Abstract
A majority of patients infected with hepatitis C virus (HCV) do not sustain an effective T-cell response, and viremia persists. The mechanism leading to failure of the HCV-specific CD8+ T-cell response in patients developing chronic infection is unclear. We investigated apoptosis susceptibility of HCV-specific CD8+ T cells during the acute and chronic stages of infection. Although HCV-specific CD8+ T cells in the blood during the acute phase of infection and in the liver during the chronic phase were highly activated and expressed an effector phenotype, the majority was undergoing apoptosis. In contrast, peripheral blood HCV-specific CD8+ T cells during the chronic phase expressed a resting memory phenotype. Apoptosis susceptibility of HCV-specific CD8+ T cells was associated with very high levels of programmed death-1 (PD-1) and low CD127 expression and with significant functional T-cell deficits. Further evaluation of the “death phase” of HCV-specific CD8+ T cells during acute HCV infection showed that the majority of cells were dying by a process of cytokine withdrawal, mediated by activated caspase 9. Contraction during the acute phase occurred rapidly via this process despite the persistence of the virus. Remarkably, in the chronic phase of HCV infection, at the site of infection in the liver, a substantial frequency of caspase 9-mediated T-cell death was also present. This study highlights the importance of cytokine deprivation-mediated apoptosis with consequent down-modulation of the immune response to HCV during acute and chronic infections.
Hepatitis C virus (HCV) infects more than 150 million people worldwide (3, 61) and is a major cause of liver failure in the United States (41). Current treatments for chronic HCV infection cure only approximately 50% of persons infected with genotype 1 virus, the most prevalent genotype in the United States (49). In addition to limitations in efficacy, current antiviral therapy is prolonged and associated with disabling side effects, highlighting the need for improved therapeutic options.
The adaptive T-cell immune response is important in mediating HCV clearance (10, 14, 19, 36, 42, 55), yet the reason that a majority of infected patients develop only a weak, narrow, or nonpersistent adaptive response to acute infection is not well understood. Studies of some patients acutely infected with HCV show impairment of cytokine production and proliferation of HCV-specific CD8+ T cells during the acute phase even though antigen-specific cells are present at a high frequency during this phase of infection (55). A period of “stunning” of HCV-specific CD8+ T cells during the earliest time points has been observed (36). During the chronic phase of HCV infection, HCV-specific CD8+ T cells also display significant functional deficits including impaired cytokine production and proliferative capacity (21, 62).
Programmed death receptor 1 (PD-1) is an inhibitory receptor in the CD28 family that is expressed on antigen-specific T cells during acute and chronic viral infections (reviewed in reference 31). The level of expression of PD-1 has been shown to correlate with impaired functionality of antigen-specific CD8+ T cells (31), and in vitro blockade of PD-1 signaling during chronic infection has been shown to improve cytokine production and proliferative capacity of these antigen-specific CD8+ T cells (4, 13, 20, 29, 47, 56, 57, 59). Interestingly, the level of PD-1 expression has also been shown to be an important determinant of apoptosis susceptibility of HIV-specific CD8+ T cells during chronic infection, with higher expression associated with higher apoptosis sensitivity, as measured by annexin V surface exposure (47). The importance of apoptosis susceptibility in modulating immune responses after PD-1 ligation in HCV infection is incompletely understood but may represent a mechanism by which the immune response fails to control viremia. Apoptosis susceptibility mediated by PD-1 may have particular relevance for T cells responding to hepatotropic viral infections for several reasons. First, the liver is a site where there is a high level of apoptosis of activated T cells (11). Second, antigen-driven CD8+ T cells from mice deficient for PD-1 and its ligand (PD-L1) display impaired apoptosis in the liver and accelerated hepatocyte damage in experimental autoimmune hepatitis (15). Third, PD-L1 expression, upregulated on hepatocytes by adenoviral infection or interferon (IFN), enhances apoptosis of Jurkat T cells (43). And finally, HCV-specific CD8+ T cells that infiltrate the liver of patients with HCV infection express very high levels of PD-1 (20, 48).
With these observations in mind, we investigated apoptosis susceptibility of responding HCV-specific CD8+ T cells during acute and chronic infections. We found remarkable similarity in the activation state of HCV-specific CD8+ T cells in the blood during the acute phase of infection and in the liver during chronic infection. This was in marked contrast to the resting memory phenotype of HCV-specific CD8+ T cells from the peripheral blood during chronic infection. Although HCV-specific CD8+ T cells were present at high frequency and were highly activated at the earliest phase of acute HCV infection in the blood and in the liver during chronic infection, they showed significant deficits in cytokine production, proliferation, and production of the cytotoxic molecule, perforin. These deficits corresponded to spontaneous apoptosis susceptibility and very high expression of PD-1. Investigating the mechanism of cell death during the acute phase of infection, we found that the HCV-specific CD8+ T-cell death was mediated by caspase 9, even in nonresolving infection, and that cells could be rescued by provision of the cytokines interleukin-2 (IL-2), IL-7, or IL-15. Similarly, a significant percentage of liver-infiltrating CD8+ T cells expressed activated caspase 9, indicating cytokine withdrawal-mediated death at the site of infection.
MATERIALS AND METHODS
Subjects.
Two patients with acute HCV infection, as evidenced by HCV antibody seroconversion in the presence of a clinical syndrome of acute hepatitis, and 16 patients with chronic HCV infection (HCV antibody and HCV PCR positive) were enrolled in the study from the Emory/Crawford Long, Atlanta Veterans Administration (VA), or Grady Hospitals and Clinics. Patients 802, 240, 168, 216, 232, 642, 671, 688, and 691 were HLA-A2 positive by fluorescence-activated cell sorting (FACS) analysis, which enabled analysis utilizing HLA-A2-restricted tetramers. The patient characteristics are summarized in Table 1. Neither patient 240 nor patient 802 resolved acute HCV infection spontaneously. Patient 802 began therapy with pegylated IFN and ribavirin at day 80 of the study. Patient 802 had human immunodeficiency virus (HIV) well controlled on highly active antiretroviral therapy (HAART) for approximately 9 months prior to our analysis. There were no changes in HIV viral load (remained undetectable throughout the study). Patient 240 had not been treated for HCV infection during the course of this study. All patients provided informed consent, and the protocol (Institutional Review Board #1358-2004) was approved by the local ethics committees of Emory University, the Atlanta VA Medical Center, and Grady Hospital and Clinics.
TABLE 1.
Patient characteristics
| Patient | Genderb | Age (yr) | HIV statusc | HCV genotype | Baseline viral load (IU/ml) | ALT (U/liter)d | Liver biopsy scoree
|
|
|---|---|---|---|---|---|---|---|---|
| Inflammation | Fibrosis | |||||||
| 802a | M | 41 | + | 1 | 3,355,000 | 957 | NP | NP |
| 240a | F | 51 | − | 1 | 10,550,000 | 237 | NP | NP |
| 140 | F | 48 | − | 1 | 8,980,000 | 170 | 2/2 | 2 |
| 168 | F | 47 | + | NPf | 3,690,000 | 84 | 4 | 4 |
| 204 | M | 45 | − | 1 | 2,590,000 | 116 | 2 | 3 |
| 215 | M | 43 | + | 1 | 5,570,000 | 56 | 3 | 2 |
| 216 | M | 53 | + | 1 | 9,780,000 | 19 | 2/3 | 0 |
| 232 | F | 52 | − | 1 | 1,220,000 | 94 | 2/2 | 2 |
| 642 | M | 62 | − | 1 | 460,000 | 53 | 2 | 2 |
| 646 | M | 57 | − | 2 | 8,470,000 | 171 | 2 | 4 |
| 647 | M | 60 | − | 1 | 28,700,000 | 197 | 2 | 2 |
| 671 | M | 50 | − | 1 | 1,060,000 | 50 | 2 | 1 |
| 688 | M | 50 | − | 1 | 16,800,000 | 43 | 2 | 2 |
| 691 | M | 57 | − | 2 | 9,780,000 | 49 | 0 | 4 |
| 695 | M | 53 | − | 1 | 104,000 | 87 | 2 | 3 |
| 696 | M | 52 | − | 1 | 9,080 | 22 | 2/0 | 2 |
| 697 | M | 53 | − | 1 | 2,890,000 | 32 | 1/2 | 1 |
| 698 | M | 62 | − | 1 | 753,000 | 85 | 1 | 1 |
Patient with acute HCV infection. For acute infection, viral load and alanine aminotransferase are shown for the first time point sampled.
M, male; F, female.
+, infected; −, not infected. For patient 802, HIV viral load was undetectable (<50 copies/ml) (on HAART), and the CD4 count was 220 cells/μl. For patient 215, HIV viral load was 42,000 copies/ml, and the CD4 count was 520 cells/μl (not on HAART). For patient 216, HIV viral load was 490 copies/ml, and the CD4 count was 195 cells/μl (on HAART). For patient 168, HIV viral load was undetectable (on HAART), and the CD4 count was 321 cells/μl.
ALT, alanine aminotransferase.
Both scores are based on the Scheuer scoring system. Where two numbers are given for the inflammation score, the first is the portal/periportal grade, and the second is lobular grade.
NP, not performed.
HCV antibody testing, viral load determination, and genotyping.
HCV antibody testing by enzyme-linked immunosorbent assay was performed at the Emory Immunology Laboratory using a kit, per the manufacturer's instructions (Abbott Diagnostics, Abbott Park, IL), and at the Atlanta VA Immunology Laboratory (Bio-Rad Laboratories, Hercules, CA). HCV viral load quantification was performed at the Emory Molecular Laboratory and Atlanta VA laboratory using a real-time reverse transcription-PCR assay (Roche Molecular Systems, Alameda, CA). HCV genotyping was performed at the Emory Molecular Laboratory using a commercially available assay (Siemens Medical Solutions Diagnostics) and at the Atlanta VA laboratory using a line probe assay (Bayer Diagnostics, Research Triangle Park, NC).
PBMCs.
