Abstract
While human immunodeficiency virus type 1 (HIV-1) infection is associated with hyperimmune activation and systemic depletion of CD4+ T cells, simian immunodeficiency virus (SIV) infection in sooty mangabeys or chimpanzees does not exhibit these hallmarks. Control of immune activation is thought to be one of the major components that govern species-dependent differences in the disease pathogenesis. A previous study introduced the idea that the resistance of chimpanzees to SIVcpz infection-induced hyperimmune activation could be the result of the expression of select sialic acid-recognizing immunoglobulin (Ig)-like lectin (Siglec) superfamily members by chimpanzee T cells. Siglecs, which are absent on human T cells, were thought to control levels of T-cell activation in chimpanzees and were thus suggested as a cause for the pathogenic differences in the course of SIVcpz or HIV-1 infection. As in human models of T-cell activation, stimulation had been attempted using an anti-CD3 monoclonal antibody (MAb) (UCHT1; isotype IgG1), but despite efficient binding, UCHT1 failed to activate chimpanzee T cells, an activation block that could be partially overcome by MAb-induced Siglec-5 internalization. We herein demonstrate that anti-CD3 MAb-mediated chimpanzee T-cell activation is a function of the anti-CD3 MAb isotype and is not governed by Siglec expression. While IgG1 anti-CD3 MAbs fail to stimulate chimpanzee T cells, IgG2a anti-CD3 MAbs activate chimpanzee T cells in the absence of Siglec manipulations. Our results thus imply that prior to studying possible differences between human and chimpanzee T-cell activation, a relevant model of chimpanzee T cell activation needs to be established.
The finding that simian immunodeficiency viruses (SIVs) in their natural hosts replicate with high efficiency but do not cause AIDS-like symptoms has sparked significant interest. It is thought that a better understanding of this phenomenon could result in the development of novel immune-based therapies for human immunodeficiency virus type 1 (HIV-1) infection. For example, SIV infection in sooty mangabeys (Cercocebus atys) has a benign clinical course compared to the pathology of HIV-1 infection (23). Possible explanations for this finding have been intensely investigated.
As in HIV-1-infected patients, severe depletion of mucosal CD4+ T cells is observed in sooty mangabeys during the acute infection period (8), but this acute depletion is not sufficient to induce an AIDS-like pathology (15). The observed relative AIDS resistance of naturally SIV-infected sooty mangabeys seems to be mostly independent of the virus-specific CD8+ T-cell response, as transient monoclonal antibody (MAb)-induced depletion of cytotoxic CD8 T cells in infected sooty mangabeys resulted in only minor changes in the levels of plasma viremia (2). In addition, the magnitude of SIV-specific CD8 T-cell responses in SIV-infected mangabeys is markedly reduced compared to HIV-infected humans (7). However, the absence of perturbations of cell cycle control in T cells in infected sooty mangabeys could be correlated with the disease-resistant phenotype (17). Generally, SIV-infected sooty mangabeys exhibit lower relative levels of immune activation and bystander T-cell apoptosis (6, 22, 24).
Much less is known about SIV infection in chimpanzees (Pan troglodytes) (19). However, a recent publication suggested a role for the expression of sialic acid-recognizing immunoglobulin (Ig)-like lectin (Siglec) molecules in the control of the immune response to SIV infection, thus offering an intriguing possible explanation for how chimpanzees escape the immune hyperstimulation seen in HIV-1-infected patients. This report found that chimpanzee T cells, in comparison to human T cells, express high levels of several members of the Siglec superfamily, which are usually involved in the downregulation of innate immune cell activation (5, 28) and are reasoned, in turn, to contribute to the downregulation of adaptive immune responses. The study provided some functional evidence that Siglecs, in particular Siglec-5, could control the degree of T-cell activation in chimpanzees mediated through the T-cell receptor (TCR)/CD3 pathway (16). In an experimental setting, such as in the above study, activation of T cells with anti-CD3 in the presence of costimulation by anti-CD28 antibodies is considered to be a good physiological correlate of antigen-specific T-cell stimulation by antigen-presenting cells (APCs) (20, 21). Since a series of previous studies has documented differences in the mechanisms and degree of stimulation between various anti-CD3 MAbs (27), we revisited the above-mentioned study to determine whether, indeed, differences in Siglec expression profiles would explain the observed differences in the response of human versus chimpanzee T cells to TCR/CD3 stimulation or whether the reduced ability of UCHT1 to optimally induce activation of chimpanzee T cells may, in fact, be related to the epitope specificity or the isotype of the anti-CD3 MAb being utilized.
We herein demonstrate that the previously reported differences in T-cell activation between human and chimpanzee T cells in response to anti-CD3 MAb stimulation is unlikely to be a function of protein sequence differences influencing anti-CD3 MAb binding or to be controlled by other molecules such as Siglecs but can be mostly attributed to the isotype of the anti-CD3 MAb being used in the analysis. Our studies further suggest that future research involving chimpanzee T-cell activation through the TCR/CD3 pathway will have to first establish a relevant experimental model for CD3-mediated T-cell activation that will be comparable to existing human models of T-cell activation.
