect than on other cell lines such as BSC1 and RK13 cells.
Two vaccinia proteins, A55R and C2L, were previously reported to affect calcium-independent cell adhesion and the formation of long projections (1, 28). Both proteins belong to the kelch protein family with a POZ/BTB domain at the N terminus and multiple kelch repeats at the C terminus. Both proteins are intracellular proteins that were nonessential for virus growth in cell cultures and were not packaged into virions; a loss of either protein had no effect on virus virulence in an intranasal infection mouse model (1, 28). However, deletion of the C2 or A55 protein caused a lesion in mice that took longer to heal in an intradermal infection mouse model (1, 28). The third kelch protein in vaccinia virus is F3 protein, but deletion of this had no effect on adhesion behavior of the virus-infected cells, suggesting that F3 protein function is distinct from that of the A55 and C2 proteins (10). Viral kelch proteins self-interacted but did not interact with other kelch proteins in yeast two-hybrid analyses (1). In contrast to the above viral kelch proteins, WR53.5 protein does not contain any kelch homology sequences, and the protein is an envelope protein on IMV particles and is also expressed on the cell surface, suggesting a different mode of regulation of cell adhesion from that of viral kelch proteins. Whether the WR53.5 protein interacts with other viral or cellular proteins to mediate cell adhesion is not known. Although calcium-independent adhesion could be mediated by integrin (22), immunofluorescence staining of WR53.5 protein did not show any increased staining at focal adhesions (data not shown). Also, we have transiently expressed the WR53.5 protein alone in 293T cells but failed to observe any corresponding enhancement in cell adhesion (data not shown), implying that WR53.5 requires other viral proteins to function in cell adhesion. Alternatively, it could be that the level of WR53.5 protein expressed in these transfected cells was not sufficient to mediate cell adhesion.
During our manuscript preparation, we found an independent work by Zhang et al., who described construction of a recombinant vaccinia virus of the LIVP strain, GLV-1h68, by inserting three marker genes into the F14.5, J2R, and A56R loci of the viral genome (36). The recombinant virus, GLV-1h68, which was subsequently injected intravenously into tumor-bearing nude mice, exhibited enhanced tumor targeting specificity and reduced toxicity and was a better oncolytic viral therapeutic agent than its parental LIVP strain (36). Although our experimental designs were different from their study, our results that WR53.5/F14.5 contributes to virus virulence in BALB/c mice are consistent with their observations.
Inactivation of WR53.5 did not block virus growth in cultures but reduced virus virulence in mice, suggesting that the WR53.5 protein may interfere with host antiviral pathways in animals. Cell adhesion could act as a regulator of intracellular signaling cascades that may play a role in cytokine response (12, 29) or suppression of apoptosis (9). Cell adhesion molecules could also play important roles in the recruitment of leukocytes and in inflammatory responses associated with tissue injury (21, 30). Whether the poxviruses evolved a novel adhesion mechanism that is distinct from cell adhesion regulation remains to be determined.
Acknowledgments
We thank Sue-Ping Lee for help in confocal microscorpy and Ya-Fan Chung and Che-Sheng Chung for help with the mouse virulence experiments. We thank Harry Wilson of Academia Sinica for manuscript editing.
This work was supported by grants from the Academia Sinica and the National Science Council (NSC96-2627-M-001-004) of R.O.C.
Footnotes
Published ahead of print on 6 August 2008.