EDTA- and heparin-anticoagulated blood was collected from each patient and either used directly for FACS staining or for peripheral blood mononuclear cell (PBMC) isolation. PBMCs were isolated using Ficoll-Paque Plus density gradient (Amersham, Oslo, Norway), washed twice in phosphate-buffered saline, and either analyzed immediately or cryopreserved in medium containing 90% fetal calf serum (HyClone, Logan, UT) and 10% dimethyl sulfoxide (Sigma-Aldrich, St. Louis, MO).
Liver biopsy.
Liver tissue was obtained by either ultrasound-guided needle biopsy or via a transjugular fluoroscopic technique and was immediately put into RPMI 1640 medium (Gibco) containing 10% fetal calf serum for immunological assays. Another fragment was fixed in formalin for histological examination. Classification of the histological changes was performed using the Scheurer scoring system at the Emory and Atlanta VA pathology laboratories.
Intrahepatic T-cell isolation.
The liver biopsy sample obtained in RPMI 1640 medium (Gibco, Carlsbad, CA) containing 10% fetal calf serum was washed three times with the same medium to remove cell debris and red blood cells. Isolation of liver-infiltrating lymphocytes was performed using an automated, mechanical disaggregation system (Medimachine, Becton Dickinson). The sample was inserted into a 50-μm Medicon and inserted into the Medimachine and run for 15 s. Disaggregated cells were removed using a syringe in the syringe port. The Medicon was rinsed twice with RPMI medium containing 10% fetal calf serum to ensure maximum cell recovery. Cells were used immediately for FACS staining.
Antibodies, HLA-A2 tetramers, and flow cytometry.
Cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, peridinin chlorophyll protein-, and allophycocyanin-labeled monoclonal antibodies or tetramers according to the manufacturers' instructions, and flow cytometry was performed using a FACSCalibur (Becton Dickinson, San Jose, CA). FACS data were analyzed with FlowJo software (Treestar). The following monoclonal antibodies from BD Pharmingen (BD Biosciences, San Jose, CA) were used: anti-CD8 peridinin chlorophyll protein, CD69 FITC, CD38 FITC, HLA-DR PE, Ki67 FITC, granzyme B PE, perforin FITC, and Bcl-2 PE. Anti-CD3 FITC and CD127 PE were obtained from Beckman Coulter (Fullerton, CA). Anti-PD-1 PE-conjugated antibody (clone EH12) was generated as described previously (16). HLA-A2 tetramers were specific for the following CD8+ T-cell epitopes: for HCV NS3 peptide consisting of residues 1073 to 1081 (NS3 1073-1081; CINGVCWTV); for HCV NS3 1406-1415 (KLVALGINAV); for the cytomegalovirus peptide NLVPMVATV (CMV NLV); for the Epstein-Barr virus peptide GLCTLVAML (EBV GLC), and for the HIV Gag peptide SLYNTVATL. The tetramers were generated at the National Tetramer Core Facility at Emory University School of Medicine. Flow cytometric collection was performed on a FACSCalibur, and analysis was performed using FlowJo software (version 8.5.3).
Intracellular cytokine staining.
A total of 1 × 106 PBMCs in 1 ml of RPMI 1640-10% fetal calf serum medium were cultured overnight with or without stimulation in the presence of Golgi Plug. For stimulation, the following were used: the peptide HCV NS3 1073-1081 (10 μg/ml), HCV NS3 1406-1415, or CMV NLV (10 μg/ml) or staphylococcal enterotoxin B (SEB) (2 μg/ml; Sigma-Aldrich). After stimulation, PBMCs were surface stained for 20 min at room temperature. The cells were washed twice with FACS buffer (phosphate-buffered saline containing 5% bovine serum albumin and 0.1% NaN3), permeabilized for 10 min at room temperature with 500 μl of FACS-Perm (BD Biosciences), washed with FACS buffer, stained with IFN-γ, IL-2, or tumor necrosis factor alpha (BD Biosciences) for 20 min at room temperature, washed again, and fixed with 1% paraformaldehyde before acquisition on a FACSCalibur. FACS data were analyzed with FlowJo software.
Apoptosis assays.
For direct ex vivo analysis of apoptosis susceptibility, either PBMCs or liver-derived lymphocytes were stained with annexin V-FITC (BD Biosciences) per the manufacturer's instructions. Dead cells were excluded with a 7-aminoactinomycin D (7-AAD) stain (BD Biosciences). Susceptibility to apoptosis was also assessed by detection of surviving antigen-specific CD8+ T cells by tetramer analysis after brief in vitro culture in the presence of medium, medium supplemented with IL-2 (50 U/ml), or medium supplemented with IL-15 (10 ng/ml) or IL-7 (10 ng/ml). For the analysis of apoptosis of HCV-specific CD8+ T cells based on CD127 expression, 10 × 106 PBMCs were sorted on a FACSAria (BD Biosciences) into HCV-specific CD8+ T cells expressing high and low levels of CD127 (CD127high and CD127low, respectively), after exclusion of dead cells with 7-AAD. HCV-specific CD8+ T cells were identified by tetramer staining. Purity of the sorted cells was >90% for each population. Cells were then cultured overnight with 1 × 106 irradiated, tetramer-negative homologous PBMCs as feeder cells, and the frequency of surviving HCV-specific CD8+ T cells was assessed on a FACSCalibur (BD Biosciences).
Activated caspase detection.
For the detection of activated caspase 8 and caspase 9, PBMCs or liver-derived lymphocytes were cultured with FITC-IETD-fluoromethylketone (caspase 8) or FITC-LEHD-fluoromethylketone (caspase 9) per the manufacturer's protocol (Calbiochem). Cells were washed twice in the provided wash buffer and stained with tetramer and anti-CD8 PE (BD Bioscience) for 20 min at room temperature. Cells were washed again with the provided wash buffer and analyzed on a FACSCalibur. Dead cells were excluded by 7-AAD staining.
Statistical analysis.
Results were graphed and analyzed using GraphPad Prism (version 4). Comparison between blood and liver was made using paired t tests, and the two-tailed P values are shown.
RESULTS
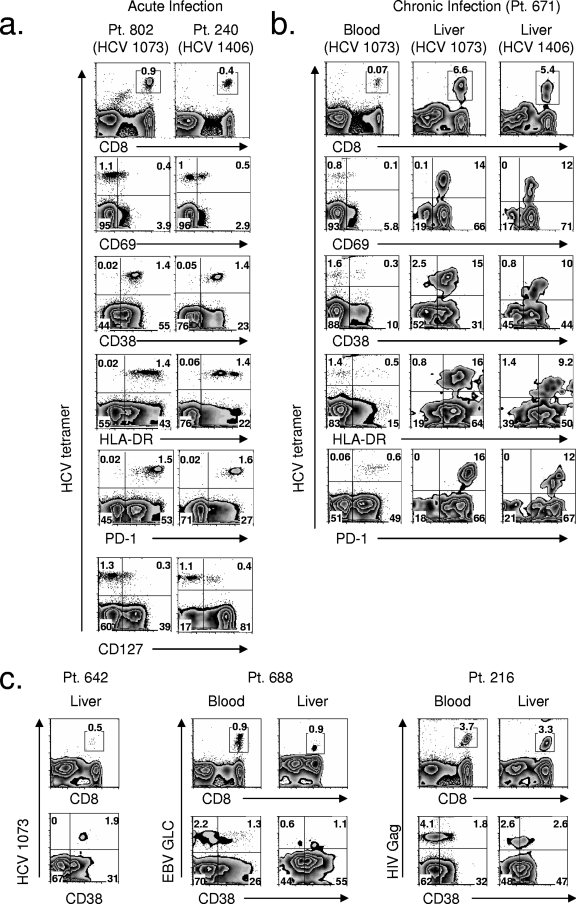
The activation phenotype of HCV-specific CD8+ T cells from the peripheral blood during acute infection differs from that of the peripheral blood during chronic infection but mirrors that found in the liver during chronic infection.
The characteristics of two patients (patients 802 and 240) with acute HCV infection and 16 patients with chronic infection are shown in Table 1. Nearly all HCV-specific CD8+ T cells evaluated from the peripheral blood of the patients with acute infection, at the earliest time points sampled (day 0 for both patients), expressed the activation markers CD38 and HLA-DR, and a significant percentage expressed the early activation marker CD69 (Fig. 1a). The activation was HCV specific since influenza-specific CD8+ T cells at the same time point did not show an activated phenotype (data not shown). In stark contrast to the activated phenotype seen during the acute phase of infection, during chronic infection a majority of HCV-specific CD8+ T cells from the peripheral blood were not activated and did not express the activation markers CD38, HLA-DR, or CD69 (Fig. 1b).
FIG. 1.
Activation phenotype and PD-1 expression of HCV-specific CD8+ T cells in blood during acute infection are mirrored in the liver during chronic infection. (a) FACS plots of the phenotype of HCV-specific CD8+ T cells from two patients during the acute phase of infection. The tetramer epitope is indicated below the patient (Pt.) number. (b) FACS plots of the phenotype of HCV-specific CD8+ T cells during chronic infection from patient 671 in the blood and the liver. The tetramer epitope is indicated in parentheses. (c) FACS plots of CD38 expression on HCV-, EBV-, and HIV-specific CD8+ T cells from three patients with chronic HCV infection. Comparison of liver and blood is shown for patient 688 and patient 216. Patient 642 did not have detectible peripheral blood HCV-specific CD8+ T cells. The tetramer epitopes are indicated on vertical axes. For all FACS plots, CD8 plots were gated on total lymphocytes; others were gated on CD8+ lymphocytes. HCV 1073, epitope of HCV NS3 1073-1081; HCV 1406, epitope of HCV NS3 1406-1415.
Interestingly, HCV-specific CD8+ T cells in the liver during chronic infection expressed the activation markers CD69, CD38, and HLA-DR (Fig. 1b) and mirrored the activated state of peripheral blood HCV-specific CD8+ T cells during the acute phase of infection (Fig. 1a). Furthermore, the uniformly high activation state of HCV-specific CD8+ T cells seen in the liver during the chronic phase was unique to HCV. In patient 671 (Fig. 1b) and patient 642 (Fig. 1c), nearly all of the HCV-specific CD8+ T cells in the liver expressed the activation marker CD38. In contrast, for patient 688 and patient 216, only 50 to 60% of EBV- or HIV-specific CD8+ T cells expressed this activation marker in the liver (Fig. 1c). Though the level of CD38 expression on liver EBV- and HIV-specific CD8+ T cells was greater than for peripheral blood (Fig. 1c), it was not uniformly increased as found for HCV-specific CD8+ T cells. Hence, non-HCV-specific CD8+ T cells from the liver of patients with chronic HCV infection showed increased activation in the liver compared to the peripheral blood, but their activation was clearly less than HCV-specific CD8+ T cells in the liver.