MATERIALS AND METHODS
Cell culture.
Human peripheral blood mononuclear cells (PBMCs) were derived from heparinized blood of normal adult human volunteers after informed consent was obtained from the donors. Chimpanzee blood samples were collected at the Yerkes National Primate Research Center, Emory University (Atlanta, GA). The studies are part of a protocol approved by the Yerkes/Emory Institutional Animal Care and Use Committee. PBMCs were obtained by Ficoll-Hypaque gradient centrifugation. All cells were cultured in RPMI 1640 medium supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, and 10% heat-inactivated fetal bovine serum in the presence of 30 U/ml recombinant human interleukin-2 (Roche Scientific, Palo Alto, CA).
Antibodies and other agents.
All anti-CD3 and anti-CD28 MAbs (clones UCHT1 [isotype IgG1] and HIT3a [isotype IgG2a] from Pharmingen; clones BB11 [IgG1] and SP34 [IgG3] from Santa Cruz, CA) were purchased commercially, except the anti-CD3 MAb clone OKT3 (isotype IgG2a), which was derived from cell culture supernatants of the OKT3 hybridoma (ATCC). Antibody concentration in the supernatants was determined using a RapidQuant IgG assay (GUAVA Technologies). Goat anti-mouse IgG beads were obtained from Miltenyi, Inc (Auburn, CA). Phytohemagglutinin-L (PHA-L) was obtained from Sigma (St. Louis, MO). The cell membrane labeling dye PKH26 was obtained from Sigma, and staining of the PBMCs was performed according to the manufacturer's instructions. Recombinant human interleukin-2 was obtained from R&D Systems (Minneapolis, MN). The enhanced green fluorescent protein (GFP) reporter virus HIV-1 NL-ENG1 has been described elsewhere (10, 11).
Flow cytometric analysis was performed using an EasyCyte flow cytometer (GUAVA, Hayward, CA). PKH26 fluorescence was recorded in the yellow fluorescence channel. Data analysis was performed using CellQuestPro software (Becton & Dickinson, San Jose, CA).
Computational mutagenesis and side chain repacking.
The gene sequences were as follows: human CD3ɛ (NCBI GeneID916; accession number CAA27516), chimpanzee CD3ɛ (NCBI GeneID 742330; accession number XP_001160698); OKT3 Fab-human CD3ɛ complex (Protein Data Bank identifier [PDB ID] 1SY6), and UCHT1 Fab-human CD3ɛ complex (PDB ID 1XIW). To predict structural changes due to the G47S residue mutation for both complexes, we modeled side chain conformational changes on a flexible protein backbone. We used ROSETTA++ to perform optimization of backbone displacement and backbone-dependent side chain rotamer conformations. ROSETTA++ is a software suite intended for predicting and designing protein structures, protein folding mechanisms, and protein-protein interactions (25). The scoring function included van der Waals and solvation interactions, hydrogen bonding, residue-residue pair statistics, and rotamer probabilities. All side chain atoms were fully represented, and simultaneous optimization of backbone displacement and side chain conformations were performed. An alignment of the antibody binding subsequences of known antibody-antigen complexes was incorporated into the docking to enrich likely complementarity-determining region loop orientations.
RESULTS AND DISCUSSION
Chimpanzee T-cell activation by anti-CD3 MAbs.
The profound in vivo differences in the pathological outcomes between SIV infection of the natural African hosts of SIV including chimpanzees and HIV-1 infection in humans are thought to hold one of the keys to our understanding of how hyperimmune activation during HIV-1 infection could potentially be therapeutically targeted to improve disease outcome.
In vivo, T-cell activation is induced by antigen-specific stimulation of the TCR/CD3 complex, followed by the activation of costimulatory molecules, most importantly, CD28. Activation of intracellular pathways following CD28 ligation is commonly provided by B7.1 (CD80) and B7.2 (CD86) molecules expressed on APCs. In an experimental setting, costimulation of T cells with anti-CD3 and anti-CD28 antibodies is generally considered to be a good physiological correlate of antigen-specific T-cell stimulation by APCs (20, 21). In a recent publication, Nguyen et al. (16) reported that the anti-CD3 MAb UCHT1, which was raised against human CD3 and previously shown to recognize the CD3ɛ chain complexed with either the CD3δ or CD3γ chain (1, 9), mostly failed to activate chimpanzee T cells in the presence of costimulation with an anti-CD28 MAb. The chimpanzee CD3ɛ chain, which is 94.2% homologous to human CD3ɛ, is recognized by UCHT1 as is human CD3ɛ, which can be demonstrated by flow cytometric analysis. The inability of UCHT1 to activate chimpanzee T cells was attributed to the expression of a select number of Siglecs by chimpanzee T cells (expression of Siglec-3, -5, -7, and -9) but not human T cells. Of interest was the study's finding that ligation of Siglec-5 by its corresponding MAb on chimpanzee T cells led to antibody-mediated internalization of Siglec-5, which allowed for partial UCHT1-mediated T-cell activation. However, these studies overlooked the potential role of the nature of CD3 ligation, which could have influenced the outcome of the experiments. This may be relevant as (i) previous publications indicate that various anti-CD3 MAbs confer activation of even human T cells with different outcomes (27) and (ii) the chimpanzee CD3ɛ chain has a Gly/Ser47 exchange, with Gly47 being reported to be involved in UCHT1 binding of human CD3ɛ (1, 9). We thus investigated whether the recalcitrance of chimpanzee T cells to UCHT1 activation is secondary to the function of the Siglec profile or could be explained by the specific clone of anti-CD3 MAb that was utilized in these studies.