REFERENCES
- 1.Beard, P. M., G. C. Froggatt, and G. L. Smith. 2006. Vaccinia virus kelch protein A55 is a 64 kDa intracellular factor that affects virus-induced cytopathic effect and the outcome of infection in a murine intradermal model. J. Gen. Virol. 871521-1529. [DOI] [PubMed] [Google Scholar]
- 2.Blasco, R., and B. Moss. 1991. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 655910-5920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bray, M., and M. Buller. 2004. Looking back at smallpox. Clin. Infect. Dis. 38882-889. [DOI] [PubMed] [Google Scholar]
- 4.Chung, C. S., C. H. Chen, M. Y. Ho, C. Y. Huang, C. L. Liao, and W. Chang. 2006. Vaccinia virus proteome: identification of proteins in vaccinia virus intracellular mature virion particles. J. Virol. 802127-2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Condit, R. C., N. Moussatche, and P. Traktman. 2006. In a nutshell: structure and assembly of the vaccinia virion. Adv. Virus Res. 6631-124. [DOI] [PubMed] [Google Scholar]
- 6.da Fonseca, F. G., E. J. Wolffe, A. Weisberg, and B. Moss. 2000. Characterization of the vaccinia virus H3L envelope protein: topology and posttranslational membrane insertion via the C-terminal hydrophobic tail. J. Virol. 747508-7517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Damon, I. K. 2007. Poxviruses, p. 2947-2975. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Strauss (ed.), Fields virology, 5th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 8.Fenner, F. 1990. Poxviruses, p. 2113-2133. In B. Fields and D. M. Knipe (ed.), Virology. Raven Press, New York, NY.
- 9.Frisch, S. M., and R. A. Screaton. 2001. Anoikis mechanisms. Curr. Opin. Cell Biol. 13555-562. [DOI] [PubMed] [Google Scholar]
- 10.Froggatt, G. C., G. L. Smith, and P. M. Beard. 2007. Vaccinia virus gene F3L encodes an intracellular protein that affects the innate immune response. J. Gen. Virol. 881917-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goebel, S. J., G. P. Johnson, M. E. Perkus, S. W. Davis, J. P. Winslow, and E. Paoletti. 1990. The complete DNA sequence of vaccinia virus. Virol. 179247-266. [DOI] [PubMed] [Google Scholar]
- 12.Goldberg, G. S., Z. Jin, H. Ichikawa, A. Naito, M. Ohki, W. S. El-Deiry, and H. Tsuda. 2001. Global effects of anchorage on gene expression during mammary carcinoma cell growth reveal role of tumor necrosis factor-related apoptosis-inducing ligand in anoikis. Cancer Res. 611334-1337. [PubMed] [Google Scholar]
- 13.Heuser, J. 2005. Deep-etch EM reveals that the early poxvirus envelope is a single membrane bilayer stabilized by a geodetic “honeycomb” surface coat. J. Cell Biol. 169269-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hollinshead, M., A. Vanderplasschen, G. L. Smith, and D. J. Vaux. 1999. Vaccinia virus intracellular mature virions contain only one lipid membrane. J. Virol. 731503-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsiao, J. C., C. S. Chung, and W. Chang. 1999. Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 738750-8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hu, X., L. J. Carroll, E. J. Wolffe, and B. Moss. 1996. De novo synthesis of the early transcription factor 70-kilodalton subunit is required for morphogenesis of vaccinia virions. J. Virol. 707669-7677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Husain, M., and B. Moss. 2001. Vaccinia virus F13L protein with a conserved phospholipase catalytic motif induces colocalization of the B5R envelope glycoprotein in post-Golgi vesicles. J. Virol. 757528-7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ichihashi, Y., T. Tsuruhara, and M. Oie. 1982. The effect of proteolytic enzymes on the infectivity of vaccinia virus. Virology 122279-289. [DOI] [PubMed] [Google Scholar]
- 19.Izmailyan, R. A., C. Y. Huang, S. Mohammad, S. N. Isaacs, and W. Chang. 2006. The envelope G3L protein is essential for entry of vaccinia virus into host cells. J. Virol. 808402-8410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Joklik, W. K. 1962. The purification of four strains of poxvirus. Virology 189-18. [DOI] [PubMed] [Google Scholar]