Reciprocal and dynamic changes in PD-1 and CD127 expression on HCV-specific CD8+ T cells during the acute phase of infection.
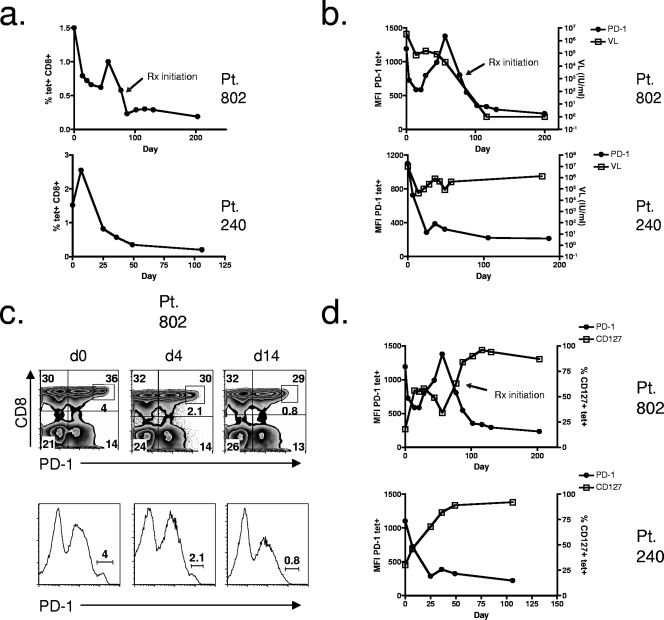
High expression levels of PD-1 and low expression levels of CD127 (IL-7 receptor-α) have been linked to a functionally “exhausted” phenotype of HCV-specific CD8+ T cells in chronic infection (48), and expression of CD127 during acute resolving infection has been associated with long-lived functional memory T-cell development (25, 28). Some studies have linked upregulation of CD127 or a decrease in PD-1 expression to the likelihood of viral clearance during the acute phase of HCV infection (58, 59). We examined longitudinal changes in tetramer frequency and the expression of PD-1 and CD127 on HCV-specific CD8+ T cells during the acute phase of infection. During the first month of evaluation, both patients displayed an overall fall in tetramer frequency (Fig. 2a). For patient 802, HCV-specific CD8+ T cells decreased by 47% (from 1.5% of CD8+ T cells on day 0 to 0.79% of CD8+ T cells on day 14) (Fig. 2a). No change in CMV-specific CD8+ T cells from day 0 to day 14 was seen, and the frequency of CMV-specific CD8+ T cells remained at approximately 2% of total lymphocytes (data not shown). HCV-specific CD8+ T-cell frequency increased again at day 60 for patient 802 (Fig. 2a). This was followed by a rapid fall coinciding with treatment initiation. Interestingly, a fall in CMV-specific CD8+ T cells, to approximately 1% of total lymphocytes for patient 802, was noted with IFN and ribavirin therapy (day 130) (data not shown). For patient 240, HCV-specific CD8+ T cells decreased by 70% (from 2.6% of CD8+ T cells on day 7 to 0.82% of CD8+ T cells on day 25) (Fig. 2a). After this, HCV-specific CD8+ T-cell tetramer frequency continued to decrease, eventually reaching nearly undetectable levels (Fig. 2a). Patient 240 never underwent antiviral therapy for HCV infection during the course of this study.
FIG. 2.
Longitudinal analysis of HCV-specific CD8+ T-cell frequency, HCV viral load, and PD-1 and CD127 expression during acute HCV infection. (a) HCV-specific CD8+ T-cell frequency over time during the acute phase for two patients with acute HCV infection. Frequency of HCV tetramer-positive T cells of total CD8+ lymphocytes is shown. (b) PD-1 expression on HCV-specific CD8+ T cells in relation to HCV viral load over time during the acute phase. MFI of PD-1 on HCV tetramer-positive cells and viral load (VL) are indicated on the y axes. (c) PD-1 expression on total CD8+ T cells shown by FACS plots and histogram plots at three time points for patient 802 during acute HCV infection. The population expressing high levels of PD-1 is indicated by the inset box in the top plots and by the line gate in the lower plots. d, day. (d) PD-1 expression in relation to CD127 expression on HCV-specific CD8+ T cells over time during the acute phase of infection. PD-1 expression is shown as MFI of PD-1 on tetramer-positive cells, and CD127 is shown as the frequency of tetramer-positive cells that expressed CD127 (y axes). Pt, patient; tet+, tetramer-positive.
The majority of HCV-specific CD8+ T cells from both acutely infected patients expressed PD-1 at the earliest time points (Fig. 1a), and expression was at a high level, with mean fluorescence intensities (MFIs) of 1,190 and 1,100 for patients 802 and 240, respectively (Fig. 2b). For patient 802, there was an initial rapid fall in the MFI of PD-1 on HCV-specific CD8+ T cells (Fig. 2b). This was followed by a rise at day 60 and again a fall with antiviral therapy (Fig. 2b). The dynamic intensity of PD-1 mirrored the level of viremia (Fig. 2b) except at day 60, when the PD-1 level peaked, but viremia continued to fall (Fig. 2b). The peak in PD-1 at day 60 coincided with an increase in tetramer frequency (Fig. 2a) and an increase in activation of HCV-specific CD8+ T cells (data not shown). PD-1 expression on HCV-specific CD8+ T cells from patient 240 fell during the acute phase with no further increase (Fig. 2b). It should be noted that for both patients during the acute phase of infection, although the expression level of PD-1 decreased, as measured by MFI, nearly 100% of HCV-specific CD8+ T cells remained PD-1 positive at all time points (data not shown).
We evaluated PD-1 expression on bulk CD8+ T cells for both patients during acute HCV infection and found three distinct populations, one with low-level PD-1 expression, one with intermediate expression, and one with high-level PD-1 expression (Fig. 2c). In fact, at the earliest time point for patient 802 and patient 240, 4.0% and 1.3%, respectively, of total CD8+ T cells expressed the highest level of PD-1 (Fig. 2c, inset box, for patient 802; data not shown for patient 240). A population expressing high levels of PD-1 was never seen on bulk CD4+ T cells (data not shown). The high-level PD-1-expressing CD8+ T-cell population from patient 802 rapidly disappeared and by day 14 was virtually absent (Fig. 2c). Similarly, for patient 240 this population was not present at the next blood sampling at day 25 (data not shown). Hence, early during acute infection, there was a marked loss in HCV-specific CD8+ T cells and, in particular, a loss of the CD8+ T cell population that expressed PD-1 at high levels. Comparing expression levels of PD-1 on HCV-specific CD8+ T cells in blood during acute infection (Fig. 1a) and liver during chronic infection (Fig. 1b) showed that the expression level in liver was similarly high (MFI of 1,207 for the epitope of HCV NS3 1073-1081) in contrast to lower intensity PD-1 expression in peripheral blood during chronic infection (MFI of 411 for the epitope of HCV NS3 1073-1081) (Fig. 1b).
Evaluation of the expression of CD127 on HCV-specific CD8+ T cells revealed remarkably reciprocal changes to PD-1 expression (Fig. 2d). At the earliest time points, when the PD-1 expression level was high, the majority of HCV-specific CD8+ T cells from both patients did not express CD127 (Fig. 1a and 2d). CD127 expression was present on only 18% and 27% of HCV-specific CD8+ T cells from patient 802 and patient 240, respectively (Fig. 1a and 2d). For patient 802, the frequency of HCV-specific CD8+ T cells expressing CD127 increased to approximately 50% at week 2, coinciding with a fall in PD-1 expression (Fig. 2d). When PD-1 increased at day 60 (Fig. 2d), CD127 fell to approximately 30% of HCV-specific CD8+ T cells, coinciding with a second peak in PD-1 expression (Fig. 2d). After initiation of antiviral therapy, CD127 expression increased to >80%, and PD-1 expression was at its nadir. This coincided with a rapid virologic response and negative HCV viral load at 4 weeks. For patient 240, CD127 expression on HCV-specific CD8+ T cells increased rapidly during the acute phase of infection, from approximately 30% expression at the earliest time point to 50% by day 7, nearly 70% by day 25, and >80% after day 36 (Fig. 2d). This rise in CD127 coincided with a reciprocal fall in PD-1 expression (Fig. 2d). Importantly, expression of CD127 in patient 240 increased to >80% of HCV-specific CD8+ T cells in the absence of therapy and despite persistent viremia. Hence, in this patient, a rise in CD127 expression on HCV-specific CD8+ T cells was not a marker of viral clearance.
HCV-specific CD8+ T cells are not fully functional despite a highly activated phenotype in the peripheral blood during acute infection and in the liver during chronic infection.