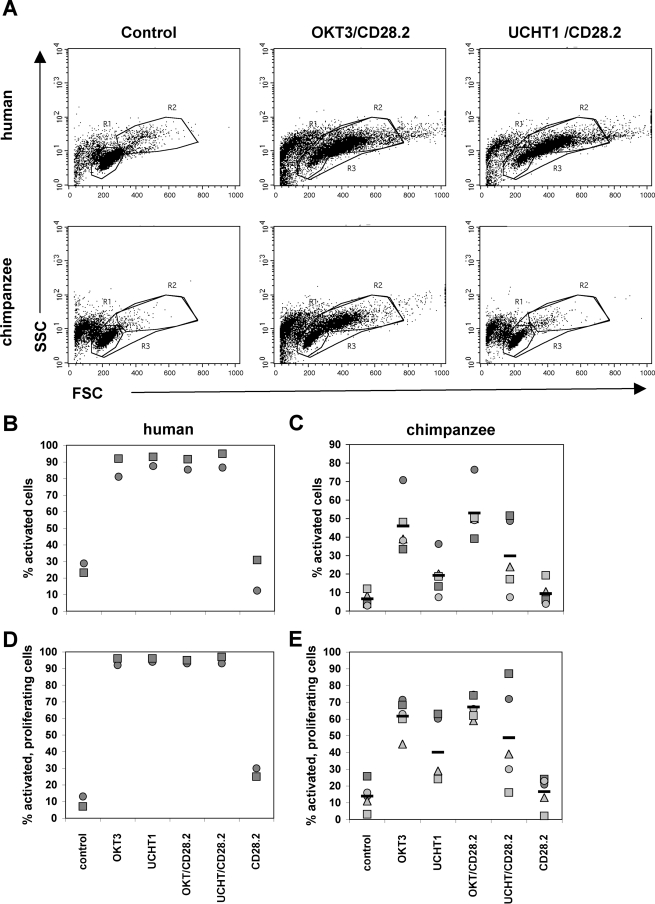
For this purpose, we initially stimulated PBMCs from five chimpanzees with the anti-CD3 MAbs UCHT1 or OKT3, either in the presence or absence of a costimulatory anti-CD28 MAb (clone CD28.2). Controls consisted of in vitro stimulation of PBMCs from two healthy human donors with the same combination of antibodies, performed in parallel. Activation of the T-cell population was then measured as an increase in cell size and granularity, which is generally associated with T-cell activation, by determining the forward scatter ([FSC] cell size) versus side scatter ([SSC] cell granularity) profile using flow cytometric analysis. Proliferation of the T-cell population was determined using the membrane stain PKH26. The results of these experiments are presented in Fig. 1.
FIG. 1.
Activation of chimpanzee PBMCs with anti-CD3 MAbs. PBMCs from two human donors and five chimpanzees were stimulated with anti-CD3 MAbs (OKT3, isotype IgG2a; UCHT1, isotype gG1) at 0.3 μg/ml, with an anti-CD28 MAb (CD28.2) at 1 μg/ml, or with combinations of the same concentrations of the anti-CD3/CD28 MAbs. For control purposes, some cells were left unstimulated. (A) T-cell activation was then determined using flow cytometric analysis for an increase in the FSC/SSC pattern (cell size/cell granularity). Resting cells are located in R1, whereas activated cells are located in R2. Cell activation levels as determined by changes in the FSC/SSC pattern and the frequency of activated cells undergoing proliferation based on PKH26 staining are plotted for the human donors (B and D, respectively) and the chimpanzees (C and E, respectively).Each symbol indicates a different donor.
As expected, a majority (>90%) of the human T cells increased in size following stimulation with either anti-CD3 MAb alone or with the combination of anti-CD3/CD28 MAbs (Fig. 1A and B). Of the activated human T-cell population, >90% responded to activation with proliferation (Fig. 1D). Costimulation with anti-CD28 MAb and cross-linking did not induce a detectable enhancement of the observed effect with human T cells (data not shown). As described previously, chimpanzee T cells responded poorly to stimulation with the clone UCHT1 anti-CD3 MAb (Fig. 1A and C). The failure of UCHT1 to induce optimal stimulation of chimpanzee T cells was not dependent on the dose of anti-CD3 MAb or kinetics (data not shown). The addition of the anti-CD28 MAb for purposes of providing costimulation with anti-CD3 MAb failed to rescue cell activation. However, stimulation with either the clone OKT3 anti-CD3 MAb alone or a combination of OKT3/CD28.2 MAbs triggered chimpanzee T-cell activation although activation at this OKT3 concentration (0.3 μg/ml) was somewhat less efficient than observed for human T cells (Fig. 1C and D).