- 21.Kitagawa, A., Y. Miura, R. Saura, M. Mitani, H. Ishikawa, A. Hashiramoto, S. Yoshiya, S. Shiozawa, and M. Kurosaka. 2006. Anchorage on fibronectin via VLA-5 (α5β1 integrin) protects rheumatoid synovial cells from Fas-induced apoptosis. Ann. Rheum Dis. 65721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lallier, T., and M. Bronner-Fraser. 1992. Alpha 1 beta 1 integrin on neural crest cells recognizes some laminin substrata in a Ca2+-independent manner. J. Cell Biol. 1191335-1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, C. L., C. S. Chung, H. G. Heine, and W. Chang. 2000. Vaccinia virus envelope H3L protein binds to cell surface heparan sulfate and is important for intracellular mature virion morphogenesis and virus infection in vitro and in vivo. J. Virol. 743353-3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manes, N. P., R. D. Estep, H. M. Mottaz, R. J. Moore, T. R. Clauss, M. E. Monroe, X. Du, J. N. Adkins, S. W. Wong, and R. D. Smith. 2008. Comparative proteomics of human monkeypox and vaccinia intracellular mature and extracellular enveloped virions. J. Proteome Res. 7960-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nalca, A., A. W. Rimoin, S. Bavari, and C. A. Whitehouse. 2005. Reemergence of monkeypox: prevalence, diagnostics, and countermeasures. Clin. Infect. Dis. 411765-1771. [DOI] [PubMed] [Google Scholar]
- 26.Niles, E. G., and J. Seto. 1988. Vaccinia virus gene D8 encodes a virion transmembrane protein. J. Virol. 623772-3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pedersen, K., E. J. Snijder, S. Schleich, N. Roos, G. Griffiths, and J. K. Locker. 2000. Characterization of vaccinia virus intracellular cores: implications for viral uncoating and core structure. J. Virol. 743525-3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pires de Miranda, M., P. C. Reading, D. C. Tscharke, B. J. Murphy, and G. L. Smith. 2003. The vaccinia virus kelch-like protein C2L affects calcium-independent adhesion to the extracellular matrix and inflammation in a murine intradermal model. J. Gen. Virol. 842459-2471. [DOI] [PubMed] [Google Scholar]
- 29.Prickett, T. D., and D. L. Brautigan. 2007. Cytokine activation of p38 mitogen-activated protein kinase and apoptosis is opposed by alpha-4 targeting of protein phosphatase 2A for site-specific dephosphorylation of MEK3. Mol. Cell. Biol. 274217-4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prozialeck, W. C., and J. R. Edwards. 2007. Cell adhesion molecules in chemically-induced renal injury. Pharmacol. Ther. 11474-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resch, W., K. K. Hixson, R. J. Moore, M. S. Lipton, and B. Moss. 2007. Protein composition of the vaccinia virus mature virion. Virology 358233-247. [DOI] [PubMed] [Google Scholar]
- 32.Rosel, J. L., P. L. Earl, J. P. Weir, and B. Moss. 1986. Conserved TAAATG sequence at the transcriptional and translational initiation sites of vaccinia virus late genes deduced by structural and functional analysis of the HindIII H genome fragment. J. Virol. 60436-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ward, G. A., C. K. Stover, B. Moss, and T. R. Fuerst. 1995. Stringent chemical and thermal regulation of recombinant gene expression by vaccinia virus vectors in mammalian cells. Proc. Natl. Acad. Sci. USA 926773-6777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeh, W. W., B. Moss, and E. J. Wolffe. 2000. The vaccinia virus A9L gene encodes a membrane protein required for an early step in virion morphogenesis. J. Virol. 749701-9711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yoder, J. D., T. S. Chen, C. R. Gagnier, S. Vemulapalli, C. S. Maier, and D. E. Hruby. 2006. Pox proteomics: mass spectrometry analysis and identification of vaccinia virion proteins. Virol. J. 310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang, Q., Y. A. Yu, E. Wang, N. Chen, R. L. Danner, P. J. Munson, F. M. Marincola, and A. A. Szalay. 2007. Eradication of solid human breast tumors in nude mice with an intravenously injected light-emitting oncolytic vaccinia virus. Cancer Res. 6710038-10046. [DOI] [PubMed] [Google Scholar]
- 37.Zhang, W. H., D. Wilcock, and G. L. Smith. 2000. Vaccinia virus F12L protein is required for actin tail formation, normal plaque size, and virulence. J. Virol. 7411654-11662. [DOI] [PMC free article] [PubMed] [Google Scholar]