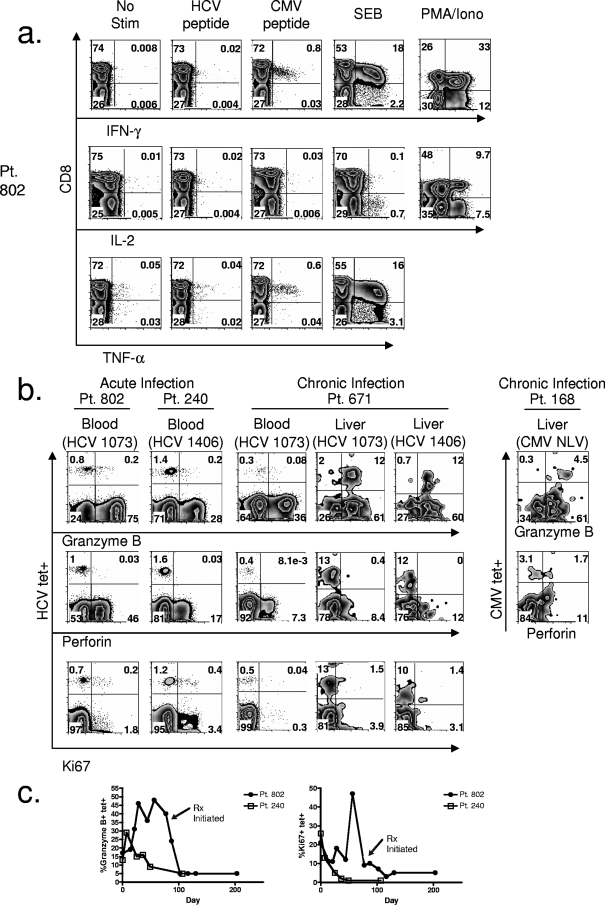
Cytokine production during early, acute HCV infection was evaluated by intracellular cytokine assay. HCV-specific CD8+ T cells at the earliest time points for patient 802 (Fig. 3a) and patient 240 (data not shown) did not produce IFN-γ, tumor necrosis factor alpha, or IL-2 in response to cognate peptide stimulation (Fig. 3a). Lack of cytokine production was specific for HCV since CMV-specific CD8+ T cells produced IFN-γ in response to CMV peptide (Fig. 3a). Given the lack of IL-2 production by CD8+ T cells in the SEB control, we included a phorbol myristate acetate-ionomycin control as well and showed IL-2 production by CD8+ T cells after stimulation with this mitogen in the setting of acute HCV infection (Fig. 3a).
FIG. 3.
Impaired function of HCV-specific CD8+ T cells during the earliest time points of acute HCV infection in blood and in the liver during chronic infection. (a) FACS plots of cytokine production of HCV-specific CD8+ T cells shown after no stimulation (No stim) or stimulation with HCV peptide (NS3 1073-1081). Comparisons to cytokine production of CMV-specific CD8+ T cells after stimulation with CMV NLV peptide, SEB, and phorbol myristate acetate-ionomycin (PMA/Iono) controls are also shown. Plots were gated on CD3+ lymphocytes. (b) FACS plots of the expression of granzyme B, perforin, and Ki67 in HCV-specific CD8+ T cells from two patients at the earliest time points of acute HCV infection and for patient 671 in blood and liver during chronic infection. Also shown is granzyme B and perforin production by CMV-specific CD8+ T cells in the liver of patient 168. Plots were gated on CD8+ lymphocytes. (c) Longitudinal expression of frequency of HCV-specific CD8+ T cells expressing granzyme B and Ki67 for two patients with acute HCV infection. Minimal perforin production of HCV-specific CD8+ T cells was seen at all the time points tested for both patients with acute HCV. Pt, patient; tet+, tetramer-positive; TNF-α, tumor necrosis factor alpha; Rx, antiviral therapy; HCV 1073, epitope of HCV NS3 1073-1081; HCV 1406, epitope of HCV NS3 1406-1415.
We assessed cytotoxicity by direct intracellular staining for granzyme B and perforin during acute HCV infection (Fig. 3b). Though approximately 12 to 30% of HCV-specific CD8+ T cells at the earliest time points produced granzyme B, there was a total lack of perforin production in both patients (Fig. 3b). The lack of perforin production for both patient 802 and patient 240 was evident not only at the earliest time point (Fig. 3b) but also throughout the entire acute phase of infection at all time points tested (data not shown). We also assessed the possibility that other granzymes compensated for the low level of granzyme B during early acute HCV infection since differential expression of these molecules during T-cell differentiation has been previously demonstrated (7, 54). For patient 240, we were unable to detect granzyme A or K at the early time point, demonstrating a lack of compensation for the dearth in granzyme B at this time point (data not shown). Granzyme B expression increased for patient 802 after the earliest time point sampled and peaked at day 60 (Fig. 3c). Expression fell after antiviral therapy (Fig. 3c). We also assessed granzyme A and K production for patient 802 at day 60 since we found a high level of granzyme B at this time point. At day 60, a significant percentage of HCV-specific CD8+ T cells produced granzyme A in addition to granzyme B, but there was only minimal granzyme K production (data not shown). For patient 240, granzyme B expression continued to fall (Fig. 3c). Evaluating liver-infiltrating HCV-specific CD8+ T cells during chronic infection, we found that the majority expressed granzyme B, but there was a total lack of perforin expression (Fig. 3b). This contrasted with the peripheral blood HCV-specific CD8+ T cells during chronic HCV that expressed neither granyzme B nor perforin (Fig. 3b). In contrast to the lack of perforin production for HCV-specific CD8+ T cells in the liver, CMV-specific CD8+ T cells in the liver showed perforin production (38% of CMV-specific CD8+ T cells for patient 168) (Fig. 3b).
Proliferation was measured directly ex vivo by intracellular staining with Ki67, a marker of cycling cells. Only modest proliferation was noted at the earliest time points sampled for patient 802 and patient 240, with approximately 20% of HCV-specific CD8+ T cells expressing Ki67 (Fig. 3b). For patient 802, proliferation of HCV-specific CD8+ T cells increased after the earliest time point, with an obvious peak at day 60 (Fig. 3c). For patient 240, there was only a continuous fall in proliferating cells from the initial modest level (Fig. 3c). Similar to the early time points during acute HCV infection, during chronic infection, the majority of both blood- and liver-infiltrating HCV-specific CD8+ T cells were not proliferating (Fig. 3b).
Hence, at the earliest time points sampled during acute HCV infection, HCV-specific CD8+ T cells displayed significant functional deficits despite being present at a high frequency. For patient 802, some of the deficits improved during the acute phase, but for patient 240, this improvement was not seen. Similar to these findings in the peripheral blood, HCV-specific CD8+ T cells in the liver during the chronic phase also displayed significant functional deficits despite being present at a high frequency.
HCV-specific CD8+ T cells were highly apoptotic in the peripheral blood during the acute phase of infection and in the liver during the chronic phase of infection.
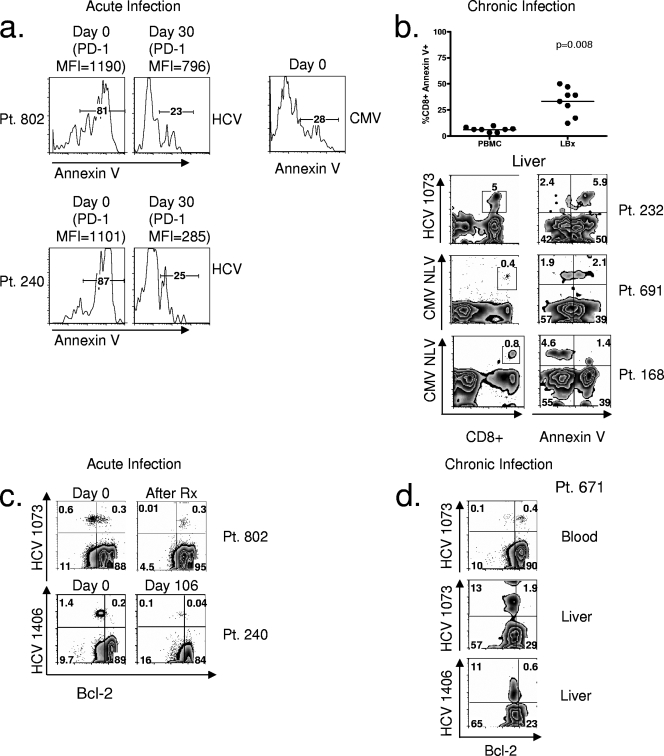
Since we noted a rapid decrease in the frequency of HCV-specific CD8+ T cells from the peripheral blood during the earliest time points of acute HCV infection sampled (Fig. 2a), as well as the early loss of the high-level PD-1-expressing populations (Fig. 2c), we investigated whether these cells were undergoing apoptosis. Approximately 80% of HCV-specific CD8+ T cells at the earliest time points for patient 802 and patient 240 were annexin V positive (Fig. 4a). At later time points, even as early as 30 days, the frequency of annexin V staining was only 15 to 30% (Fig. 4a). This high level of annexin positivity was specific to HCV since CMV-specific CD8+ T cells at the earliest time point of HCV infection expressed low levels of annexin V (Fig. 4a). Therefore, at the earliest time point of infection, the majority of HCV-specific CD8+ T cells were apoptotic. Furthermore, this apoptosis susceptibility was associated with the expression of the highest level of PD-1 on HCV-specific CD8+ T cells (Fig. 4a). At day 0, the PD-1 MFIs on HCV-specific CD8+ T cells were 1,190 and 1,101 for patients 802 and 240, respectively. When apoptosis susceptibility decreased at day 30, the PD-1 MFIs on HCV-specific CD8+ T cells decreased to 796 and 285 for patients 802 and 240, respectively (Fig. 4a).
FIG. 4.
HCV-specific CD8+ T cells during the earliest time points of acute HCV infection and in the liver during chronic HCV infection are highly apoptotic. (a) Histogram plots of annexin V expression on HCV-specific CD8+ T cells at day 0 and day 30 for two patients with acute HCV infection. Comparison with annexin V surface expression on CMV-specific CD8+ T cells at day 0 for patient 802 is also shown. The MFIs of PD-1 on HCV-specific CD8+ T cells at day 0 and day 30 are indicated above the plots. (b) Comparison of annexin V expression on total CD8+ lymphocytes in liver (LBx, liver biopsy) and peripheral blood (top summary plot). Also shown are FACS plots of tetramer frequency (first column) and annexin V expression (second column) on HCV-specific and CMV-specific CD8+ T cells in the liver of three patients with chronic HCV infection. The first row of FACS plots was gated on total lymphocytes; the second was gated on CD8+ lymphocytes. Necrotic cells were excluded by 7-AAD staining. (c) FACS plots of Bcl-2 expression on HCV-specific CD8+ T cells for two patients with acute HCV infection. Day 0 for both patients is shown. For patient 802, the time point 2 weeks after beginning antiviral therapy (Rx) is shown. For patient 240, who did not begin antiviral therapy, day 106 is shown. Plots were gated on CD8+ lymphocytes. (d) FACS plots of Bcl-2 expression on HCV-specific CD8+ T cells from patient 671 in blood and liver. Plots were gated on CD8+ lymphocytes. Tetramer epitopes are shown on the y axis. Pt, patient; HCV 1073, epitope of HCV NS3 1073-1081; HCV 1406, epitope of HCV NS3 1406-1415.