Susceptibility of chimpanzee T cells to HIV-1/SIVcpz infection following anti-CD3 MAb stimulation.
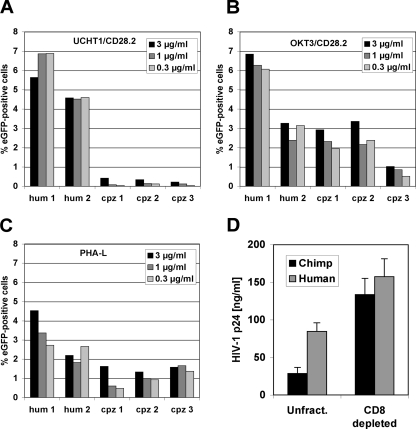
We next investigated whether levels of achievable cell activation would correspond to levels of achievable infection by infecting activated T cells from the two human donors and three chimpanzees using an enhanced GFP HIV-1 reporter virus (10, 11). As expected, the two stimulatory conditions allowed for readily detectable infection of human T cells with the reporter virus, with achievable infectivity levels varying between donors. In contrast, while UCHT1 stimulation, consistent with its inability to provide optimal activation, did not allow for relevant infection of chimpanzee PBMCs with the reporter virus, incubation of chimpanzee T cells with the OKT3 MAb allowed for variable but readily detectable levels of infection of chimpanzee PBMCs. Titration of the anti-CD3 MAbs revealed that the failure of UCHT1 to promote HIV-1 infection is not a function of the MAb concentration used (Fig. 2A and B). Stimulation with PHA-L rendered both cell types susceptible to infection, but, again, human T cells were relatively more susceptible than chimpanzee T cells. This decreased susceptibility of chimpanzee T cells could be explained by the slightly decreased achievable level of T-cell activation. However, we also considered the possibility that the previously reported noncytolytic anti-HIV-1 activity of CD8+ T cells could be developed to different degrees in the two species and could explain the differences between human and chimpanzee T cells in HIV-1 replication following OKT3 stimulation (14, 29, 30). To test this idea, we compared the ability of the HIV-1 HxB2 strain to replicate in unfractionated and CD8+ T-cell-depleted PBMCs from three human donors and three chimpanzees following stimulation with OKT3. Interestingly, removal of CD8+ T cells from the chimpanzee PBMC cultures compensated for most of the difference in the achievable HIV-1 replication levels. Experiments using the HIV-1 TYBE strain produced similar results (data not shown) (18, 31). The observed differences in HIV-1 replication in unfractionated PBMC cultures of human and chimpanzee origin are thus not just a function of stimulation levels but are greatly influenced by the proportionally higher noncytolytic effect exerted by CD8+ chimpanzee T cells.
FIG. 2.
Susceptibility of human and chimpanzee T cells to HIV-1 infection following in vitro stimulation with anti-CD3 MAbs. PBMCS from two human donors and three chimpanzees were stimulated with various concentrations of anti-CD3 MAb UCHT1(A), anti-CD3 MAb OKT3 (B), and PHA-L (C), and after 2 days infected with a GFP-expressing HIV-1 reporter virus. On day 4, levels of HIV-1 infection were determined as the percentage of GFP-positive cells. (D) Unfractionated (Unfract) and CD8+ T-cell-depleted PBMCs from three human donors and three chimpanzees were stimulated with OKT3 and infected with HIV-1 HxB2. Culture supernatants were harvested on day 7 postinfection and subjected to HIV-1 Gag p24 enzyme-linked immunosorbent assay. The histogram presents the mean p24 concentration of three donors ± standard deviation. Hum, human; cpz, chimpanzee.
OKT3/CD28.2 stimulated human and chimpanzee PBMCs were also found susceptible to infection with HIV-1 SG3 (X4 virus) and with SIVcpz GAB2 (X4 virus), but, consistent with current literature, stimulation did not render chimpanzee PBMCs susceptible to infection with an R5-tropic HIV-1 (YU2) (data not shown) (4, 19).
In summary, these finding suggest that the in vitro activation of T cells from chimpanzees, which is mediated through the TCR/CD3 pathway, is not dominated by molecules such as Siglecs (16) but could be a function of the antibody affinity or the antibody isotype (for OKT3, IgG2a; for UCHT1, IgG1).
Anti-CD3 MAb isotype determines the ability to stimulate chimpanzee T cells.
