Abstract
Members of the family Pospiviroidae, like Citrus exocortis viroid (CEVd), replicate through an RNA-based asymmetric rolling-circle mechanism in which oligomeric plus-strand [(+)] RNA intermediates are cleaved to monomeric linear (ml) RNA and then circularized. Here we show, by rapid amplification of 5′ and 3′ cDNA ends and in vitro ligation assays, that ml CEVd (+) RNA resulting from cleavage of a dimeric transcript transgenically expressed in Arabidopsis thaliana contains 5′-phosphomonoester and 3′-hydroxyl termini. The nature of these termini and the double-stranded structure previously proposed as the substrate for cleavage in vivo suggest that a type III RNase catalyzes cleavage and an RNA ligase distinct from tRNA ligase promotes circularization.
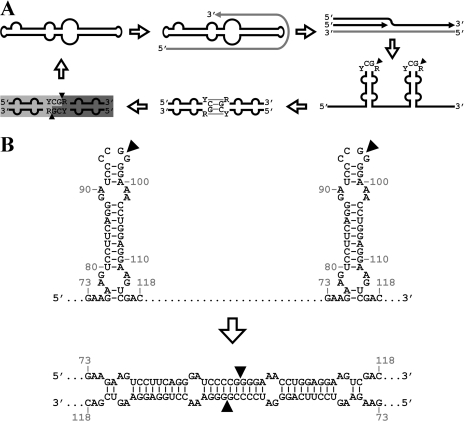
Viroids are plant pathogens consisting of a non-protein-coding small circular RNA (246 to 401 nucleotides [nt]) (5, 8, 13, 26). The approximately 30 viroid species known are classified into two families, Pospiviroidae and Avsunviroidae, whose members replicate in the nucleus and chloroplast, respectively (13). Viroid replication occurs through an RNA rolling-circle mechanism characterized by the reiterative RNA-RNA transcription of circular templates and processing of the resulting oligomeric RNAs of one or both polarities (2). In members of the family Pospiviroidae, like Potato spindle tuber viroid (PSTVd), the most abundant circular RNA in vivo to which plus-strand [(+)] polarity is assigned arbitrarily, is transcribed into complementary oligomeric minus-strand [(−)] RNAs. These strands serve directly as templates for the synthesis of oligomeric (+) RNAs that are processed into monomeric linear (ml) and monomeric circular (mc) RNAs (1). In members of the family Avsunviroidae, like Avocado sunblotch viroid, the oligomeric (−) RNAs are processed into mc (−) RNAs, which through a second rolling circle direct the synthesis of oligomeric (+) RNAs, with subsequent processing producing the mc (+) RNAs (7). Whereas in the family Avsunviroidae, cleavage of the oligomeric RNAs of both polarities is mediated by hammerhead ribozymes that generate ml RNAs with 5′-hydroxyl (5′-OH) and 2′,3′-cyclic phosphodiester (2′,3′>P) termini (11), how this step happens in the family Pospiviroidae is unclear. Previous work showed that viroid RNA processing occurs accurately in transgenic lines of the viroid nonhost Arabidopsis thaliana expressing dimeric transcripts of representative members of the family Pospiviroidae (6). With this experimental system, the processing site of oligomeric (+) RNA intermediates has been mapped at a position within a conserved double-stranded structure that two consecutive hairpin I motifs can promote (Fig. 1) (14).
FIG. 1.
(A) Asymmetric rolling-circle replication cycle followed by members of the family Pospiviroidae that includes the kissing-loop interaction between two contiguous hairpin I motifs and the subsequent formation of a double-stranded structure proposed to be the substrate for cleavage (14). (B) Hairpin I and double-stranded structure corresponding to CEVd (+) RNA. Black arrowheads indicate the processing sites located 2 nt apart in each strand. Nucleotide numbering refers to CEVd sequence variant (with GenBank sequence number M34917) with a deleted G between positions 70 and 74.