This high level of apoptosis susceptibility during the acute phase was recapitulated in the liver during the chronic phase of infection (Fig. 4b). Comparison of apoptosis susceptibility of total CD8+ T cells in patients with chronic HCV infection showed that liver CD8+ T cells were more susceptible to apoptosis than peripheral blood CD8+ T cells, as measured by annexin V positivity (Fig. 4b); these findings agreed with prior studies demonstrating an enrichment of apoptotic T cells detected by terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling assay in the liver of patients with HCV infection (45). Assessing antigen-specific CD8+ T cells in the liver demonstrated that greater than 70% of HCV-specific CD8+ T cells expressed annexin V (Fig. 4b). HCV-specific CD8+ T cells showed greater apoptosis susceptibility than CMV-specific CD8+ T cells in the liver from two patients with chronic HCV infection (50% and 23% of CMV-specific CD8+ T cells for patients 691 and 168, respectively, were annexin V positive) (Fig. 4b). Thus, mirroring the increase in activation of HCV-specific CD8+ T cells in the liver compared with non-HCV-specific epitopes (Fig. 1d), there was an increase in apoptosis susceptibility in comparison to other antigen specificities.
We evaluated the expression of the antiapoptotic molecule Bcl-2 during the acute phase of infection in the blood (Fig. 4c) and in the liver during the chronic phase (Fig. 4d). The majority of HCV-specific CD8+ T cells at the earliest time points of acute HCV infection, for both patient 802 and patient 240, expressed only low levels of Bcl-2, with approximately 70% to 80% of cells expressing low levels of Bcl-2 (Fig. 4c). Interestingly, for patient 802 at this early time point, 0.3% of lymphocytes was HCV specific and expressed high levels of Bcl-2 (Fig. 4c). After HCV antiviral treatment, the only remaining HCV-specific CD8+ T cells were all expressing higher Bcl-2 levels. The frequency of the remaining HCV-specific CD8+ T cells (0.3%) was identical to the frequency at the early time point that had increased Bcl-2 (0.3%). It is plausible that at the early time point, HCV-specific memory CD8+ T cells had already been selected for survival. For patient 240 who had not undergone antiviral therapy, even at later time points of up to 100 days, the expression of Bcl-2 in the majority of HCV-specific CD8+ T cells remained low (Fig. 4c). In the liver during the chronic phase of infection, the majority of HCV-specific CD8+ T cells also expressed Bcl-2 at low levels (Fig. 4d). This contrasted with Bcl-2 expression for HCV-specific CD8+ T cells during chronic infection in the peripheral blood, where the majority expressed Bcl-2 (Fig. 4d), again highlighting the phenotypic similarities between HCV-specific CD8+ T cells from the periphery in acute infection and the liver in chronic infection.
Cytokine withdrawal contributes to cell death during the acute phase in the peripheral blood and in the liver during the chronic phase.
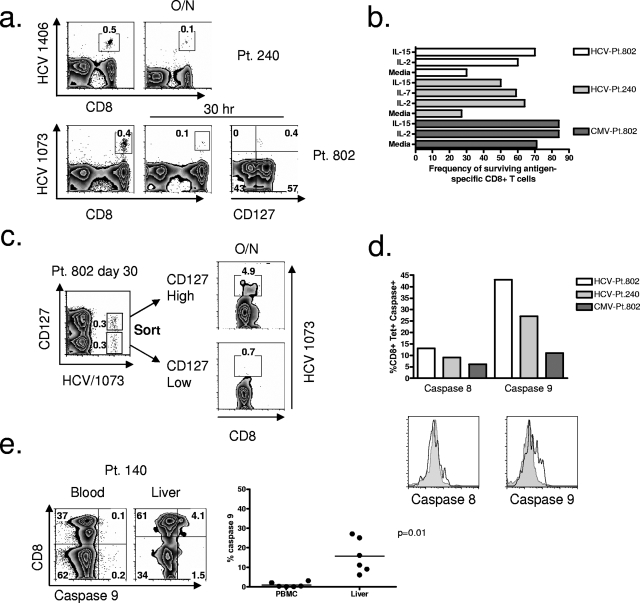
Brief in vitro culture of HCV-specific CD8+ T cells during the earliest phase of acute HCV infection confirmed their apoptosis susceptibility (Fig. 5a). For patient 240 approximately 0.5% of total lymphocytes were specific for the epitope of HCV NS3 1406-1415 (Fig. 5a). After overnight culture, only 0.1% could be detected (Fig. 5a). Similarly, for patient 802, 0.4% of total lymphocytes were HCV specific at the earliest time point directly ex vivo. After in vitro culture for 30 h, only 0.1% could be detected. Importantly, all HCV-specific CD8+ T cells that survived short in vitro culture were CD127 positive (Fig. 5a). The loss of antigen-specific CD8+ T cells was specific for HCV since at the same time point, after overnight culture, the majority of CMV-specific CD8+ T cells could be detected (Fig. 5b). Furthermore, loss of HCV-specific CD8+ T cells could be partially reversed by coculture with either IL-2 or IL-15 for both patient 240 and patient 802 (Fig. 5b). Additionally, we tested the ability of IL-7 to reverse apoptosis and found that IL-7 also enhanced survival of HCV-specific CD8+ T cells from patient 240 (Fig. 5b). Since CMV-specific CD8+ T cells were not highly apoptotic, the addition of cytokine minimally enhanced survival of CMV-specific CD8+ T cells (Fig. 5b). Sorting HCV-specific CD8+ T cells based on CD127 expression confirmed that expression of this receptor identified a population of cells capable of surviving in vitro culture (Fig. 5c). After overnight culture, only the CD127high population of HCV-specific CD8+ T cells could be detected (Fig. 5c).
FIG. 5.
Cytokine deprivation-mediated apoptosis in blood during acute HCV infection and in the liver during chronic HCV infection. (a) FACS plots showing frequency of HCV-specific CD8+ T cells before and after brief in vitro culture are shown for two patients with acute HCV infection. For patient 240, cells were cultured overnight (O/N). For patient 802 cells were cultured for 30 h, and CD127 expression on HCV-specific CD8+ T cells after in vitro culture is also shown. (b) Frequency of surviving HCV- and CMV-specific CD8+ T cells, sampled at the earliest time point of acute HCV infection for patient 802 and patient 240, after 30 h in vitro culture in the presence of medium alone, IL-2, or IL-15. For patient 240, culture with IL-7 is also included. (c) FACS plots of cell sorting on CD127+ versus CD127− HCV-specific CD8+ T cells for patient 802, sampled at day 30, showing the surviving frequency of HCV-specific CD8+ T cells after in vitro overnight culture with irradiated autologous feeder cells. Plots were gated on CD8+ lymphocytes. (d) Frequency of activated caspase 8 and caspase 9 expression in HCV-specific CD8+ T cells for patient 802 and patient 240 at the earliest time points of acute HCV infection. Activated caspase expression in CMV-specific CD8+ T cells for patient 802 is shown for comparison. The histograms compare the MFI of expression of activated caspase 8 or caspase 9 on HCV-specific (unshaded) versus CMV-specific (shaded) CD8+ T cells at the earliest time point sampled for patient 802. (e) FACS plots showing the frequency of activated caspase 9 expression in bulk CD8+ T cells from the blood versus the liver of a patient with chronic HCV infection (patient 140). Plots were gated on 7-AAD-negative, CD3+ lymphocytes. Also shown is a summary plot comparing activated caspase 9 expression on CD8+ T cells from blood and liver of patients with chronic HCV infection. Pt, patient; tet+, tetramer-positive; HCV 1073, epitope of HCV NS3 1073-1081; HCV 1406, epitope of HCV NS3 1406-1415.
Given that CD127low, HCV-specific CD8+ T cells expressing high levels of PD-1 were highly apoptotic at the earliest time point of acute HCV infection, we assessed apoptosis susceptibility of HCV-specific CD8+ T cells at day 60 for patient 802, a time point when PD-1 expression increased to a high level a second time, and CD127 expression was decreased (Fig. 2d). Interestingly, we found that approximately 60% of HCV-specific CD8+ T cells at day 60 were susceptible to apoptosis, as measured by annexin V assay (data not shown). CMV-specific CD8+ T cells at day 60 were not highly apoptotic (15% annexin V staining). In the cell culture assay, after 30 h in culture, only IL-15 had a significant impact on apoptosis reversal of HCV-specific CD8+ T cells at day 60, with IL-2 providing only modest benefit and IL-7 providing no benefit (data not shown). It appeared that cytokine deprivation-mediated apoptosis at this time point, though present, was not as pronounced as at the earlier time point.
Two general types of apoptosis of activated T cells have been described (reviewed in reference 34). The first is activation-induced cell death (AICD) and the second is activated T-cell autonomous death (ACAD). AICD is triggered by persistent antigen stimulation and death receptor signaling and is mediated by activation of caspase 8. ACAD, in contrast, occurs after the withdrawal of antigen and/or cytokines such as IL-2 and is mediated by activation of caspase 9. We investigated the expression of activated caspases 8 and 9 in HCV-specific CD8+ T cells during the acute phase of infection and found increased expression of caspase 9 for both patient 240 and patient 802 (Fig. 5d). CMV-specific CD8+ T cells at the same time point of acute HCV infection did not express high levels of activated caspase 8 or caspase 9 (Fig. 5d). Investigating expression of activated caspase 9 in the liver during the chronic phase of infection, we found a significant population of CD8+ T cells expressing activated caspase 9 (Fig. 5e). In comparison, expression of activated caspase 9 on CD8+ T cells in the blood during chronic infection was low or not detectible (Fig. 5e). Hence, during the acute phase of infection and despite persistence of HCV, HCV-specific CD8+ T cells died by a process of cytokine withdrawal-mediated death (ACAD). In the liver during the chronic phase, ACAD also accounted for a significant frequency of CD8+ T-cell death.
