Abstract
Gag-specific cytotoxic T lymphocytes (CTLs) exert strong suppressive pressure on human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) replication. However, it has remained unclear whether they can actually contain primary viral replication. Recent trials of prophylactic vaccines inducing virus-specific T-cell responses have indicated their potential to confer resistance against primary SIV replication in rhesus macaques, while the immunological determinant for this vaccine-based viral control has not been elucidated thus far. Here we present evidence implicating Gag-specific CTLs as responsible for the vaccine-based primary SIV control. Prophylactic vaccination using a Gag-expressing Sendai virus vector resulted in containment of SIVmac239 challenge in all rhesus macaques possessing the major histocompatibility complex (MHC) haplotype 90-120-Ia. In contrast, 90-120-Ia-positive vaccinees failed to contain SIVs carrying multiple gag CTL escape mutations that had been selected, at the cost of viral fitness, in SIVmac239-infected 90-120-Ia-positive macaques. These results show that Gag-specific CTL responses do play a crucial role in the control of wild-type SIVmac239 replication in vaccinees. This study implies the possibility of Gag-specific CTL-based primary HIV containment by prophylactic vaccination, although it also suggests that CTL-based AIDS vaccine efficacy may be abrogated in viral transmission between MHC-matched individuals.
Despite tremendous efforts to develop AIDS vaccines eliciting virus-specific T-cell responses, whether this approach actually does result in controlling human immunodeficiency virus (HIV) replication remains unknown. Recent trials have shown reductions in postchallenge viral loads by prophylactic vaccination eliciting virus-specific T-cell responses in macaque AIDS models (19, 22, 34), but the first advanced human trial of a T-cell-based vaccine was halted because of a lack of efficacy (5). Hence, it is quite important to determine which T-cell responses are responsible for primary HIV control.
Cytotoxic T-lymphocyte (CTL) responses have been indicated to play an important role in the control of HIV and simian immunodeficiency virus (SIV) infections (2, 9, 10, 17, 23, 29). Above all, the potential of Gag-specific CTL responses to contribute to viral control has been suggested by a cohort study indicating an association of HIV control with the breadth of Gag-specific CTL responses (15). In support of this, a recent in vitro study revealed their ability to rapidly respond to SIV infection (28). However, it has remained unclear whether Gag-specific CTL-based viral containment can be achieved by prophylactic vaccination.
We previously developed a prophylactic AIDS vaccine regimen consisting of a DNA prime followed by a boost with a Sendai virus (SeV) vector expressing SIVmac239 Gag (SeV-Gag) (22, 32). Our trial showed potential for efficiently inducing Gag-specific T-cell responses and containment of SIVmac239 challenge in a group of Burmese rhesus macaques sharing the major histocompatibility complex class I (MHC-I) haplotype 90-120-Ia (22). A follow-up study revealed the reappearance of plasma viremia at >1 year postchallenge in some of these 90-120-Ia-positive SIV controllers. In these transient controllers, multiple CTL escape mutations were accumulated in the viral gag gene, resulting in viremia reappearance and thus suggesting the involvement of Gag206-216 (IINEEAADWDL) epitope-specific, Gag241-249 (SSVDEQIQW) epitope-specific, and Gag373-380 (APVPIPFA) epitope-specific CTLs in sustained viral control (12). Nonetheless, it has remained undetermined whether such Gag-specific CTL responses were responsible for the vaccine-based primary SIV control in 90-120-Ia-positive vaccinees. In the present study, we challenged the 90-120-Ia-positive vaccinees with SIVs carrying the gag CTL escape mutations to determine the role of Gag-specific CTLs in primary SIVmac239 control.
MATERIALS AND METHODS
Viral competition assay.
SIV molecular clone DNAs with gag mutations were constructed by site-directed mutagenesis from the wild-type SIVmac239 (14) molecular clone DNA. Virus stocks were obtained by transfection of COS-1 cells with wild-type or mutant SIV molecular clone DNAs, and their titers were measured by reverse transcription (RT) assay as described previously (25, 33). For analysis of viral replication, HSC-F cells (herpesvirus saimiri-immortalized macaque T-cell line) (1) were infected with wild-type or mutant SIVs (normalized by RT activity), and virus production was monitored by measuring RT activity in the culture supernatants. For competition, HSC-F cells were coinfected with two SIVs at a ratio of 1:1 or 1:4, and the culture supernatants were harvested every other day and used for RT assays. On day 6, the supernatant was added to fresh HSC-F cells to start the second culture. Similarly, on day 12 after the initial coinfection, the second culture supernatant was added to fresh HSC-F cells to start the third culture. RNAs were extracted from the initial culture supernatant on day 6 and from the third culture supernatant on day 18 postcoinfection. The fragment (nucleotides 1231 to 2958 in SIVmac239 [GenBank accession number M33262]) containing the entire gag region was amplified from the RNA by RT-PCR and sequenced. Alternatively, it was subcloned into plasmids to determine dominant sequences.