Our initial results suggested two possibilities to explain the inability of UCHT1 to optimally activate chimpanzee T cells: (i) a Gly-47/Ser-47 change in the chimpanzee CD3ɛ chain preferentially affects the affinity of UCHT1, resulting in a failure of UCHT1 to trigger the conformational changes in the CD3ɛ/CD3γ or CD3ɛ/CD3δ heterodimers needed to induce T-cell activation; (ii) stimulation by anti-CD3 MAbs is isotype dependent, suggesting differences in the Fc receptor profiles between the two species.
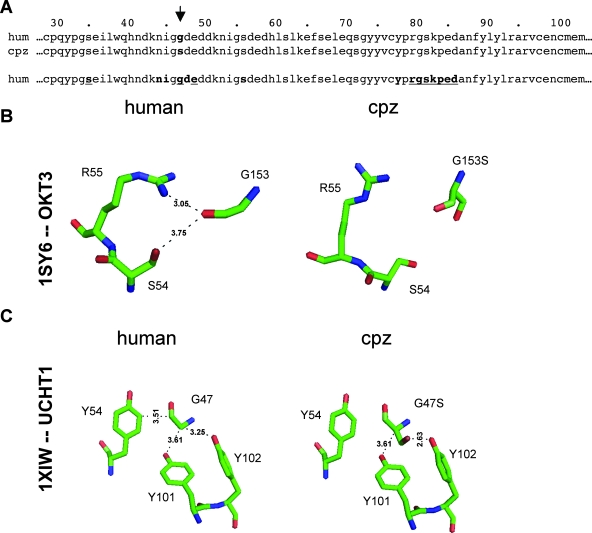
UCHT1 and OKT3 both recognize an immunodominant epitope in the CD3ɛ chain, which is associated with the formation of heterodimers with CD3δ or with CD3γ. For both antibodies the points of interaction are described based on crystal structures (1, 9). Figure 3A presents the amino acid alignment of human and chimpanzee CD3ɛ sequences. Relative to the amino acid sequence used for previously published crystallization experiments of human CD3ɛ with UCHT1 or OKT3, chimpanzee CD3ɛ has a Gly/Ser change at position 47, with Gly47 reported to be involved in binding of both UCHT1 and OKT3 (1, 9). This amino acid change could thus cause a decrease in the MAb affinity, resulting in the inability of the MAb to induce conformational changes despite binding. To investigate this possibility, we modeled the interactions of the chimpanzee CD3ɛ chain with the respective Fab fragments, using the previously published crystal structures of human CD3ɛ with the Fab fragments as templates (1, 9).
FIG. 3.
Species-specific differences in the UCHT1/CD3ɛ chain complex or OKT3/CD3ɛ chain complex. (A) Amino acid sequence alignment of the human (hum) and chimpanzee (cpz) CD3ɛ chains. The partial sequences represent the sequence that in previously published crystal structures was shown to contribute to human CD3 interaction with the anti-CD3 MAbs OKT3 and UCHT1. The arrow indicates the Gly/Ser-47 change in chimpanzee CD3ɛ (residues are in boldface). In the bottom sequence, CD3ɛ contact residues with UCHT1 are printed in bold, while CD3ɛ contact residues with OKT3 are underlined. To predict structural changes due to the G47S residue mutation for both complexes, we modeled side chain conformational changes on a flexible protein backbone, by using ROSETTA++ to perform optimization of backbone displacement and backbone-dependent side chain rotamer conformations. (B) Predicted conformational changes due to the G47S exchange in the OKT3/CD3ɛ complex. (C) Predicted conformational changes due to the G47S exchange in the UCHT1/CD3ɛ complex.
In the original crystal structure for the OKT3-CD3 interaction (PDB ID 1SY6), the CD3ɛ G47 makes contact with the Fab residues S54 and R55 by the criterion that two side chains make contact if at least one non-hydrogen atom pair lies within 4 Å of each other, which essentially represents a hydrogen-bonding cutoff between the donor and acceptor. In the repacked model of the complex with the single mutation G47S, both contacts are lost (Fig. 3B).
In the original crystal structure for the UCHT1-CD3 interaction (PDB ID 1XIW), the G47 residue makes contact with the three tyrosine Fab residues (Y54, Y101, and Y102) (Fig. 3C, left). In the repacked model of the complex with the single amino acid change G47S, the contact with Y54 is lost (Fig. 3C, right). However, the majority of the predicted interactions between the chimpanzee CD3ɛ chain and the UCHT1 fragment are not affected by the Gly/Ser-47 exchange. So, while binding of both anti-CD3 MAbs, OKT3 and UCHT1, could be affected by the Gly/Ser change at position 47 in chimpanzee CD3ɛ, the overall reduction in affinity is likely to be relatively low and thus unlikely to explain the failure of UCHT1 to optimally trigger chimpanzee T-cell activation.