DISCUSSION
HCV establishes a persistent infection in the majority of people infected, yet the reasons for the success of the virus are still incompletely understood. Clear distinctions in the adaptive T-cell response during the acute phase of infection in those individuals resolving infection versus those developing persistent infection have been described (10, 14, 19, 36, 42, 55). For example, the lack of a broad and strong CD4+ and CD8+ T-cell response has been linked to persistence. However, the reason one person develops a strong adaptive immune response while another does not is largely unknown. That one person's response is prolonged and effective while another's is transient and ineffective may be due to particular aspects of the virus, such as viral escape mutation or direct interference with the IFN response by the NS3/4 protease, but the difference in response also may involve specific attributes of the host immune system. In general, development of a strong and persistent T-cell response requires three signals to be delivered to the T cell (reviewed in reference 40). The first is signaling via the T-cell receptor (TCR), the second is signaling via costimulatory molecules expressed on T cells and antigen-presenting cells, and the third is signaling via cytokines in the vicinity of the responding T cells. Deficiency of one or more of these signals could play a role in the dysfunction of T cells in HCV infection.
In this study, we carefully evaluated the adaptive CD8+ T-cell response at the earliest time points of acute HCV infection in two patients. Prior studies of the earliest time points of acute HCV infection have demonstrated a period of dysfunction of HCV-specific CD8+ T cells, called stunning (36). We found at the earliest time points that HCV-specific CD8+ T cells expressed the highest level of PD-1, and the high level of expression of PD-1 occurred during a period of significant dysfunction of HCV-specific CD8+ T cells, despite a high antigen-specific T-cell frequency. Furthermore, the cells expressing high levels of PD-1 were also mostly apoptotic. Though prior studies have also reported high-level PD-1 expression during the acute phase of infection, our study highlights the dynamic nature of this expression and indicates that a high level of PD-1 expression is not always associated with dysfunction. For example, we found that differences in blood sampling of only 4 days saw marked changes in the intensity of PD-1 staining. In addition, for patient 802, there was a rapid fall in PD-1 expression on HCV-specific CD8+ T cells at the earliest time point but a reexpression to a high level at day 60 (Fig. 2b). Unlike the dysfunction of HCV-specific CD8+ T cells seen at the earliest time point associated with high PD-1 levels (Fig. 2), at day 60, HCV-specific CD8+ T cells expressed a high level of granzyme B, and nearly half were proliferating, as measured by Ki67 expression (Fig. 3c), despite a very high level of PD-1 expression. Further insight into the dynamic signals given to responding antigen-specific CD8+ T cells during acute infection may be needed to better understand this observation. Perhaps in some circumstances, PD-1 upregulation that occurs in the setting of enhanced activation and copious supporting cytokines is unable to provide an overall inhibitory signal to the T cells, but in another scenario with lower costimulatory signaling or a limited cytokine milieu, inhibitory PD-1 signals impair T-cell responses.
In our study, the activation kinetics, functionality, and apoptosis susceptibility of HCV-specific CD8+ T cells during acute HCV infection in the presence or absence of HIV coinfection did not seem to differ. This may have been related to patient 802's treatment with HAART and an undetectable HIV viral load for approximately 9 months prior to acute HCV infection; however, larger cohorts of patients with and without HIV coinfection will need to be studied to determine whether differences in apoptosis susceptibility exist. Some studies have demonstrated quantitative and qualitative deficits in the adaptive T-cell response to HCV in the setting of HIV coinfection (17, 22, 32, 33) while others have not (1). Given that uncontrolled HIV viremia and a low CD4 count might be expected to lead to greater cytokine withdrawal-mediated apoptosis of HCV-specific CD8+ T cells, studying apoptosis susceptibility in the setting of HIV coinfection may be highly relevant.
Our study highlights the reciprocal expression pattern of PD-1 and CD127 (IL-7 receptor-α) during longitudinal analysis of the acute phase of infection (Fig. 2d). When PD-1 intensity increased, CD127 expression fell, and vice versa. This became even more pronounced after antiviral therapy, which led to a rapid fall in PD-1 intensity while nearly 100% of cells expressed CD127. We also found that apoptosis susceptibility of HCV-specific CD8+ T cells correlated with low expression of CD127. In fact, HCV-specific CD8+ T cells during the acute phase of infection that survived short in vitro culture showed increased CD127 expression. Since upregulation of CD127 has been linked to formation of functional memory T cells (25, 28), we hypothesize that these CD127+ HCV-specific CD8+ T cells seen during early, acute HCV infection represent a population of either effector cells with memory T-cell potential or a population of bona fide memory T cells. Our evaluation of PD-1 and CD127 expression during the acute phase of infection also showed that expression levels of these surface receptors were dynamic and seemed to gauge the level of viremia. With high levels of viremia, there appeared to be an expansion of effector CD8+ T cells expressing high levels of PD-1 high and low levels of CD127. When viremia fell, PD-1 expression decreased and CD127 expression increased. However, during progression to the chronic phase in nonresolving infection, CD127 expression on HCV-specific CD8+ T cells from the peripheral blood continued to increase despite continued viremia (patient 240). Why the CD127 gauge failed in the peripheral blood during progression to chronic infection is not yet understood, but prior studies have shown that in chronic infection in the liver, these HCV-specific CD8+ T cells remain CD127low (48).
We report remarkable similarity in the activation state and functional impairments of HCV-specific CD8+ T cells in the blood during the acute phase and in the liver during the chronic phase of infection. HCV-specific CD8+ T cells in the blood and liver were highly activated, and prior studies have shown a similar pattern of very high PD-1 and low CD127 expression on liver-infiltrating HCV-specific CD8+ T cells (20, 48). These liver-infiltrating HCV-specific CD8+ T cells also displayed significant functional deficits. The majority of HCV-specific CD8+ T cells in the liver were not proliferating. In addition, though the majority of HCV-specific CD8+ T cells produced granzyme B, there was a total lack of detectible perforin production. Interestingly, this same pattern of undetectable perforin but abundant granzyme was seen in CD8+ T cells infiltrating rectal, gut-associated lymphoid tissue of patients with HIV infection (52). The authors hypothesized that the lack of perforin at this site might serve to protect the tissue from unwanted immune damage but that certain sexually transmitted viruses might capitalize on this immune perturbation for their benefit (52). The same immune perturbation in the liver might play a role in protection against overzealous immune responses but might also be important in allowing persistence of HCV and other liver-tropic viruses. Impaired maturation of antigen-specific cells might explain the deficit in perforin production (2), and in HIV infection further maturation of T cells as evidenced by CD27 downregulation was associated with perforin production (23). In HCV infection, the majority of HCV-specific cells in acute and chronic infection and those infiltrating the liver remained CD27+ (data not shown) and may not have “matured” into perforin-producing cells. However, the importance of perforin in HCV viral clearance is incompletely understood, and it should be noted that the perforin/granzyme pathway has been shown to be critical in clearance of some viral infections but not others (51).
A prior study demonstrated enhancement of total CD3+ T-cell apoptosis in the liver of patients with chronic HCV infection (45), and our study supports the hypothesis that highly activated effector T cells in the liver are also apoptotic. Importantly, we expand on this study by showing that HCV-specific CD8+ T cells at the site of infection show a higher level of apoptosis susceptibility than other antigen-specific CD8+ T cells and that these cells are branded by very high levels of PD-1 expression. Indeed, we found that the majority of HCV-specific CD8+ T cells in blood at the earliest time points during acute infection and in liver during chronic infection were highly apoptotic, expressed the highest level of PD-1, and expressed only low levels of the antiapoptotic molecule Bcl-2.
This contrasted with HCV-specific CD8+ T cells in blood during chronic infection that were not activated, expressed intermediate PD-1 levels, and displayed increased expression of Bcl-2. Hence, during chronic infection, a distinct compartmentalization of the activated HCV-specific CD8+ T cells to the liver was present. During early acute HCV infection, liver homing of HCV-specific CD8+ T cells might also have explained the loss of high-level PD-1-expressing T cells from the periphery; nevertheless, based on our studies, these cells were highly susceptible to apoptosis.
PD-1 was first identified in a hybridoma undergoing apoptosis (27), but the inhibitory function of PD-1 has been more definitively linked to deactivation of downstream TCR signaling intermediates rather than apoptosis induction (35). A prior study in HIV infection has demonstrated that PD-1 signaling can lead to enhanced apoptosis susceptibility of HIV-specific CD8+ T cells during chronic infection (47), and our study further highlights the importance of apoptosis in antigen-specific CD8+ T cells expressing high levels of PD-1. In our studies of spontaneous apoptosis, rescue with cytokine could be detected by identification of surviving HCV-specific CD8+ T cells by tetramer analysis. Since PD-1/PD-L1 signaling is tightly linked to TCR stimulation, we were unable to show rescue by PD-L1 blockade since TCR stimulation in brief culture leads to downregulation of TCR and impaired ability to detect antigen-specific cells by tetramer analysis. Future studies will be needed to improve our understanding of the mechanism of apoptosis induction after PD-1 ligation.
We further evaluated the mechanism of apoptosis during the acute and chronic phases of HCV infection. Either AICD- or ACAD-mediated death or a combination of both could be involved. The process of AICD was first demonstrated by studying immature T cells in thymic cultures that died by apoptosis after stimulation by antibodies to CD3/TCR (53). Subsequently, it was shown that mature T cells also die by apoptosis after activation by a phenomenon of peripheral deletion (30). This process can be mimicked in vitro by initial TCR ligation in the presence of IL-2, followed several days later by reactivation (37, 38). AICD is triggered by persistent antigen stimulation and death receptor signaling and is mediated by activation of caspase 8. Conversely, ACAD, or passive cell death, occurs after withdrawal of cytokine and/or antigen. This form of cell death is mediated by caspase 9. During the contraction phase of a resolving, acute human viral infection it has been shown that T cells contract via a mechanism of cytokine withdrawal (9). However, the mechanism of contraction during nonresolving HCV infection had not been reported. We show that despite persistent infection, HCV-specific CD8+ T cells also contracted by a cytokine withdrawal mechanism and that this could be reversed by coculture with IL-2, IL-7, or IL-15. The ability to reverse the death process with IL-7 has particular relevance, given a recent study that demonstrated that IL-7 treatment during lymphocytic choriomeningitis virus infection enhanced the number of memory CD8+ T cells when its administration was restricted to the contraction phase of the response (44).