Animal experiments.
Burmese rhesus macaques (Macaca mulatta) were maintained in accordance with the guidelines for animal experiments performed at the National Institute of Infectious Diseases (26). Three animals, R01-007, R02-003, and R02-012, that received a prophylactic DNA prime/SeV-Gag boost vaccine and contained SIVmac239 challenge have been reported previously (22). In the present study, macaques R06-015, R06-035, R06-041, R05-004, R05-027, and R07-005 also received the DNA prime/SeV-Gag boost vaccine. The DNA used for the vaccination, CMV-SHIVdEN, was constructed from env- and nef-deleted simian-human immunodeficiency virus SHIVMD14YE molecular clone DNA (SIVGP1) (31, 32) and has the genes encoding SIVmac239 Gag, Pol, Vif, and Vpx, SIVmac239-HIV chimeric Vpr, and HIV Tat and Rev. At the DNA vaccination, animals received 5 mg of CMV-SHIVdEN DNA intramuscularly. Six weeks after the DNA prime step, animals received a single boost intranasally with 6 × 109 cell infectious units of F-deleted replication-defective SeV-Gag (21, 32). Approximately 3 months after the boost, animals were challenged intravenously with 1,000 50% tissue culture infective doses of SIVmac239, SIVmac239Gag216S244E, or SIVmac239Gag216S244E247L312V373T. The challenge virus stocks were prepared by virus propagation on rhesus macaque peripheral blood mononuclear cells (PBMCs). Sequence analysis confirmed the absence of gag mutations except for the two or five mutations in the challenge viruses.
Immunostaining of CD4+ T-cell memory subsets.
PBMCs were subjected to immunofluorescence staining by using fluorescein isothiocyanate-conjugated anti-human CD28, phycoerythrin-conjugated anti-human CD95, peridinin chlorophyll protein-conjugated anti-human CD4, and allophycocyanin-conjugated anti-human CD3 monoclonal antibodies (Becton Dickinson, Tokyo, Japan). The central memory subset of CD4+ T cells was defined by possession of a CD28+ CD95+ phenotype, as described previously (13, 27).
Measurement of virus-specific CD8+ T-cell responses.
We measured virus-specific CD8+ T-cell levels by flow cytometric analysis of gamma interferon (IFN-γ) induction after specific stimulation, as described previously (13, 22). In brief, PBMCs were cocultured with autologous herpesvirus papio-immortalized B-lymphoblastoid cell lines infected with a vaccinia virus vector expressing SIVmac239 Gag for Gag-specific stimulation or a vesicular stomatitis virus G protein-pseudotyped SIVGP1 for SIV-specific stimulation. The pseudotyped virus was obtained by cotransfection of COS-1 cells with a vesicular stomatitis virus G protein expression plasmid and the SIVGP1 DNA. Alternatively, B-lymphoblastoid cell lines were pulsed with 1 to 10 μM peptides for peptide-specific stimulation (11, 12). The 15-mer Gag367-381 peptide was used to detect Gag367-381-specific CTLs, including Gag373-380-specific CTLs. Intracellular IFN-γ staining was performed using a Cytofix Cytoperm kit (Becton Dickinson). Peridinin chlorophyll protein-conjugated anti-human CD8, allophycocyanin-conjugated anti-human CD3, and phycoerythrin-conjugated anti-human IFN-γ antibodies (Becton Dickinson) were used. Specific T-cell levels were calculated by subtracting nonspecific IFN-γ+ T-cell frequencies from those after Gag-specific, SIV-specific, or peptide-specific stimulation. Specific T-cell levels of <100 cells per million PBMCs were considered negative.
Statistical analysis.
Statistical analysis was performed with Prism software, version 4.03, with significance set at P values of <0.05 (GraphPad Software, Inc., San Diego, CA). Central memory CD4+ T-cell counts before challenge were not significantly different between the wild-type SIV-challenged (n = 4) and the mutant SIV-challenged (n = 5) macaques (P = 0.70 by unpaired two-tailed t test with Welch's correction and P = 0.73 by nonparametric Mann-Whitney U test). Ratios of the central memory CD4+ T-cell counts from a few months postchallenge to those prechallenge were log transformed and compared between the two groups by an unpaired two-tailed t test and the Mann-Whitney U test. Gag-specific CD8+ T-cell frequencies postvaccination (prechallenge) or postchallenge were also log transformed and compared between the two groups in the same statistical manner.