Here, using transgenic A. thaliana expressing a dimeric transcript of Citrus exocortis viroid (CEVd) (genus Pospiviroid, family Pospiviroidae), we have determined the termini of the ml (+) RNA accumulating in vivo by rapid amplification of 5′ and 3′ cDNA ends (5′- and 3′-RACE) (25) and by in vitro ligation assays. The nature of these termini, 5′-phosphomonoester (5′-P) and 3′-OH, has deep implications concerning the host enzymes that mediate processing of the replicative intermediates.
5′- and 3′-RACE of the ml CEVd (+) RNA.
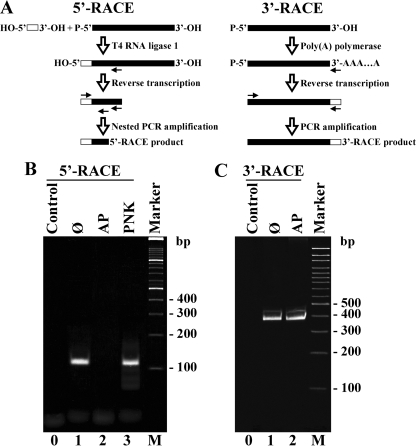
An RNA preparation enriched in viroid RNAs was obtained by chromatography on nonionic cellulose CF11 (21) from an A. thaliana transgenic line expressing constitutively a dimeric head-to-tail CEVd (+) transcript (sequence variant with GenBank nucleotide sequence accession number M34917 with a deleted G between positions 70 and 74) starting at position 40 and ending at position 39 (6). The preparation was separated by double polyacrylamide gel electrophoresis (PAGE), first in native conditions and then in denaturing conditions (12), and the fraction of RNAs comigrating in the region of a ml CEVd (+) RNA standard purified from CEVd-infected gynura (Gynura aurantiaca DC) was eluted. Northern blot hybridization revealed that the ml (+) RNA was the only CEVd species present in the fraction (data not shown). To characterize the 5′ terminus of the ml CEVd (+) RNA, three aliquots of this fraction were ligated to the adaptor oligoribonucleotide PI [5′-r(CGACUGGAGCACGAGGACACUGACAUGGACUGAAGGAGUA GAAA)-3′, where r indicates that the sequence corresponds to an oligoribonucleotide] with T4 RNA ligase 1 (Epicentre) that requires 5′-P and 3′-OH termini. The first aliquot did not receive any previous treatment, while the second was pretreated with T4 polynucleotide kinase (Roche) and the third was pretreated with calf alkaline phosphatase (Roche). The ligation products were used as templates in reverse transcription (RT) reactions with Superscript III reverse transcriptase (Invitrogene) and the viroid-specific primer PII (5′-GCTTCAGCGACGATCGGATGTGGAGCC-3′) complementary to CEVd sequence between positions 203 and 229 and then subjected to PCR amplification with the High Expand DNA polymerase mixture (Roche), an adaptor-specific primer PIII (5′-GGAGCACGAGGACACTGACATGG-3′) with the same sequence as that underlined in PI and a nested viroid-specific primer PIV (5′-TTCTCCGCTGGACGCCAGTGATCCGC-3′) complementary to CEVd sequence between positions 147 and 172 (Fig. 2A, left). Separation of RT-PCR products by PAGE showed amplification of a single prominent cDNA in the reaction mixture containing the untreated aliquot and in the mixture pretreated with polynucleotide kinase (Fig. 2B, lanes 1 and 3). Conversely, no amplification products were detected in the reaction mixture containing the aliquot pretreated with alkaline phosphatase (Fig. 2B, lane 2). The amplification products resulting from the untreated and polynucleotide kinase-treated aliquots were eluted from the gel and cloned in plasmid pTZ57R/T (Fermentas). Sequencing of several clones corresponding to each product showed that they were identical and consisted of a 115-bp DNA that, in addition to the adaptor, included the CEVd sequence from G97 to A172. This result confirmed that processing of the dimeric CEVd (+) RNA occurs between positions G96 and G97 as previously determined by primer extension (14), and it also showed that the resulting ml (+) RNA intermediate has a 5′-P terminus, since it was ligated to the adaptor without pretreatment with polynucleotide kinase but not after pretreatment with alkaline phosphatase.