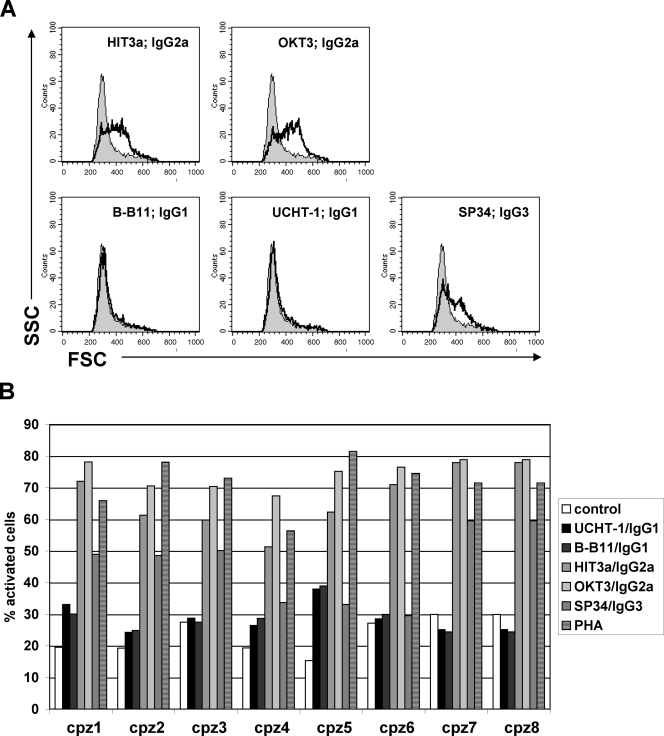
We thus finally investigated the possibility that differences in the antibody isotype are responsible for the differences in the ability of UCHT1 (IgG1) or OKT3 (IgG2a) to activate chimpanzee T cells. For this purpose, we stimulated PBMCs from three human donors and eight chimpanzees with a panel of five anti-CD3 antibodies (1 μg/ml), representing the isotypes IgG1 (MAbs UCHT1 and B-B11), IgG2a (OKT3 and HIT3a), and IgG3 (SP34). For the experiments, the antibodies were added to the PBMCs and remained in culture. One hour after the addition of the anti-CD3 MAbs to the PBMCs, an aliquot of the PBMCs from each culture was taken, and the level of MAb binding was determined. All antibodies were found to recognize CD3 expression on cells of both species with similar efficiency (data not shown). T-cell activation was then determined as a change in the FSC/SSC profile. Results from a representative flow cytometry experiment depicting the response of T cells from one chimpanzee to activation with the various anti-CD3 MAbs is shown in Fig. 4A. A summary of the responses to antibody-mediated T-cell activation of cells from eight chimpanzees is shown in Fig. 4B. The results demonstrate that while anti-CD3 MAbs of the IgG1 isotype stimulate human T cells, they fail to provide T-cell activation in chimpanzee T cells. Anti-CD3 MAbs of the IgG2a isotype provide T-cell activation in a species-independent manner, strongly suggesting that previously reported failure of chimpanzee T cells to optimally respond to anti-CD3 MAb-mediated stimulation is primarily a function of the isotype of the anti-CD3 MAb being utilized.
FIG. 4.
Anti-CD3 MAb-mediated in vitro T-cell activation in chimpanzees is dependent on the MAb isotype. PBMCs from three human donors (data not shown) and eight chimpanzees were stimulated in 24-well plates with a panel of anti-CD3 MAbs of various isotypes (MAb concentration, 1.0 μg/ml; cell density, 1 × 106 cells/ml). (A) T-cell activation was quantified by flow cytometric analysis as an increase in the FSC/SSC pattern. (B) Summary of the T-cell activation levels in response to stimulation with the indicated anti-CD3 MAbs for the eight tested animals.
These findings are not entirely surprising as a series of previous reports already demonstrated that Fc receptor profiles can be the crucial determinants for the stimulatory potential of mouse-derived anti-CD3 MAbs although the MAbs recognized the same epitope area or even had identical epitope recognition and only varying Fc domains (3, 12, 13). While little is known about the expression profiles of chimpanzee Fc receptors, there are some differences between the protein sequences of human and chimpanzee Fc receptors. The low-affinity Fc-gamma-R2A and -R2B sequences (NP_001009077 and XP_001153863, respectively) of the two species are identical in the parts that have previously been described to be involved in IgG binding. However, the chimpanzee high-affinity Fc-gamma-R1 (CD64) has an L114P substitution in the B/C loop and an R159H substitution right next to the F/G loop domain, regions that have been reported to be involved in IgG binding (26). These amino acid exchanges may be sufficient to account for affinity differences to IgG1 MAbs and could explain the observed inability of IgG1 anti-CD3 MAbs to optimally stimulate chimpanzee T cells.
While we can clearly demonstrate that it is possible to stimulate chimpanzee T cells through the CD3 pathway using IgG2a anti-CD3 MAbs, in vitro culture conditions for optimal activation of T cells from chimpanzees remain to be defined (Fig. 1 and 4). In this regard it should be noted that blood samples from nonhuman primates compared with humans are mostly, if not always, obtained following restraint and anesthesia, which may promote the release of endogenous steroids that are known to influence studies of immune function. In any case, whether Siglecs truly play a role in restricting chimpanzee T-cell activation and as a result in SIV pathogenesis would have to be reevaluated (16).