RESULTS
Comparison of viral fitness in wild-type and mutant SIVs.
We used two mutant SIVs for challenge of the 90-120-Ia-positive vaccinees. The first, designated SIVmac239Gag216S244E, carries two gag mutations, GagL216S and GagD244E, leading to a leucine (L)-to-serine (S) substitution at the 216th amino acid (aa) and an aspartic acid (D)-to-glutamic acid (E) substitution at the 244th aa in Gag. The second, designated SIVmac239Gag216S244E247L312V373T, carries five gag mutations, GagL216S, GagD244E, GagI247L (isoleucine [I] to L at the 247th aa), GagA312V (alanine [A] to valine [V] at the 312th aa), and GagA373T (A to threonine [T] at the 373rd aa). In our previous study (12), the former became dominant in the early phase (at approximately 4 months postchallenge) during the period of viral control, and the latter was dominant at viremia reappearance in a transient controller. GagL216S, GagD244E and GagI247L, and GagA373T mutations result in viral escape from recognition by Gag206-216-specific, Gag241-249-specific, and Gag373-380-specific CTLs, respectively, while it remains unclear whether GagA312V was selected for by CTLs.
We first compared viral fitness in wild-type and mutant SIVs. In HSC-F cells (a macaque T-cell line), not only the wild type but also the mutant SIVs were able to replicate, but SIVmac239Gag216S244E replication was less efficient than that of wild-type SIVmac239, and SIVmac239Gag216S244E247L312V373T replication was even less efficient (Fig. 1A). In competitions between two SIVs, HSC-F cells were coinfected with both viruses, and viral genome sequences in the culture supernatants were assessed to establish which SIV became predominant. In culture supernatants of HSC-F cells after coinfection with SIVmac239 and SIVmac239Gag216S244E inoculated at a ratio of 1:1, the wild type rapidly became dominant (at day 6) (Fig. 1B). Coinfection at a ratio of 1:4 resulted in equivalence at day 6, but the wild type again dominated by day 18 (Fig. 1C). These results indicate a lower replicative ability of SIVmac239Gag216S244E than of wild-type SIVmac239. In addition, competition between SIVmac239Gag216S244E and SIVmac239Gag216S244E247L312V373T showed the lower replicative ability of the latter (Fig. 1B and C).
FIG. 1.
Replication of mutant SIVs in vitro. (A) Wild-type and mutant SIV replication kinetics in HSC-F cells. HSC-F cells were infected with SIVmac239 (closed circles), SIVmac239Gag216S244E (asterisks), or SIVmac239Gag216S244E247L312V373T (open triangles). Virus production was monitored by measuring RT activity in the culture supernatants. Representative results from three sets of experiments are shown. (B) Viral competition assay. HSC-F cells were coinfected with SIVmac239 and SIVmac239Gag216S244E (left) or with SIVmac239Gag216S244E and SIVmac239Gag216S244E247L312V373T (right) at a ratio of 1:1. Viral gag fragments were amplified by RT-PCR from viral RNAs from the culture supernatants at days 6 and 18 postinfection and then sequenced. Dominant amino acid sequences at the 216th and 244th aa (left) or the 247th, 312th, and 373rd aa (right) in Gag in three sets of experiments are shown. Wt, only the wild-type sequence was detected; Wt (mt), the wild type was dominant, but the mutant was detectable (the mutant/wild-type ratio was <1/2). (C) Viral competition assay. HSC-F cells were coinfected with SIVmac239 and SIVmac239Gag216S244E (left) or with SIVmac239Gag216S244E and SIVmac239Gag216S244E247L312V373T (right) at a ratio of 1:4. The amplified gag fragments were subcloned into plasmids and sequenced. Frequencies of the indicated SIV clones (number of indicated clone per total number of clones) are shown. Changes in RT levels in the culture supernatants are shown in the bottom panels. The arrows indicate the time points of coinfection (at day 0) and viral passage for the second (at day 6) and the third (at day 12) cultures.
Challenge of 90-120-Ia-positive vaccinees with wild-type or mutant SIVs.
