Abstract
The interaction between the HIV-1 Rev protein and the Rev-Responsive Element (RRE) RNA is an attractive target for anti-viral therapy. We have designed α-helical peptidomimetics of Rev-like peptides using sidechain-sidechain macrolactam formation between positions i and i+4. One peptidomimetic having an appropriate location, orientation, and length of the macrolactam exhibited both significant helical character and specific RRE binding. This molecule displays two-fold greater RNA specificity than the wild-type Rev peptide and more than 20-fold greater specificity than an uncyclized control peptide. Thus, specific, high affinity recognition of the RRE is feasible utilizing a small, relatively rigid peptidomimetic scaffold.
While RNA molecules provide potentially useful targets for drug design, there are few examples of small, relatively rigid molecules able to discriminate between RNA sites with high specificity. One such target is the HIV-1 Rev-Responsive Element (RRE) RNA, which is recognized by the Arginine-Rich Motif (ARM) of the Rev protein2,3, primarily via insertion of the α-helical ARM into the widened major groove of an asymmetric internal bulge in stem-loop IIB.4 Binding specificity for RRE correlates with peptide helicity5, suggesting that correctly positioning a set of interacting side chains within a rigid structural framework can lead to the desired discrimination. Given that the Rev-RRE interaction facilitates the nuclear export of incompletely processed viral transcripts and is essential for viral replication6, it provides an attractive target for anti-HIV therapy.
Previous work has shown that pre-stabilizing the Rev ARM α-helix within a zinc finger scaffold generates tight RRE-binders.7 We wished to create a new class of conformationally constrained α-helical peptidomimetics that are short, relatively rigid, and that might be effective competitors of the Rev-RRE interaction. Previously, peptidomimetics of a BIV Tat peptide, which binds to its cognate TAR RNA in a β-turn conformation,8 were designed using head-to-tail cyclization to enforce the conformational constraint.9,10 Here we report the first α-helical peptidomimetics targeted to a viral RNA using macrolactam constraints between amino acid side chains (i, i+4), which have previously been shown to induce helicity in small peptides.11
We generated peptidomimetics based upon a highly specific RRE binding peptide, R6QR7, identified in a genetic selection experiment.12 When helically stabilized, this peptide binds the RRE with an affinity similar to the Rev ARM and contains a glutamine in place of the asparagine at the central position in the Rev ARM (Fig. 1). The R6QR7 α-helix is presumably oriented further from the RNA major groove than the Rev ARM, potentially allowing more space for a macrolactam constraint.
Figure 1.
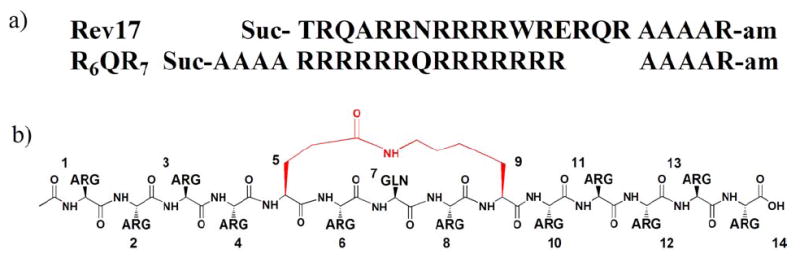
(a) Sequence comparison of Rev17 and R6QR7 peptides, helically stabilized by terminal modifications as previously reported.4,11 (b) Schematic of R6QR7 peptidomimetic 8 with (i, i+4) macrolactam constraint in red.