FIG. 2.
Analysis of the 5′ and 3′ termini of the ml CEVd (+) RNA purified from transgenic A. thaliana expressing a dimeric CEVd (+) transcript. (A) Scheme depicting 5′- and 3′-RACE procedures. (B and C) RNA aliquots not treated or subjected to different pretreatments were subjected to 5′-RACE (B) and 3′-RACE (C), with the resulting products being separated by PAGE. Lane 0, control without RNA; lane 1, untreated RNA (Ø); lanes 2 and 3, RNA treated with alkaline phosphatase (AP) and polynucleotide kinase (PNK), respectively; lane M, DNA markers (M) with sizes (in base pairs) indicated to the right of the gels.
This approach was extended to two additional members of the family Pospiviroidae expressed in transgenic A. thaliana (6): Apple scar skin viroid and Hop stunt viroid, the type species of the genera Apscaviroid and Hostuviroid, respectively (13). The results showed that the 5′ termini of the purified ml viroid (+) RNAs also have a 5′-P group and confirmed the processing sites between positions G90 and A91 (Apple scar skin viroid, sequence variant with GenBank accession number AF421195) and G82-G83 (Hop stunt viroid, sequence variant with GenBank accession number D13764) (data not shown) as previously identified by primer extension (14). The same strategy was finally applied to the ml CEVd (+) RNA purified from infected gynura. In this case, six or seven cDNAs were amplified, indicating that the population of ml CEVd (+) RNA is more heterogeneous in an infected plant. However, this population included the 115-bp product, thus confirming the presence of an RNA starting and ending at positions G97 and G96, respectively, and with a 5′-P terminus.
To characterize the 3′ terminus of the ml CEVd (+) RNA purified from transgenic A. thaliana, two aliquots of the preparation were 3′ polyadenylated with yeast poly(A) polymerase (USB) that adds AMP residues to an RNA with a terminal 3′-OH group. The first aliquot did not receive any previous treatment, while the second was pretreated with alkaline phosphatase. The polyadenylation products were subjected to RT with a poly(A) tail-complementary primer PV (5′-CCGGATCCTCTAGATCGGCCGCT17V-3′, where V is A, C, or G) and to PCR amplification with this same primer and the CEVd-specific PVI primer (5′-GGAAACCTGGAGGAAGTCG-3′) homologous to the CEVd sequence between positions 98 and 116 (Fig. 2A, right). PAGE separation of the resulting RT-PCR products showed the amplification of the same two DNAs in both reaction mixtures (Fig. 2C, lanes 1 and 2). The cDNAs amplified from the untreated RNA aliquot were eluted from the gel and cloned. Sequencing of several clones revealed that the largest and less abundant product was a 408-bp DNA including the CEVd sequence from G98 to U370 and from C1 to G96 and that the smaller and most abundant product was a 362-bp DNA including the CEVd sequence from G98 to U370 and from C1 to G50. The sequence of the largest amplification product confirmed again that processing of oligomeric CEVd (+) RNA in transgenic A. thaliana occurs between positions G96 and G97 (14). Moreover, since the ml CEVd (+) RNA was polyadenylated without any pretreatment, this RNA must contain a 3′-OH free terminus, together with the already characterized 5′-P. On the other hand, the sequence of the smaller amplification product predicted a second 3′-OH free terminus at position G50 that had not been detected in previous experiments (14). The observation that CEVd contains a poly(A)-rich sequence (positions 51 to 61) next to G50 suggests that the smaller amplification product most likely resulted from hybridization of the poly(dT) 3′ tail of primer PV with this poly(A)-rich region and that, consequently, position G50 does not actually correspond to a ml CEVd (+) RNA terminus.
In vitro ligation assays.