In contrast to our finding of caspase 9-mediated death of HCV-specific CD8+ T cells, in a transgenic hepatitis model expression of HCV structural proteins in murine hepatocytes resulted in enhanced apoptosis of activated T cells that was associated with an upregulation of FasL and was inhibited by anti-FasL antibody (26). Furthermore, utilizing reverse transcription-PCR a study of Fas and FasL expression in patients with chronic HCV infection demonstrated a progressive increase in Fas/FasL during stages of liver disease from chronic hepatitis to cirrhosis (6). In our acutely infected patients, although we noted that nearly 100% of the HCV-specific CD8+ T cells expressed CD95 (Fas), we were unable to detect caspase 8-mediated death signaling. In addition, we found high-level rescue of cell death by provision of cytokines, pointing to a critical role for a caspase 9-mediated death pathway. Though our evaluation of the mechanism of apoptosis of liver-infiltrating HCV-specific CD8+ T cells was limited by biopsy sample size, we offer evidence that the cytokine withdrawal death pathway also plays an important role at the site of infection during the chronic phase. Further studies characterizing apoptosis of HCV-specific CD8+ T cells in the liver of patients with chronic HCV infection are needed to determine the exact contribution of each death pathway.
An important unanswered question is how HCV-specific CD8+ T cells during chronic HCV infection are maintained since limited proliferation (measured by Ki67 expression) was found, and high levels of apoptosis were seen (for liver HCV-specific CD8+ T cells). We propose that in the peripheral blood, the majority of HCV-specific CD8+ T cells are long-lived resting memory cells (expressing high levels of CD127) and are maintained homeostatically. For the liver, it is not currently known whether a stable population of HCV-specific CD8+ T cells exists or whether these populations fluctuate, displaying discrete periods of proliferation and nonproliferation. Perhaps HCV-specific CD8+ T cells infiltrating the liver had proliferated at a nonliver site (e.g., lymph node) and homed to the liver later. Longitudinal human studies utilizing serial liver biopsy would be informative but obviously not easily performed.
What might be mediating cytokine withdrawal during HCV infection and enhancing apoptosis susceptibility of responding T cells? A compilation of recent reports suggests that CD4+ CD25+ FoxP3+ T regulatory cells are integral in inhibiting T-cell responses in HCV infection (5, 8, 18, 39, 50, 60). High numbers of CD4+ CD25+ FoxP3+ T regulatory cells have been reported in the liver of patients with chronic infection (60), and a very recent report suggests that CD4+ CD25+ FoxP3+ T regulatory inhibition of effector T cells is mediated by induction of cytokine deprivation apoptosis (46). Based on these studies and our report, we hypothesize that during progression to chronic HCV infection, these regulatory T cells create an environment of low IL-2 availability and induce apoptosis of responding HCV-specific CD8+ T cells. Future studies will need to explore a mechanistic link between the level of PD-1 signaling and cytokine deprivation-mediated apoptotic pathways.
For effective immune responses to pathogens, a balance of TCR signaling, costimulatory/coinhibitory signals, and the cytokine milieu dictates the level of response. During the height of infection, high levels of PD-1 expression may be limiting immune responses, but a high level of TCR signaling and cytokine availability override this inhibitory PD-1 signaling. During the contraction phase of acute responses to resolving viral infection, reduced TCR signaling and decreased cytokine availability do not override negative signals, and the immune response wanes. During nonresolving acute infection, we hypothesize that despite continued TCR signaling, a particular dearth of cytokines prevents continued immune responses, and the response also wanes even at the site of infection. This lack of cytokine may be related to an infiltration of CD4+ CD25+ FoxP3+ T regulatory cells or perhaps to a lack of CD4+ T-cell help. Prior studies of liver immune responses have indicated that the liver may be a preferential site of apoptosis of activated T cells (24), and our findings support this. Moreover, our report highlights the relevance of this process for HCV-specific CD8+ T cells' infiltrating the liver of patients with chronic infection. T-cell apoptosis in the liver has been hypothesized to be a critical component in the induction of liver tolerance (11, 12), and it is conceivable that HCV and other chronic hepatotropic viruses take advantage of this process for their own survival. Further understanding of the possible pathogenic role for apoptosis of HCV-specific CD8+ T cells in the failure to control or clear HCV infection could lead to better interventions aimed at enhancing current antiviral treatments.
Acknowledgments
We thank Dimitri Fillos for excellent technical assistance and Francie Lasseter, Beverly Weaver, and Ellen Katz for patient cohort coordination. We thank Enrique Martinez for his contribution to this research.
We acknowledge the support from the Grand Challenges in Global Health Initiative (G.F.), EVC/CFAR Flow Cytometry Core P30 AI050409, Cancer Research Institute Investigator Award (A.G.), Woodruff Health Sciences Fund (A.G.), the Yerkes Research Center Base Grant RR-00165, and the Public Health Service (K08 AI072191 to H.R., AI56299 to G.F., and AI070101 to A.G.).
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Alatrakchi, N., C. S. Graham, Q. He, K. E. Sherman, and M. J. Koziel. 2005. CD8+ cell responses to hepatitis C virus (HCV) in the liver of persons with HCV-HIV coinfection versus HCV monoinfection. J. Infect. Dis. 191702-709. [DOI] [PubMed] [Google Scholar]
- 2.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8379-385. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, G. L., A. Wasley, E. P. Simard, G. M. McQuillan, W. L. Kuhnert, and M. J. Alter. 2006. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann. Intern. Med. 144705-714. [DOI] [PubMed] [Google Scholar]
- 4.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439682-687. [DOI] [PubMed] [Google Scholar]
- 5.Bolacchi, F., A. Sinistro, C. Ciaprini, F. Demin, M. Capozzi, F. C. Carducci, C. M. Drapeau, G. Rocchi, and A. Bergamini. 2006. Increased hepatitis C virus (HCV)-specific CD4+ CD25+ regulatory T lymphocytes and reduced HCV-specific CD4+ T cell response in HCV-infected patients with normal versus abnormal alanine aminotransferase levels. Clin. Exp. Immunol. 144188-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bortolami, M., A. Kotsafti, R. Cardin, and F. Farinati. 2008. Fas/FasL system, IL-1β expression and apoptosis in chronic HBV and HCV liver disease. J. Viral Hepat. 15515-522. [DOI] [PubMed] [Google Scholar]
- 7.Bratke, K., M. Kuepper, B. Bade, J. C. Virchow, Jr., and W. Luttmann. 2005. Differential expression of human granzymes A, B, and K in natural killer cells and during CD8+ T cell differentiation in peripheral blood. Eur. J. Immunol. 352608-2616. [DOI] [PubMed] [Google Scholar]
- 8.Cabrera, R., Z. Tu, Y. Xu, R. J. Firpi, H. R. Rosen, C. Liu, and D. R. Nelson. 2004. An immunomodulatory role for CD4+ CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 401062-1071. [DOI] [PubMed] [Google Scholar]
- 9.Callan, M. F., C. Fazou, H. Yang, T. Rostron, K. Poon, C. Hatton, and A. J. McMichael. 2000. CD8+ T-cell selection, function, and death in the primary immune response in vivo. J. Clin. Investig. 1061251-1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10439-449. [DOI] [PubMed] [Google Scholar]
- 11.Crispe, I. N. 2003. Hepatic T cells and liver tolerance. Nat. Rev. Immunol. 351-62. [DOI] [PubMed] [Google Scholar]
- 12.Crispe, I. N., T. Dao, K. Klugewitz, W. Z. Mehal, and D. P. Metz. 2000. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol. Rev. 17447-62. [DOI] [PubMed] [Google Scholar]
- 13.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 14.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 3461006-1007. [DOI] [PubMed] [Google Scholar]
- 15.Dong, H., G. Zhu, K. Tamada, D. B. Flies, J. M. van Deursen, and L. Chen. 2004. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity 20327-336. [DOI] [PubMed] [Google Scholar]
- 16.Dorfman, D. M., J. A. Brown, A. Shahsafaei, and G. J. Freeman. 2006. Programmed death-1 (PD-1) is a marker of germinal center-associated T cells and angioimmunoblastic T-cell lymphoma. Am. J. Surg. Pathol. 30802-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dutoit, V., D. Ciuffreda, D. Comte, J. J. Gonvers, and G. Pantaleo. 2005. Differences in HCV-specific T cell responses between chronic HCV infection and HIV/HCV co-infection. Eur. J. Immunol. 353493-3504. [DOI] [PubMed] [Google Scholar]
- 18.Ebinuma, H., N. Nakamoto, Y. Li, D. A. Price, E. Gostick, B. L. Levine, J. Tobias, W. W. Kwok, and K. M. Chang. 2008. Identification and in-vitro expansion of functional antigen-specific CD25+ FoxP3+ regulatory T-cells in hepatitis C virus infection. J. Virol. 825043-5053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerlach, J. T., H. M. Diepolder, M. C. Jung, N. H. Gruener, W. W. Schraut, R. Zachoval, R. Hoffmann, C. A. Schirren, T. Santantonio, and G. R. Pape. 1999. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C. Gastroenterology. 117933-941. [DOI] [PubMed] [Google Scholar]