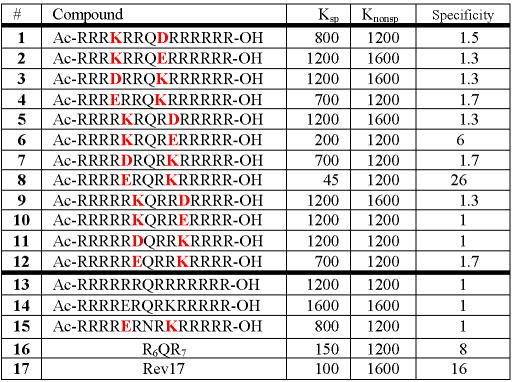
A series of macrolactam-constrained R6QR7 peptidomimetics (Fig. 1 and Table 1) was synthesized to investigate both the helical propensity and the specific binding of molecules with different length, location and orientation of the lactam ring. All peptides were synthesized using Fmoc solid phase synthesis with orthogonal Pd(0) labile protecting groups on the relevant lactam precursors, followed by on-resin lactam formation.13 Only one peptidomimetic (8) recognized the RRE with high affinity and specificity. Interestingly, peptide 8 exhibits a two-fold higher affinity for RRE than a helical Rev peptide stabilized by terminal modifications (17).4 Importantly, the unconstrained analog of 8, peptide 14, does not specifically recognize the RRE and thus adding the conformational constraint results in a >25-fold increase in binding specificity. Peptide 6, which, like peptide 8, contains a lysine-glutamate macrolactam straddling the essential glutamine, also specifically recognizes the RRE, albeit not as tightly, due to lack of helicity as described below. A helical (Fig. S3) asparagine variant (15) constrained with the same linkage as 8, does not specifically bind the RRE. This is consistent with previous results showing the importance of the extra methylene group in the unconstrained R6QR7 peptide context.11
Table 1.
Composition and Binding of R6QR7 Peptidomimetics
|
Compounds 1-12 are the designed peptidomimetics; red residues are involved in the macrolactam constraint. Peptide 13 is a general linear R6QR7 control peptide. RRE binding Kd’s were determined by electrophoretic mobility shift assays, with specificity defined as the ratio of dissociation constants of wild-type RRE IIB and a C46-G74 mutant RRE, shown not to specifically recognize Rev or R6QR7 peptides.4,11
Circular dichroism (CD) spectra of only three peptidomimetics (peptides 4, 8 and 12) exhibited a classical α-helical curve shape with dual minima at 222nm and 208nm, whereas the unconstrained R6QR7 peptide 13, along with remaining peptidomimetics, exhibited a single minimum at 202nm, typical of short, unstructured peptides (Fig. 2a and Fig. S3).14
Figure 2.
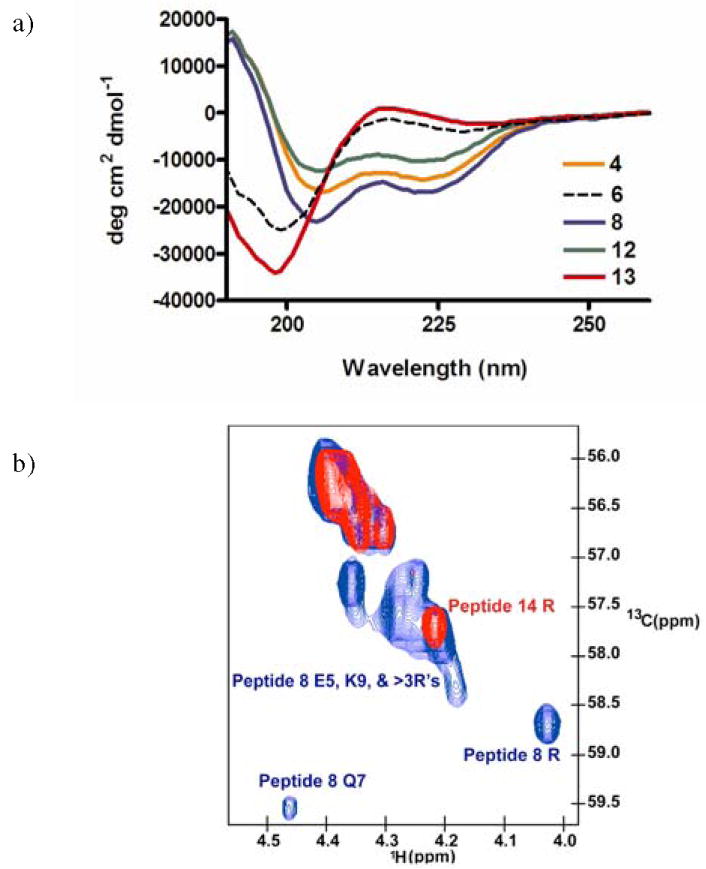
Structure of R6QR7 Peptidomimetics. (a) CD spectra of selected peptidomimetics and unconstrained control peptide (13). CD spectra were recorded at 4°C in 10mM sodium phosphate, 100mM KF, pH 7.5. (b) Natural abundance 13C HSQC comparing chemical shifts in the Cα-Hα region between peptide 8 (blue) and uncyclized peptide 14 (red).