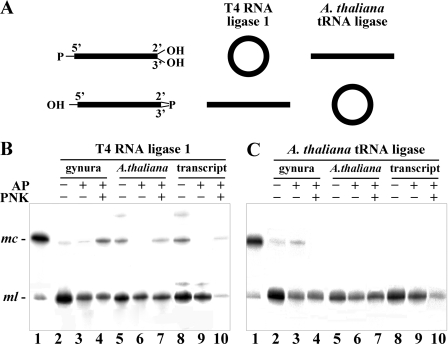
Next, the susceptibility of the ml CEVd (+) RNA purified from transgenic A. thaliana to be ligated by two enzymes with distinct specificities was assayed: the T4 RNA ligase 1 (Rnl1) that requires 5′-P and 3′-OH termini (9, 28, 29) and the A. thaliana tRNA ligase that requires 5′-OH and 2′,3′>P termini (10) (Fig. 3A). Additionally, ml CEVd (+) RNA purified from CEVd-infected gynura, as well as RNA synthesized in vitro by transcription and subsequent treatment with tobacco acid pyrophosphatase (Epicentre)—resulting in a full-length ml CEVd (+) RNA from positions G97 to U370 and from C1 to G96 with 5′-P and 3′-OH termini—were used as a controls. Aliquots of these three RNAs that were either untreated, pretreated with alkaline phosphatase, or pretreated first with alkaline phosphatase and then with polynucleotide kinase were assayed for ligation with T4 RNA ligase 1 and A. thaliana tRNA ligase. For this purpose, a recombinant version of the latter (GenBank accession number NP_172269) with a carboxy-terminal His tag was expressed in Escherichia coli and purified. Ligation products were separated by denaturing PAGE and examined by Northern blot hybridization with a cRNA probe labeled with 32P. The ml CEVd (+) RNA from infected gynura was circularized by the T4 RNA ligase 1 in all instances (Fig. 3B, lanes 2 to 4), whereas that from transgenic A. thaliana was ligated when untreated (Fig. 3B, lane 5) or pretreated with alkaline phosphatase plus polynucleotide kinase (Fig. 3B, lane 7), but not when pretreated only with alkaline phosphatase (Fig. 3B, lane 6). The ml CEVd (+) RNA control synthesized in vitro with 5′-P and 3′-OH termini behaved similarly to the RNA from A. thaliana (Fig. 3B, lanes 8 to 10).
FIG. 3.
(A) Diagram depicting results of ligation by T4 RNA ligase 1 and A. thaliana tRNA ligase of two RNAs with different termini. (B and C) Ligation in vitro of ml CEVd (+) RNA purified from infected gynura and transgenic A. thaliana expressing a dimeric CEVd (+) transcript. Products generated by T4 RNA ligase 1 (B) and A. thaliana tRNA ligase (C) were separated by denaturing PAGE and analyzed by Northern blot hybridization. Prior to ligation, aliquots of ml CEVd (+) RNA from infected gynura (lanes 2 to 4) or transgenic A. thaliana (lanes 5 to 7) and ml CEVd (+) RNA synthesized in vitro with 5′-P and 3′-OH termini (lanes 8 to 10) were not treated (−) (lanes 2, 5, and 8) or pretreated (+) with alkaline phosphatase (AP) (lanes 3, 6, and 9) or pretreated (+) with alkaline phosphatase and polynucleotide kinase (PNK) (lanes 4, 7, and 10). Lane 1, RNA from infected gynura containing mc and ml CEVd (+) RNAs with their positions indicated to the left of the gels. The product with the lowest mobility in lanes 5 and 8 most likely corresponds to the dimeric linear CEVd RNA.
On the other hand, A. thaliana tRNA ligase circularized, although with low efficiency, the ml CEVd (+) RNA from infected gynura (Fig. 3C, lane 2). Circularization of this RNA also occurred after pretreatment with alkaline phosphatase (Fig. 3C, lane 3), but not when additionally pretreated with polynucleotide kinase (Fig. 3C, lane 4). Neither the ml CEVd (+) RNA from transgenic A. thaliana (Fig. 3C, lanes 5 to 7) nor the CEVd (+) RNA synthesized in vitro with 5′-P and 3′-OH termini (Fig. 3C, lanes 8 to 10) were circularized by the A. thaliana tRNA ligase. Altogether, these results indicate that the ml CEVd (+) RNA from transgenic A. thaliana contains essentially 5′-P and 3′-OH termini, whereas its counterpart from infected gynura is more heterogeneous and composed by molecules with 5′-P and 3′-OH termini and with 5′-OH and 2′,3′>P termini. The 5-P termini of some molecules from gynura may be buried within the secondary structure, thus explaining their circularization with T4 RNA ligase 1 after pretreatment with alkaline phosphatase (Fig. 3B, lane 3).