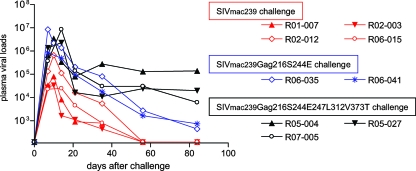
Next, we challenged 90-120-Ia-positive macaques with the mutant SIVs after DNA prime/SeV-Gag vaccination. Remarkably, all three vaccinees (R05-004, R05-027, and R07-005) challenged with SIVmac239Gag216S244E247L312V373T failed to control viral replication and showed high set point plasma viral loads, while all four vaccinees (R01-007, R02-003, R02-012, and R06-015) challenged with wild-type SIVmac239 contained viral replication, with undetectable set point plasma viral loads (Fig. 2). Even the two vaccinees (R06-035 and R06-041) challenged with SIVmac239Gag216S244E failed to contain viral replication, although with lower plasma viral loads, at approximately 103 RNA copies/ml at 3 months postchallenge. Central memory CD4+ T-cell counts before challenge were not significantly different between the wild-type SIV-challenged (n = 4) and mutant SIV-challenged (n = 5) macaques, but ratios of the counts at a few months postchallenge to prechallenge for the latter group were significantly lower than those for the former (P = 0.0021 by unpaired t test and P = 0.0159 by Mann-Whitney U test) (Fig. 3). Thus, 90-120-Ia-positive vaccinees can contain wild-type SIVmac239 but not SIVmac239Gag216S244E or SIVmac239Gag216S244E247L312V373T challenge.
FIG. 2.
Plasma viral loads after wild-type or mutant SIV challenge. The 90-120-Ia-positive vaccinees were challenged with SIVmac239 (red lines), SIVmac239Gag216S244E (blue lines), or SIVmac239Gag216S244E247L312V373T (black lines). Plasma viral loads (SIV gag RNA copies/ml plasma) were determined as described before (22). The lower limit of detection is approximately 4 × 102 copies/ml.
FIG. 3.
Changes in central memory CD4+ T-cell counts after wild-type or mutant SIV challenge. (A) Peripheral central memory CD4+ (CD4+ CD95+ CD28+) T-cell counts (/μl) prechallenge (pre-C) and a few months postchallenge (post-C). (B) Statistical comparison of central memory CD4+ T-cell loss between the wild-type SIV-challenged (Wt) and the mutant SIV-challenged (Mt) macaques. The ratios of central memory CD4+ T-cell counts postchallenge to those prechallenge are plotted. The longer bars indicate geometric mean values, and the regions between the shorter bars indicate the 95% confidence intervals. The ratios in the mutant group (n = 5) were significantly lower than those in the wild-type group (n = 4) (P = 0.0021 by unpaired t test and P = 0.0159 by Mann-Whitney U test).
Viral gag sequence analysis confirmed the rapid selection for the GagL216S mutation in all wild-type SIVmac239-challenged macaques, as described previously (22). All of the gag mutations in the challenge mutant viruses were maintained during the observation period (Table 1). SIVmac239Gag216S244E247L312V373T-challenged macaques showed no additional dominant gag mutations, whereas animals challenged with SIVmac239Gag216S244E rapidly selected viruses with a GagV145A (V to A at the 145th aa) mutation. Recovery of viral fitness by this mutation was not observed, and whether it was selected for by CTLs was unclear in our previous study (12).
TABLE 1.
Dominant sequences in SIV Gag in macaques after challenge
| Macaque | Time (wk) of plasma sample | Amino acid change in Gag at positiona:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 140 | 145 | 206 | 216 | 244 | 247 | 312 | 341 | 373 | ||
| R01-007 | 5 | L216S | ||||||||
| R02-003 | 5 | L216S | ||||||||
| R02-012 | 5 | L216S | ||||||||
| R06-015 | 5 | (I206M) | L216S | |||||||
| R06-035 | 5 | L216S* | D244E* | |||||||
| 12 | V145A | L216S* | D244E* | (N341Y) | ||||||
| R06-041 | 5 | (V145A) | L216S* | D244E* | ||||||
| 12 | V145A | L216S* | D244E* | |||||||
| R05-004 | 5 | L216S* | D244E* | I247L* | A312V* | A373T* | ||||
| 12 | (I140V) | L216S* | D244E* | I247L* | A312V* | A373T* | ||||
| R05-027 | 5 | L216S* | D244E* | I247L* | A312V* | A373T* | ||||
| 12 | L216S* | D244E* | I247L* | A312V* | A373T* | |||||
| R07-005 | 5 | L216S* | D244E* | I247L* | A312V* | A373T* | ||||
| 12 | L216S* | D244E* | I247L* | A312V* | A373T* | |||||
A fragment containing the entire gag region was amplified from plasma RNA by nested RT-PCR and then sequenced. We were unable to amplify the fragment from plasmas obtained at week 12 from the wild-type SIVmac239-challenged macaques with undetectable viremia. Dominant gag mutations resulting in amino acid changes are shown. Asterisks indicate the mutations included in the challenge inoculums. Parentheses indicate that both the wild-type and mutant sequences were detected equivalently at that position.