Only these three peptidomimetics, having a glutamate–lysine linkage, with glutamate on the N-terminal side, showed any evidence of helicity, with peptide 8 exhibiting 33% helicity. Although all three peptidomimetics are helical by CD, only peptide 8 is able to specifically recognize the RRE, illustrating that only the linkage positioned exactly on the opposite side of the essential glutamine is viable for binding. The difference in binding between peptides 8 and 6 is most likely due to the lack of helical induction in peptide 6, based only on the orientation of the macrolactam.
A comparison of the 13C HSQC NMR spectra of the constrained peptide 8 and the unconstrained peptide 14 showed differences in dispersion in the Cα-Hα region (Fig. 2b), indicating a difference in backbone conformation. Peptide 8 revealed numerous peaks with characteristic downfield 13C shifts and upfield 1H shifts consistent with an α-helical secondary structure.15 Homonuclear 2D TOCSY experiments (see supplement) provided unique assignments for the lysine, glutamate, and glutamine side chains, which all appeared helical based on the dispersion. At least three arginines also showed helical downshifts but could not be assigned definitively. The NMR data on peptidomimetic 8 are consistent with the ~30% helicity observed by CD, and further indicates that the helicity is most likely localized within the constrained region of the peptide.
A fluorescence polarization competition assay was used to test whether the conformationally constrained, tight RRE binding peptide 8 could efficiently compete for RNA binding with the wild-type Rev peptide.16 Peptide 17, which is ~30% helical, and has a high affinity for the RRE, was labeled with fluorescein at its C-terminus and titrated with unlabeled competitors (Fig S4).
Of the three helical peptidomimetics (4, 8 and 12), peptide 8 was the most effective competitor, with an IC50 (~150 nM) comparable to its Kd (~40nM) (Fig. 3 and Table 1; note that the competition assay was conducted at an RNA concentration four-fold above Kd). The other peptidomimetics showed minimal competition, also consistent with the direct binding assays (Fig. 3 and Table 1).
Figure 3.
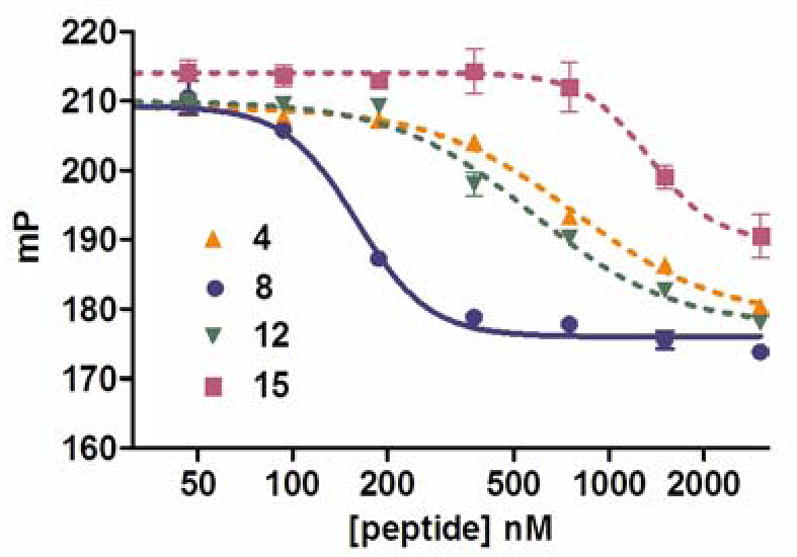
Fluorescence polarization competition assays with helical peptidomimetics. Assays were performed by titrating competitors into a mixture of RRE RNA and labeled Rev peptide in 30mM HEPES pH 7.5, 100mM KCl, 40mM NaCl, 10mM NH4OAc, 10mM Guanidinium, 2mM MgCl2, 0.5mM EDTA, 0.01% NP-40.16
The results presented here indicate that a conformationally-constrained α-helical peptidomimetic (peptide 8) can bind the RRE RNA with high affinity and specificity when the constraint is properly located and oriented. The positioning of the constraint on the α-helical face opposite the RNA major groove proves essential, as about two-thirds of the α-helix is surrounded by RNA in the Rev ARM-RRE complex.3 The observation that our designed, relatively short, constrained peptidomimetic is able to recognize the RRE IIB and compete for Rev binding suggests that the approach may be useful for generating other molecules that specifically target the Rev-RRE interaction for anti-viral therapy.