Termini of the native ml (+) RNAs from the family Pospiviroidae: comparison with previous data.
The identification of 5′-P and 3′-OH termini in the ml (+) RNA intermediate processed in transgenic A. thaliana expressing a dimeric CEVd (+) transcript is consistent with data from an early report with Chrysanthemum stunt viroid, indicating that 72% of ml Chrysanthemum stunt viroid (+) RNA purified from infected plants contains a 5′-P terminus (23). In addition, ml CEVd (+) RNA with 5′-P and 3′-OH termini obtained by in vitro transcription and treatment with alkaline phosphatase and polynucleotide kinase was shown to be highly infectious (24). However, subsequent studies on the nature and biological significance of the ml PSTVd (+) RNA based on the 5′-end labeling of this species isolated from infected tissue (22), on its ligation in vitro by wheat germ and Chlamydomonas reinhardtii RNA ligases (3, 19), and on the infectivity of ml PSTVd (+) RNA produced by artificially nicking the circular forms (16) led to the proposal that the ml PSTVd (+) RNA intermediate has 5′-OH and 2′,3′>P termini. However, the present results and those from a previous work aimed at characterizing the in vivo processing site of the oligomeric (+) RNAs of CEVd and two other members of the family Pospiviroidae (14) indicate that the ml CEVd (+) RNA population in transgenic A. thaliana is mostly homogeneous and representative of the product resulting from the processing in vivo of the dimeric transcript, possibly because viroid replication in this nonhost plant is low and RNA-RNA amplification of the mc CEVd (+) RNA is inefficient (6, 14). In contrast, in a host plant like gynura, the mc CEVd (+) RNA serves as a template for efficient RNA-RNA amplification and the population of the replicative intermediate ml CEVd (+) is contaminated with nicked by-products of the most abundant mc CEVd (+) RNA (14). Therefore, the experimental system based on transgenic A. thaliana offers the advantage that it can be programmed with a replication intermediate (6), and the fate of the intermediate can be monitored with little interference resulting from degradation of the mc viroid (+) RNA in vivo or in vitro during extraction and purification. Despite the fact that the present results suggest that the ml CEVd (+) RNA forms with 5′-OH and 2′,3′>P termini from gynura arise from nicking of the most abundant mc CEVd (+) RNA, the possibility that a fraction of these molecules may participate in the replication cycle cannot be discarded.
Host factors involved in viroid RNA processing.
In the family Avsunviroidae, hammerhead ribozymes catalyze self-cleavage of the oligomeric RNAs of both polarities in vivo (11). However, ribozyme-mediated RNA processing does not seem to operate in the family Pospiviroidae (27), and accordingly, the current consensus is that cleavage and ligation of the oligomeric (+) RNA intermediates are catalyzed by host enzymes. A good candidate to mediate cleavage is a type III RNase, because the 5′-P and 3′-OH termini identified here in the ml CEVd (+) RNA are those characteristically produced by these enzymes that act on double-stranded or highly structured RNA substrates (20). Moreover, some type III RNases cleave both strands of their substrates, generating products of approximately 20 bp with 2-nt 3′-protruding termini (15). A conserved double-stranded structure, formed by a kissing-loop interaction between two hairpin I motifs contiguous in the oligomeric (+) RNA, has been proposed as the substrate for cleavage, with the cleavage sites in each strand being 2 nt apart (Fig. 1) (14). To date, in A. thaliana, seven type III RNases have been described, including four Dicer-like proteins (DCL1 to DCL4) and three additional ones (RTL1 to RTL3) (4, 17). Regarding ligation, the resulting ml CEVd (+) RNA with 5′-P and 3′-OH termini is not a substrate of the tRNA ligase, the only plant RNA ligase described so far (10). Therefore, unless these termini are later modified, the present results suggest the existence of a second plant RNA ligase that would mediate joining of the same 5′-P and 3′-OH termini as those required by the T4 RNA ligases 1 and 2 (18, 24, 28, 29).