Gag-specific CTL responses were induced after SeV-Gag boost in all vaccinees, and there was no significant difference in the levels between the wild-type and mutant challenges (P = 0.1198 by unpaired t test and P = 0.1111 by Mann-Whitney U test). However, secondary Gag-specific CTL responses were less efficient after challenge with mutant SIV than after challenge with wild-type SIV (P = 0.0095 by unpaired t test and P = 0.0159 by Mann-Whitney U test) (Fig. 4A).
FIG. 4.
Gag-specific CD8+ T-cell responses before and after wild-type or mutant SIV challenge. Macaques R01-007, R02-003, R02-012, and R06-015 were challenged with SIVmac239; macaques R06-035 and R06-041 were challenged with SIVmac239Gag216S244E; and macaques R05-004, R05-027, and R07-005 were challenged with SIVmac239Gag216S244E247L312V373T. (A) Gag-specific CD8+ T-cell frequencies at 2 weeks postboost (postvaccination) (left) and 2 weeks postchallenge (right). (B) Gag206-216-specific, Gag241-249-specific, and Gag367-381-specific CD8+ T-cell frequencies at 2 weeks (all except for R02-012) or 4 weeks (in R02-012) postboost (postvaccination) and 5 weeks (in R01-007, R02-003, R02-012, R06-035, R06-041, and R05-004) or 6 weeks (in R06-015, R05-027, and R07-005) postchallenge. ND, not determined.
SeV-Gag boost induced efficient Gag206-216-specific and Gag241-249-specific CTL responses in all vaccinees and Gag367-381-specific CTL responses in some of them (Fig. 4B). Challenge with wild-type SIVmac239 resulted in efficient secondary responses of these three epitope-specific CTLs, whereas SIVmac239Gag216S244E247L312V373T challenge evoked none of them (Fig. 4B). SIVmac239Gag216S244E challenge did not result in secondary responses of Gag206-216-specific or Gag241-249-specific CTLs but did induce Gag367-381-specific CTL responses in one case (Fig. 4B). These results indicate that SIVmac239Gag216S244E evades recognition by Gag206-216-specific and Gag241-249-specific CTLs and that SIVmac239Gag216S244E247L312V373T evades recognition by Gag206-216-specific, Gag241-249-specific, and Gag367-381-specific CTLs.
We next examined Gag-specific and SIV-specific CTL responses after mutant SIV challenge (Fig. 5A). We used an env- and nef-deleted SHIV molecular clone DNA, SIVGP1, that has the genes encoding SIVmac239 Gag, Pol, Vif, Vpx, and a part of Vpr and measured the frequencies of CTLs responding to SIVGP1-transduced cells (referred to as SIV-specific CTLs) as described previously (13, 32). SIV-specific CTL frequencies at week 12 were much higher than those at week 2 for all five macaques challenged with mutant SIVs. In contrast, Gag-specific CTL frequencies at week 12 were lower than those at week 2 for four of five animals; the remaining macaque, R06-035, mounted Gag367-381-specific CTL responses. Importantly, in all animals challenged with mutant SIVs, SIV-specific CTL frequencies were at marginal levels or lower than Gag-specific CTL frequencies at week 2, but the former became higher than the latter at week 12. These results indicate an induction of CTL responses specific for SIV antigens other than Gag in all five macaques after mutant SIV challenge.
FIG. 5.
SIV-specific CD8+ T-cell responses after mutant SIV challenge. (A) Gag-specific (closed boxes) and SIV-specific (open boxes) CD8+ T-cell frequencies at 2 weeks or 12 weeks postchallenge. (B) Frequencies of CD8+ T cells specific for pools of SIV Gag peptides. A panel of 117 overlapping peptides (15 to 17 aa in length and overlapping by 10 to 12 aa) spanning the entire SIV Gag amino acid sequence were divided into the following 10 pools (each consisting of 11 or 12 peptides): pool 1, 1st to 65th aa in SIV Gag; pool 2, 55th to 114th aa; pool 3, 104th to 165th aa; pool 4, 155th to 213th aa; pool 5, 202nd to 265th aa; pool 6, 255th to 316th aa; pool 7, 306th to 364th aa; pool 8, 354th to 416th aa; pool 9, 406th to 464th aa; and pool 10, 453rd to 510th aa. The pools were used for stimulation to detect peptide pool-specific CD8+ T cells.