In summary, our data provide insights on differences in the requirements for optimal activation of T cells from chimpanzees and humans, which may impact the differences in clinical outcomes between natural SIV infection of chimpanzees and HIV-1 infection. However, key to studying these differences at the molecular level will be a relevant in vitro model of chimpanzee T-cell stimulation. Our results suggest that such a model is currently not available.
Acknowledgments
This work was supported by grant NIH R01AI064012 to O.K. and grant RR-000165 to the Yerkes Primate Center.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Arnett, K. L., S. C. Harrison, and D. C. Wiley. 2004. Crystal structure of a human CD3-epsilon/delta dimer in complex with a UCHT1 single-chain antibody fragment. Proc. Natl. Acad. Sci. USA 10116268-16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. P., G. Silvestri, J. T. Safrit, B. Sumpter, N. Kozyr, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2007. Depletion of CD8+ cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species. J. Immunol. 1788002-8012. [DOI] [PubMed] [Google Scholar]
- 3.Ceuppens, J. L., F. J. Bloemmen, and J. P. Van Wauwe. 1985. T cell unresponsiveness to the mitogenic activity of OKT3 antibody results from a deficiency of monocyte Fc gamma receptors for murine IgG2a and inability to cross-link the T3-Ti complex. J. Immunol. 1353882-3886. [PubMed] [Google Scholar]
- 4.Cho, M. W., R. Shibata, and M. A. Martin. 1996. Infection of chimpanzee peripheral blood mononuclear cells by human immunodeficiency virus type 1 requires cooperative interaction between multiple variable regions of gp120. J. Virol. 707318-7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crocker, P. R. 2005. Siglecs in innate immunity. Curr. Opin. Pharmacol. 5431-437. [DOI] [PubMed] [Google Scholar]
- 6.Cumont, M. C., O. Diop, B. Vaslin, C. Elbim, L. Viollet, V. Monceaux, S. Lay, G. Silvestri, R. Le Grand, M. Muller-Trutwin, B. Hurtrel, and J. Estaquier. 2008. Early divergence in lymphoid tissue apoptosis between pathogenic and nonpathogenic simian immunodeficiency virus infections of nonhuman primates. J. Virol. 821175-1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dunham, R., P. Pagliardini, S. Gordon, B. Sumpter, J. Engram, A. Moanna, M. Paiardini, J. N. Mandl, B. Lawson, S. Garg, H. M. McClure, Y. X. Xu, C. Ibegbu, K. Easley, N. Katz, I. Pandrea, C. Apetrei, D. L. Sodora, S. I. Staprans, M. B. Feinberg, and G. Silvestri. 2006. The AIDS resistance of naturally SIV-infected sooty mangabeys is independent of cellular immunity to the virus. Blood 108209-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon, S. N., N. R. Klatt, S. E. Bosinger, J. M. Brenchley, J. M. Milush, J. C. Engram, R. M. Dunham, M. Paiardini, S. Klucking, A. Danesh, E. A. Strobert, C. Apetrei, I. V. Pandrea, D. Kelvin, D. C. Douek, S. I. Staprans, D. L. Sodora, and G. Silvestri. 2007. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J. Immunol. 1793026-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kjer-Nielsen, L., M. A. Dunstone, L. Kostenko, L. K. Ely, T. Beddoe, N. A. Mifsud, A. W. Purcell, A. G. Brooks, J. McCluskey, and J. Rossjohn. 2004. Crystal structure of the human T cell receptor CD3 epsilon gamma heterodimer complexed to the therapeutic MAb OKT3. Proc. Natl. Acad. Sci. USA 1017675-7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kutsch, O., E. N. Benveniste, G. M. Shaw, and D. N. Levy. 2002. Direct and quantitative single-cell analysis of human immunodeficiency virus type 1 reactivation from latency. J. Virol. 768776-8786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levy, D. N., G. M. Aldrovandi, O. Kutsch, and G. M. Shaw. 2004. Dynamics of HIV-1 recombination in its natural target cells. Proc. Nat. Acad. Sci. 1014204-4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lobo, P. I., and H. C. Patel. 1997. Murine monoclonal IgG antibodies: differences in their IgG isotypes can affect the antibody effector activity when using human cells. Immunol. Cell Biol. 75267-274. [DOI] [PubMed] [Google Scholar]
- 13.Lubeck, M. D., Z. Steplewski, F. Baglia, M. H. Klein, K. J. Dorrington, and H. Koprowski. 1985. The interaction of murine IgG subclass proteins with human monocyte Fc receptors. J. Immunol. 1351299-1304. [PubMed] [Google Scholar]