Acknowledgments
We thank A. Ahuir for excellent technical assistance. Work in the J.-A.D. and R.F. laboratories was supported by the Ministerio de Educación y Ciencia (MEC) of Spain (grants BFU2005-06808/BMC and AGL2004-06311-C02-01) and by the Generalitat Valenciana (ACOMP07/268). M.-E.G. received predoctoral fellowships from MEC and CSIC-Bancaja, and D.M-S. received a predoctoral fellowship from MEC.
Footnotes
Published ahead of print on 13 August 2008.
REFERENCES
- 1.Branch, A. D., B. J. Benenfeld, and H. D. Robertson. 1988. Evidence for a single rolling circle in the replication of potato spindle tuber viroid. Proc. Natl. Acad. Sci. USA 859128-9132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Branch, A. D., and H. D. Robertson. 1984. A replication cycle for viroids and other small infectious RNAs. Science 223450-455. [DOI] [PubMed] [Google Scholar]
- 3.Branch, A. D., H. D. Robertson, C. Greer, P. Gegenheimer, C. Peebles, and J. Abelson. 1982. Cell-free circularization of viroid progeny RNA by an RNA ligase from wheat-germ. Science 2171147-1149. [DOI] [PubMed] [Google Scholar]
- 4.Comella, P., F. Pontvianne, S. Lahmy, F. Vignols, N. Barbezier, A. DeBures, E. Jobet, E. Brugidou, M. Echeverria, and J. Sáez-Vásquez. 2008. Characterization of a ribonuclease III-like protein required for cleavage of the pre-rRNA in the 3′ETS in Arabidopsis. Nucleic Acids Res. 361163-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daròs, J. A., S. F. Elena, and R. Flores. 2006. Viroids: an Ariadne's thread into the RNA labyrinth. EMBO Rep. 7593-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daròs, J. A., and R. Flores. 2004. Arabidopsis thaliana has the enzymatic machinery for replicating representative viroid species of the family Pospiviroidae. Proc. Natl. Acad. Sci. USA 1016792-6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daròs, J. A., J. F. Marcos, C. Hernández, and R. Flores. 1994. Replication of avocado sunblotch viroid: evidence for a symmetric pathway with two rolling circles and hammerhead ribozyme processing. Proc. Natl. Acad. Sci. USA 9112813-12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding, B., and A. Itaya. 2007. Viroid: a useful model for studying the basic principles of infection and RNA biology. Mol. Plant-Microbe Interact. 207-20. [DOI] [PubMed] [Google Scholar]
- 9.El Omari, K., J. Ren, L. E. Bird, M. K. Bona, G. Klarmann, S. F. J. LeGrice, and D. K. Stammers. 2006. Molecular architecture and ligand recognition determinants for T4 RNA ligase. J. Biol. Chem. 2811573-1579. [DOI] [PubMed] [Google Scholar]
- 10.Englert, M., and H. Beier. 2005. Plant tRNA ligases are multifunctional enzymes that have diverged in sequence and substrate specificity from RNA ligases of other phylogenetic origins. Nucleic Acids Res. 33388-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flores, R., J. A. Daròs, and C. Hernández. 2000. The Avsunviroidae family: viroids containing hammerhead ribozymes. Adv. Virus Res. 55271-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flores, R., N. Duran-Vila, V. Pallás, and J. S. Semancik. 1985. Detection of viroid and viroid-like RNAs from grapevine. J. Gen. Virol. 662095-2102. [Google Scholar]