At week 12 after mutant SIV challenge, Gag-specific CTL responses were undetectable in macaque R07-005 but were still detected in the other four macaques. We then analyzed Gag-specific CTL responses in these four macaques by using a panel of overlapping peptides spanning the entire SIV Gag amino acid sequence (Fig. 5B). In both SIVmac239Gag216S244E-challenged animals, R06-035 and R06-041, exhibiting detectable Gag367-381-specific CTL responses (data not shown), CTL responses specific for the peptide mixture corresponding to the 354th to 416th aa in SIV Gag were detected at week 12. In addition, we found Gag255-316-specific CTL responses in macaque R06-035 and Gag155-213-specific CTL responses in macaque R06-041. SIVmac239Gag216S244E247L312V373T-challenged macaques R05-004 and R05-027 showed responses specific for several Gag peptide mixtures, including Gag202-265-specific and Gag255-316-specific CTL responses. These results indicate an induction of CTL responses specific for Gag epitopes other than the Gag206-216, Gag241-249, and Gag373-380 epitopes after mutant SIV challenge.
DISCUSSION
In the present study, SIVs carrying multiple gag CTL escape mutations showed lower replicative abilities than that of the wild type; nonetheless, the 90-120-Ia-positive vaccinees were able to contain only the latter. This demonstrates that Gag-specific CTL responses did play a central role in the vaccine-based primary containment of wild-type SIVmac239 replication in 90-120-Ia-positive macaques.
Elicitation of virus-specific T-cell responses by prophylactic vaccination is believed to be a promising strategy for HIV control (3, 24); whether this approach can actually result in HIV control remains unknown. Recent studies have indicated the possibility of reductions in set point viral loads after SIV challenge by prophylactic vaccination inducing T-cell responses in rhesus macaques (19, 22, 34), yet the immune component crucial for the vaccine-based viral control has not been determined. No clear evidence for a contribution of vaccine-induced CTLs to this viral control has been forthcoming to date, although virus-specific CTL responses have been implicated in exerting strong suppressive pressure on HIV/SIV infection (9, 22). Indeed, viral replication persists even in the presence of CTL responses in the natural course of infection; it has thus remained unclear whether HIV/SIV replication can be controlled by vaccine-induced CTLs. The evidence from the present study now strongly implicates Gag-specific CTL responses as responsible for vaccine-based primary SIV control. This offers the possibility of Gag-specific CTL-based HIV containment by prophylactic vaccination and provides insight into the development of CTL-based AIDS vaccines.
The containment of SIVmac239 but failure to contain SIVmac239Gag216S244E in the vaccinees documents a crucial role for Gag206-216-specific and/or Gag241-249-specific CTL responses in vaccine-based SIVmac239 containment. Furthermore, challenge with SIVmac239Gag216S244E247L312V373T, possessing diminished viral fitness compared to SIVmac239Gag216S244E, tended to result in higher viral loads, indicating the involvement of Gag373-380-specific CTL responses in viral control, while more complete viral evasion of Gag241-249-specific CTL recognition by addition of the GagI247L mutation may also contribute to the difference between SIVmac239Gag216S244E and SIVmac239Gag216S244E247L312V373T challenge. Taken together, we conclude that these two or three epitope-specific CTL responses are crucial for primary SIVmac239 control in 90-120-Ia-positive vaccinees. Conversely, this study implies that viral evasion of recognition by two dominant epitope-specific CTLs can result in failure of primary viral containment but may not be sufficient for abrogation of vaccine efficacy. Thus, analysis of CTL-based vaccine efficacy against SIVs carrying single or multiple CTL escape mutations could contribute to an evaluation of its potential for controlling the replication of highly diversified HIVs.
Our results suggest that SIV- but non-Gag-specific CTLs became predominant after mutant SIV challenge. Additionally, CTLs recognizing Gag regions other than the Gag206-216, Gag241-249, and Gag373-380 epitopes were detected in most cases. These CTL responses may exert suppressive pressure on viral replication but are considered insufficient for controlling replication of the mutant SIVs with lower viral fitness.
Finally, this study also provides evidence indicating a possible abrogation of CTL-based AIDS vaccine efficacy in viral transmission between MHC-I-matched individuals. Indeed, even the mutant SIVs carrying multiple CTL escape mutations were able to replicate persistently in vivo, despite their diminished replicative ability. Transmission of these viruses can result in persistent viral infection and AIDS progression (30). CTL escape mutations resulting in a loss of viral fitness may revert to the wild-type sequence after transmission into MHC-I-mismatched hosts (4, 8, 9, 16, 18, 20), but such reversion does not occur rapidly; alternatively, some may be retained with additional compensatory mutations (6, 7, 30). Thus, there may be a risk of transmission and accumulation of HIV CTL escape variants even among MHC-I-mismatched individuals, resulting in abrogation of CTL-based AIDS vaccine efficacy in a population.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology, by a grant from the Japan Health Sciences Foundation, and by grants from the Ministry of Health, Labor, and Welfare in Japan.