- 20.Golden-Mason, L., B. Palmer, J. Klarquist, J. A. Mengshol, N. Castelblanco, and H. R. Rosen. 2007. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 819249-9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 755550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harcourt, G., E. Gomperts, S. Donfield, and P. Klenerman. 2006. Diminished frequency of hepatitis C virus specific interferon gamma secreting CD4+ T cells in human immunodeficiency virus/hepatitis C virus coinfected patients. Gut 551484-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haridas, V., T. W. McCloskey, R. Pahwa, and S. Pahwa. 2003. Discordant expression of perforin and granzyme A in total and HIV-specific CD8 T lymphocytes of HIV infected children and adolescents. AIDS 172313-2322. [DOI] [PubMed] [Google Scholar]
- 24.Huang, L., G. Soldevila, M. Leeker, R. Flavell, and I. N. Crispe. 1994. The liver eliminates T cells undergoing antigen-triggered apoptosis in vivo. Immunity 1741-749. [DOI] [PubMed] [Google Scholar]
- 25.Huster, K. M., V. Busch, M. Schiemann, K. Linkemann, K. M. Kerksiek, H. Wagner, and D. H. Busch. 2004. Selective expression of IL-7 receptor on memory T cells identifies early CD40L-dependent generation of distinct CD8+ memory T cell subsets. Proc. Natl. Acad. Sci. USA 1015610-5615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iken, K., L. Huang, H. Bekele, E. V. Schmidt, and M. J. Koziel. 2006. Apoptosis of activated CD4+ and CD8+ T cells is enhanced by co-culture with hepatocytes expressing hepatitis C virus (HCV) structural proteins through FasL induction. Virology 346363-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ishida, Y., Y. Agata, K. Shibahara, and T. Honjo. 1992. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 113887-3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 41191-1198. [DOI] [PubMed] [Google Scholar]
- 29.Kasprowicz, V., J. Schulze Zur Wiesch, T. Kuntzen, B. E. Nolan, S. Longworth, A. Berical, J. Blum, C. McMahon, L. L. Reyor, N. Elias, W. W. Kwok, B. G. McGovern, G. Freeman, R. T. Chung, P. Klenerman, L. Lewis-Ximenez, B. D. Walker, T. M. Allen, A. Y. Kim, and G. M. Lauer. 2008. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J. Virol. 823154-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kawabe, Y., and A. Ochi. 1991. Programmed cell death and extrathymic reduction of Vβ8+ CD4+ T cells in mice tolerant to Staphylococcus aureus enterotoxin B. Nature 349245-248. [DOI] [PubMed] [Google Scholar]
- 31.Keir, M. E., M. J. Butte, G. J. Freeman, and A. H. Sharpe. 2008. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 26677-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim, A. Y., G. M. Lauer, K. Ouchi, M. M. Addo, M. Lucas, J. Schulze Zur Wiesch, J. Timm, M. Boczanowski, J. E. Duncan, A. G. Wurcel, D. Casson, R. T. Chung, R. Draenert, P. Klenerman, and B. D. Walker. 2005. The magnitude and breadth of hepatitis C virus-specific CD8+ T cells depend on absolute CD4+ T-cell count in individuals coinfected with HIV-1. Blood 1051170-1178. [DOI] [PubMed] [Google Scholar]
- 33.Kim, A. Y., J. Schulze zur Wiesch, T. Kuntzen, J. Timm, D. E. Kaufmann, J. E. Duncan, A. M. Jones, A. G. Wurcel, B. T. Davis, R. T. Gandhi, G. K. Robbins, T. M. Allen, R. T. Chung, G. M. Lauer, and B. D. Walker. 2006. Impaired hepatitis C virus-specific T cell responses and recurrent hepatitis C virus in HIV coinfection. PLoS Med. 3e492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krammer, P. H., R. Arnold, and I. N. Lavrik. 2007. Life and death in peripheral T cells. Nat. Rev. Immunol. 7532-542. [DOI] [PubMed] [Google Scholar]
- 35.Latchman, Y., C. R. Wood, T. Chernova, D. Chaudhary, M. Borde, I. Chernova, Y. Iwai, A. J. Long, J. A. Brown, R. Nunes, E. A. Greenfield, K. Bourque, V. A. Boussiotis, L. L. Carter, B. M. Carreno, N. Malenkovich, H. Nishimura, T. Okazaki, T. Honjo, A. H. Sharpe, and G. J. Freeman. 2001. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2261-268. [DOI] [PubMed] [Google Scholar]
- 36.Lechner, F., D. K. Wong, P. R. Dunbar, R. Chapman, R. T. Chung, P. Dohrenwend, G. Robbins, R. Phillips, P. Klenerman, and B. D. Walker. 2000. Analysis of successful immune responses in persons infected with hepatitis C virus. J. Exp. Med. 1911499-1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lenardo, M., K. M. Chan, F. Hornung, H. McFarland, R. Siegel, J. Wang, and L. Zheng. 1999. Mature T lymphocyte apoptosis—immune regulation in a dynamic and unpredictable antigenic environment. Annu. Rev. Immunol. 17221-253. [DOI] [PubMed] [Google Scholar]
- 38.Lenardo, M. J. 1991. Interleukin-2 programs mouse αβ T lymphocytes for apoptosis. Nature 353858-861. [DOI] [PubMed] [Google Scholar]
- 39.Manigold, T., E. C. Shin, E. Mizukoshi, K. Mihalik, K. K. Murthy, C. M. Rice, C. A. Piccirillo, and B. Rehermann. 2006. Foxp3+ CD4+ CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood 1074424-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mescher, M. F., J. M. Curtsinger, P. Agarwal, K. A. Casey, M. Gerner, C. D. Hammerbeck, F. Popescu, and Z. Xiao. 2006. Signals required for programming effector and memory development by CD8+ T cells. Immunol. Rev. 21181-92. [DOI] [PubMed] [Google Scholar]
- 41.Miller, M. E., J. E. Everhart, and J. H. Hoofnagle. 2006. Epidemiologic research and the action plan for liver disease research. Ann. Epidemiol. 16861-865. [DOI] [PubMed] [Google Scholar]
- 42.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Muhlbauer, M., M. Fleck, C. Schutz, T. Weiss, M. Froh, C. Blank, J. Scholmerich, and C. Hellerbrand. 2006. PD-L1 is induced in hepatocytes by viral infection and by interferon-alpha and -gamma and mediates T cell apoptosis. J. Hepatol. 45520-528. [DOI] [PubMed] [Google Scholar]
- 44.Nanjappa, S. G., J. H. Walent, M. Morre, and M. Suresh. 2008. Effects of IL-7 on memory CD8 T cell homeostasis are influenced by the timing of therapy in mice. J. Clin. Investig. 1181027-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuti, S., D. Rosa, N. M. Valiante, G. Saletti, M. Caratozzolo, P. Dellabona, V. Barnaba, and S. Abrignani. 1998. Dynamics of intra-hepatic lymphocytes in chronic hepatitis C: enrichment for Vα24+ T cells and rapid elimination of effector cells by apoptosis. Eur. J. Immunol. 283448-3455. [DOI] [PubMed] [Google Scholar]
- 46.Pandiyan, P., L. Zheng, S. Ishihara, J. Reed, and M. J. Lenardo. 2007. CD4+ CD25+ Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat. Immunol. 81353-1362. [DOI] [PubMed] [Google Scholar]
- 47.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2032281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Radziewicz, H., C. C. Ibegbu, M. L. Fernandez, K. A. Workowski, K. Obideen, M. Wehbi, H. L. Hanson, J. P. Steinberg, D. Masopust, E. J. Wherry, J. D. Altman, B. T. Rouse, G. J. Freeman, R. Ahmed, and A. Grakoui. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 812545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Reichard, O., R. Schvarcz, and O. Weiland. 1997. Therapy of hepatitis C: alpha interferon and ribavirin. Hepatology 26108S-111S. [DOI] [PubMed] [Google Scholar]
- 50.Rushbrook, S. M., S. M. Ward, E. Unitt, S. L. Vowler, M. Lucas, P. Klenerman, and G. J. Alexander. 2005. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J. Virol. 797852-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russell, J. H., and T. J. Ley. 2002. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20323-370. [DOI] [PubMed] [Google Scholar]
- 52.Shacklett, B. L., C. A. Cox, M. F. Quigley, C. Kreis, N. H. Stollman, M. A. Jacobson, J. Andersson, J. K. Sandberg, and D. F. Nixon. 2004. Abundant expression of granzyme A, but not perforin, in granules of CD8+ T cells in GALT: implications for immune control of HIV-1 infection. J. Immunol. 173641-648. [DOI] [PubMed] [Google Scholar]
- 53.Smith, C. A., G. T. Williams, R. Kingston, E. J. Jenkinson, and J. J. Owen. 1989. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature 337181-184. [DOI] [PubMed] [Google Scholar]
- 54.Takata, H., and M. Takiguchi. 2006. Three memory subsets of human CD8+ T cells differently expressing three cytolytic effector molecules. J. Immunol. 1774330-4340. [DOI] [PubMed] [Google Scholar]
- 55.Thimme, R., D. Oldach, K. M. Chang, C. Steiger, S. C. Ray, and F. V. Chisari. 2001. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J. Exp. Med. 1941395-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trautmann, L., N. Chomont, and R. P. Sekaly. 2007. Inhibition of the PD-1 pathway restores the effector function of HIV-specific T cells. Med. Sci. (Paris) 2324-25. (In French.) [DOI] [PubMed] [Google Scholar]
- 57.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 121198-1202. [DOI] [PubMed] [Google Scholar]
- 58.Urbani, S., B. Amadei, P. Fisicaro, D. Tola, A. Orlandini, L. Sacchelli, C. Mori, G. Missale, and C. Ferrari. 2006. Outcome of acute hepatitis C is related to virus-specific CD4 function and maturation of antiviral memory CD8 responses. Hepatology 44126-139. [DOI] [PubMed] [Google Scholar]
- 59.Urbani, S., B. Amadei, D. Tola, M. Massari, S. Schivazappa, G. Missale, and C. Ferrari. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 8011398-11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ward, S. M., B. C. Fox, P. J. Brown, J. Worthington, S. B. Fox, R. W. Chapman, K. A. Fleming, A. H. Banham, and P. Klenerman. 2007. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J. Hepatol. 47316-324. [DOI] [PubMed] [Google Scholar]
- 61.Wasley, A., and M. J. Alter. 2000. Epidemiology of hepatitis C: geographic differences and temporal trends. Semin. Liver Dis. 201-16. [DOI] [PubMed] [Google Scholar]
- 62.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 1693447-3458. [DOI] [PubMed] [Google Scholar]