- 13.Flores, R., C. Hernández, A. E. Martínez de Alba, J. A. Daròs, and F. Di Serio. 2005. Viroids and viroid-host interactions. Annu. Rev. Phytopathol. 43117-139. [DOI] [PubMed] [Google Scholar]
- 14.Gas, M. E., C. Hernández, R. Flores, and J. A. Daròs. 2007. Processing of nuclear viroids in vivo: an interplay between RNA conformations. PLoS Pathog. 31813-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond, S. M. 2005. Dicing and slicing—the core machinery of the RNA interference pathway. FEBS Lett. 5795822-5829. [DOI] [PubMed] [Google Scholar]
- 16.Hashimoto, J., K. Suzuki, and T. Uchida. 1985. Infectivity of artificially nicked viroid molecules. J. Gen. Virol. 661545-1551. [Google Scholar]
- 17.Hiraguri, A., R. Itoh, N. Kondo, Y. Nomura, D. Aizawa, Y. Murai, H. Koiwa, M. Seki, K. Shinozaki, and T. Fukuhara. 2005. Specific interactions between Dicer-like proteins and HYL1/DRB-family dsRNA-binding proteins in Arabidopsis thaliana. Plant Mol. Biol. 57173-188. [DOI] [PubMed] [Google Scholar]
- 18.Ho, C. K., and S. Shuman. 2002. Bacteriophage T4 RNA ligase 2 (gp24.1) exemplifies a family of RNA ligases found in all phylogenetic domains. Proc. Natl. Acad. Sci. USA 9912709-12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kikuchi, Y., K. Tyc, W. Filipowicz, H. L. Sänger, and H. J. Gross. 1982. Circularization of linear viroid RNA via 2′-phosphomonoester, 3′,5′-phosphodiester bonds by a novel type of RNA ligase from wheat-germ and Chlamydomonas. Nucleic Acids Res. 107521-7529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacRae, I. J., and J. A. Doudna. 2007. Ribonuclease revisited: structural insights into ribonuclease III family enzymes. Curr. Opin. Struct. Biol. 17138-145. [DOI] [PubMed] [Google Scholar]
- 21.Pallás, V., A. Navarro, and R. Flores. 1987. Isolation of a viroid-like RNA from hop different from hop stunt viroid. J. Gen. Virol. 683201-3205. [Google Scholar]
- 22.Palukaitis, P., and M. Zaitlin. 1987. The nature and biological significance of linear potato spindle tuber viroid molecules. Virology 157199-210. [DOI] [PubMed] [Google Scholar]
- 23.Palukaitis, P., and R. H. Symons. 1980. Purification and characterization of the circular and linear forms of chrysanthemum stunt viroid. J. Gen. Virol. 46477-489. [Google Scholar]
- 24.Rigden, J. E., and M. A. Rezaian. 1992. In vitro synthesis of an infectious viroid: analysis of the infectivity of monomeric linear CEV. Virology 186201-206. [DOI] [PubMed] [Google Scholar]
- 25.Schaefer, B. C. 1995. Revolutions in rapid amplification of cDNA ends: new strategies for polymerase chain-reaction cloning of full-length cDNA ends. Anal. Biochem. 227255-273. [DOI] [PubMed] [Google Scholar]
- 26.Tabler, M., and M. Tsagris. 2004. Viroids: petite RNA pathogens with distinguished talents. Trends Plant Sci. 9339-348. [DOI] [PubMed] [Google Scholar]
- 27.Tsagris, M., M. Tabler, and H. L. Sänger. 1987. Oligomeric potato spindle tuber viroid (PSTV) RNA does not process autocatalytically under conditions where other RNAs do. Virology 157227-231. [DOI] [PubMed] [Google Scholar]
- 28.Uhlenbeck, O. C., and R. I. Gumport. 1982. T4 RNA ligase, p. 31-58. In P. Boyer (ed.), The enzymes. Academic Press, New York, NY.
- 29.Wang, L. K., B. Schwer, and S. Shuman. 2006. Structure-guided mutational analysis of T4 RNA ligase 1. RNA 122126-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]