The animal experiments were conducted through the Cooperative Research Program in Tsukuba Primate Research Center, National Institute of Biomedical Innovation, with the help of the Corporation for Production and Research of Laboratory Primates. We thank DNAVEC Corp., H. Igarashi, F. Ono, A. Hiyaoka, K. Oto, Y. Yasutomi, M. Miyazawa, A. Kimura, K. Mori, N. Yamamoto, T. Kurata, Y. Nagai, and A. Nomoto for their help.
Footnotes
Published ahead of print on 30 July 2008.
REFERENCES
- 1.Akari, H., K. Mori, K. Terao, I. Otani, M. Fukasawa, R. Mukai, and Y. Yoshikawa. 1996. In vitro immortalization of Old World monkey T lymphocytes with herpesvirus saimiri: its susceptibility to infection with simian immunodeficiency viruses. Virology 218382-388. [DOI] [PubMed] [Google Scholar]
- 2.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implication for vaccine development. Curr. Opin. Immunol. 11451-459. [DOI] [PubMed] [Google Scholar]
- 4.Brander, C., and B. D. Walker. 2003. Gradual adaptation of HIV to human host populations: good or bad news? Nat. Med. 91359-1362. [DOI] [PubMed] [Google Scholar]
- 5.Cohen, J. 2007. Did Merck's failed HIV vaccine cause harm? Science 3181048-1049. [DOI] [PubMed] [Google Scholar]
- 6.Crawford, H., J. G. Prado, A. Leslie, S. Hué, I. Honeyborne, S. Reddy, M. van der Stok, Z. Mncube, C. Brander, C. Rousseau, J. I. Mullins, R. Kaslow, P. Goepfert, S. Allen, E. Hunter, J. Mulenga, P. Kiepiela, B. D. Walker, and P. J. R. Goulder. 2007. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J. Virol. 818346-8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich, T. C., C. A. Frye, L. J. Yant, D. H. O'Connor, N. A. Kriewaldt, M. Benson, L. Vojnov, E. J. Dodds, C. Cullen, R. Rudersdorf, A. L. Hughes, N. Wilson, and D. I. Watkins. 2004. Extra-epitopic compensatory substitutions partially restore fitness to simian immunodeficiency virus variants that escape from an immunodominant cytotoxic T-lymphocyte response. J. Virol. 782581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedrich, T. C., E. J. Dodds, L. J. Yant, L. Vojnov, R. Rudersdorf, C. Cullen, D. T. Evans, R. C. Desrosiers, B. R. Mothe, J. Sidney, A. Sette, K. Kunstman, S. Wolinsky, M. Piatak, J. Lifson, A. L. Hughes, N. Wilson, D. H. O'Connor, and D. I. Watkins. 2004. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 10275-281. [DOI] [PubMed] [Google Scholar]
- 9.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4630-640. [DOI] [PubMed] [Google Scholar]
- 10.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato, M., H. Igarashi, A. Takeda, Y. Sasaki, H. Nakamura, M. Kano, T. Sata, A. Iida, M. Hasegawa, S. Horie, E. Higashihara, Y. Nagai, and T. Matano. 2005. Induction of Gag-specific T-cell responses by therapeutic immunization with a Gag-expressing Sendai virus vector in macaques chronically infected with simian-human immunodeficiency virus. Vaccine 233166-3173. [DOI] [PubMed] [Google Scholar]
- 12.Kawada, M., H. Igarashi, A. Takeda, T. Tsukamoto, H. Yamamoto, S. Dohki, M. Takiguchi, and T. Matano. 2006. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J. Virol. 801949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawada, M., T. Tsukamoto, H. Yamamoto, A. Takeda, H. Igarashi, D. I. Watkins, and T. Matano. 2007. Long-term control of simian immunodeficiency virus replication with central memory CD4+ T-cell preservation after nonsterile protection by a cytotoxic T-lymphocyte-based vaccine. J. Virol. 815202-5211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65651-662. [DOI] [PubMed] [Google Scholar]
- 15.Kiepiela, P., K. Ngumbela, C. Thobakgale, D. Ramduth, I. Honeyborne, E. Moodley, S. Reddy, C. de Pierres, Z. Mncube, N. Mkhwanazi, K. Bishop, M. van der Stok, K. Nair, N. Khan, H. Crawford, R. Payne, A. Leslie, J. Prado, A. Prendergast, J. Frater, N. McCarthy, C. Brander, G. H. Learn, D. Nickle, C. Rousseau, H. Coovadia, J. I. Mullins, D. Heckerman, B. D. Walker, and P. Goulder. 2007. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat. Med. 1346-53. [DOI] [PubMed] [Google Scholar]