- 14.Mackewicz, C. E., D. J. Blackbourn, and J. A. Levy. 1995. CD8+ T cells suppress human immunodeficiency virus replication by inhibiting viral transcription. Proc. Natl. Acad. Sci. USA 922308-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milush, J. M., J. D. Reeves, S. N. Gordon, D. Zhou, A. Muthukumar, D. A. Kosub, E. Chacko, L. D. Giavedoni, C. C. Ibegbu, K. S. Cole, J. L. Miamidian, M. Paiardini, A. P. Barry, S. I. Staprans, G. Silvestri, and D. L. Sodora. 2007. Virally induced CD4+ T cell depletion is not sufficient to induce AIDS in a natural host. J. Immunol. 1793047-3056. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen, D. H., N. Hurtado-Ziola, P. Gagneux, and A. Varki. 2006. Loss of Siglec expression on T lymphocytes during human evolution. Proc. Natl. Acad. Sci. USA 1037765-7770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paiardini, M., B. Cervasi, B. Sumpter, H. M. McClure, D. L. Sodora, M. Magnani, S. I. Staprans, G. Piedimonte, and G. Silvestri. 2006. Perturbations of cell cycle control in T cells contribute to the different outcomes of simian immunodeficiency virus infection in rhesus macaques and sooty mangabeys. J. Virol. 80634-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rana, S., G. Besson, D. G. Cook, J. Rucker, R. J. Smyth, Y. Yi, J. D. Turner, H. H. Guo, J. G. Du, S. C. Peiper, E. Lavi, M. Samson, F. Libert, C. Liesnard, G. Vassart, R. W. Doms, M. Parmentier, and R. G. Collman. 1997. Role of CCR5 in infection of primary macrophages and lymphocytes by macrophage-tropic strains of human immunodeficiency virus: resistance to patient-derived and prototype isolates resulting from the Δccr5 mutation. J. Virol. 713219-3227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuitemaker, H., L. Meyaard, N. A. Kootstra, R. Dubbes, S. A. Otto, M. Tersmette, J. L. Heeney, and F. Miedema. 1993. Lack of T cell dysfunction and programmed cell death in human immunodeficiency virus type 1-infected chimpanzees correlates with absence of monocytotropic variants. J. Infect. Dis. 1681140-1147. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz, R. H. 1992. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 711065-1068. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz, R. H. 2003. T cell anergy. Annu. Rev. Immunol. 21305-334. [DOI] [PubMed] [Google Scholar]
- 22.Silvestri, G., A. Fedanov, S. Germon, N. Kozyr, W. J. Kaiser, D. A. Garber, H. McClure, M. B. Feinberg, and S. I. Staprans. 2005. Divergent host responses during primary simian immunodeficiency virus SIVsm infection of natural sooty mangabey and nonnatural rhesus macaque hosts. J. Virol. 794043-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silvestri, G., M. Paiardini, I. Pandrea, M. M. Lederman, and D. L. Sodora. 2007. Understanding the benign nature of SIV infection in natural hosts. J. Clin. Investig. 1173148-3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvestri, G., D. L. Sodora, R. A. Koup, M. Paiardini, S. P. O'Neil, H. M. McClure, S. I. Staprans, and M. B. Feinberg. 2003. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity 18441-452. [DOI] [PubMed] [Google Scholar]
- 25.Simons, K. T., I. Ruczinski, C. Kooperberg, B. Fox, C. Bystroff, and D. Baker. 1999. Improved recognition of native-like protein structures using a combination of sequence-dependent and sequence-independent features of proteins. Proteins 3482-95. [DOI] [PubMed] [Google Scholar]
- 26.Sondermann, P., J. Kaiser, and U. Jacob. 2001. Molecular basis for immune complex recognition: a comparison of Fc-receptor structures. J. Mol. Biol. 309737-749. [DOI] [PubMed] [Google Scholar]
- 27.Van Wauwe, J. P., J. G. Goossens, and P. C. Beverley. 1984. Human T lymphocyte activation by monoclonal antibodies; OKT3, but not UCHT1, triggers mitogenesis via an interleukin 2-dependent mechanism. J. Immunol. 133129-132. [PubMed] [Google Scholar]
- 28.Varki, A., and T. Angata. 2006. Siglecs—the major subfamily of I-type lectins. Glycobiology 161R-27R. [DOI] [PubMed] [Google Scholar]
- 29.Walker, C. M., A. L. Erickson, F. C. Hsueh, and J. A. Levy. 1991. Inhibition of human immunodeficiency virus replication in acutely infected CD4+ cells by CD8+ cells involves a noncytotoxic mechanism. J. Virol. 655921-5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker, C. M., D. J. Moody, D. P. Stites, and J. A. Levy. 1986. CD8+ lymphocytes can control HIV infection in vitro by suppressing virus replication. Science 2341563-1566. [DOI] [PubMed] [Google Scholar]
- 31.Yi, Y., W. Chen, I. Frank, J. Cutilli, A. Singh, L. Starr-Spires, J. Sulcove, D. L. Kolson, and R. G. Collman. 2003. An unusual syncytia-inducing human immunodeficiency virus type 1 primary isolate from the central nervous system that is restricted to CXCR4, replicates efficiently in macrophages, and induces neuronal apoptosis. J. Neurovirol. 9432-441. [DOI] [PubMed] [Google Scholar]