- 16.Kobayashi, M., H. Igarashi, A. Takeda, M. Kato, and T. Matano. 2005. Reversion in vivo after inoculation of a molecular proviral DNA clone of simian immunodeficiency virus with a cytotoxic-T-lymphocyte escape mutation. J. Virol. 7911529-11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. St. John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10282-289. [DOI] [PubMed] [Google Scholar]
- 19.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 3121530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, B., A. D. Gladden, M. Altfeld, J. M. Kaldor, D. A. Cooper, A. D. Kelleher, and T. M. Allen. 2007. Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution. J. Virol. 81193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li, H. O., Y. F. Zhu, M. Asakawa, H. Kuma, T. Hirata, Y. Ueda, Y. S. Lee, M. Fukumura, A. Iida, A. Kato, Y. Nagai, and M. Hasegawa. 2000. A cytoplasmic RNA vector derived from nontransmissible Sendai virus with efficient gene transfer and expression. J. Virol. 746564-6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 1991709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMichael, A. J., and T. Hanke. 2003. HIV vaccines 1983-2003. Nat. Med. 9874-880. [DOI] [PubMed] [Google Scholar]
- 25.Miyagi, E., S. Opi, H. Takeuchi, M. Khan, R. Goila-Gaur, S. Kao, and K. Strebel. 2007. Enzymatically active APOBEC3G is required for efficient inhibition of human immunodeficiency virus type 1. J. Virol. 8113346-13353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National Institute of Infectious Diseases. 2007. Guides for animal experiments performed at National Institute of Infectious Diseases. National Institute of Infectious Diseases, Tokyo, Japan. (In Japanese.)
- 27.Pitcher, C. J., S. I. Hagen, J. M. Walker, R. Lum, B. L. Mitchell, V. C. Maino, M. K. Axthelm, and L. J. Picker. 2004. Development and homeostasis of T cell memory in rhesus macaques. J. Immunol. 16829-43. [DOI] [PubMed] [Google Scholar]
- 28.Sacha, J. B., C. Chung, E. G. Rakasz, S. P. Spencer, A. K. Jonas, A. T. Bean, W. Lee, B. J. Burwitz, J. J. Stephany, J. T. Loffredo, D. B. Allison, S. Adnan, A. Hoji, N. A. Wilson, T. C. Friedrich, J. D. Lifson, O. O. Yang, and D. I. Watkins. 2007. Gag-specific CD8+ T lymphocytes recognize infected cells before AIDS-virus integration and viral protein expression. J. Immunol. 1782746-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 30.Seki, S., M. Kawada, A. Takeda, H. Igarashi, T. Sata, and T. Matano. 2008. Transmission of simian immunodeficiency virus carrying multiple cytotoxic-T-lymphocyte escape mutations with diminished replicative ability can result in AIDS progression in rhesus macaques. J. Virol. 825093-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shibata, R., F. Maldarelli, C. Siemon, T. Matano, M. Parta, G. Miller, T. Fredrickson, and M. A. Martin. 1997. Infection and pathogenicity of chimeric simian-human immunodeficiency viruses in macaques: determinants of high virus loads and CD4 cell killing. J. Infect. Dis. 176362-373. [DOI] [PubMed] [Google Scholar]
- 32.Takeda, A., H. Igarashi, H. Nakamura, M. Kano, A. Iida, T. Hirata, M. Hasegawa, Y. Nagai, and T. Matano. 2003. Protective efficacy of an AIDS vaccine, a single DNA-prime followed by a single booster with a recombinant replication-defective Sendai virus vector, in a macaque AIDS model. J. Virol. 779710-9715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Willey, R. L., D. H. Smith, L. A. Lasky, T. S. Theodore, P. L. Earl, B. Moss, D. J. Capon, and M. A. Martin. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson, N. A., J. Reed, G. S. Napoe, S. Piaskowski, A. Szymanski, J. Furlott, E. J. Gonzalez, L. J. Yant, N. J. Maness, G. E. May, T. Soma, M. R. Reynolds, E. Rakasz, R. Rudersdorf, A. B. McDermott, D. H. O'Connor, T. C. Friedrich, D. B. Allison, A. Patki, L. J. Picker, D. R. Burton, J. Lin, L. Huang, D. Patel, G. Heindecker, J. Fan, M. Citron, M. Horton, F. Wang, X. Liang, J. W. Shiver, D. R. Casimiro, and D. I. Watkins. 2006. Vaccine-induced cellular immune responses reduce plasma viral concentrations after repeated low-dose challenge with pathogenic simian immunodeficiency virus SIVmac239. J. Virol. 805875-5885. [DOI] [PMC free article] [PubMed] [Google Scholar]