Supplementary Material
Details of the synthesis and purity of compounds 1-12, experimental details for gel mobility shift assay, CD, fluorescence polarization and NMR. This material is available free of charge via the Internet at http://pubs.acs.org.
Acknowledgments
This work was funded by the NIH (GM 56531), the Sandler Basic Research Foundation, and a Howard Hughes Predoctoral Fellowship to M.D.D.
References
- 2.Zapp ML, Green MR. Nature. 1989;342:714–6. doi: 10.1038/342714a0. [DOI] [PubMed] [Google Scholar]
- 3.Daly TJ, Cook KS, Gray GS, Maione TE, Rusche JR. Nature. 1989;342:816–9. doi: 10.1038/342816a0. [DOI] [PubMed] [Google Scholar]
- 4.Battiste JL, Mao H, Rao NS, Tan R, Muhandiram DR, Kay LE, Frankel AD, Williamson JR. Science. 1996;273:1547–51. doi: 10.1126/science.273.5281.1547. [DOI] [PubMed] [Google Scholar]
- 5.Tan R, Chen L, Buettner JA, Hudson D, Frankel AD. Cell. 1993;73:1031–40. doi: 10.1016/0092-8674(93)90280-4. [DOI] [PubMed] [Google Scholar]
- 6.Malim MH, Hauber J, Le SY, Maizel JV, Cullen BR. Nature. 1989;338:254–7. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 7.McColl DJ, Honchell CD, Frankel AD. Proc Natl Acad Sci U S A. 1999;96:9521–6. doi: 10.1073/pnas.96.17.9521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tamilarasu N, Huq I, Rana TM. Bioorg Med Chem Lett. 2000;10:971–4. doi: 10.1016/s0960-894x(00)00140-2. [DOI] [PubMed] [Google Scholar]
- 9.Friedler A, Friedler D, Luedtke NW, Tor Y, Loyter A, Gilon C. J Biol Chem. 2000;275:23783–9. doi: 10.1074/jbc.M002200200. [DOI] [PubMed] [Google Scholar]
- 10.Runyon ST, Puglisi JD. J Am Chem Soc. 2003;125:15704–5. doi: 10.1021/ja036344h. [DOI] [PubMed] [Google Scholar]
- 11.Geistlinger TR, Guy RK. J Am Chem Soc. 2001;123:1525–6. doi: 10.1021/ja005549c. [DOI] [PubMed] [Google Scholar]
- 12.Tan R, Frankel AD. Proc Natl Acad Sci U S A. 1998;95:4247–52. doi: 10.1073/pnas.95.8.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geistlinger TR, Guy RK. Methods Enzymol. 2003;364:223–46. doi: 10.1016/s0076-6879(03)64013-9. [DOI] [PubMed] [Google Scholar]
- 14.Greenfield NJ. Anal Biochem. 1996;235:1–10. doi: 10.1006/abio.1996.0084. [DOI] [PubMed] [Google Scholar]
- 15.Wishart DS, Sykes BD. Methods Enzymol. 1994;239:363–92. doi: 10.1016/s0076-6879(94)39014-2. [DOI] [PubMed] [Google Scholar]
- 16.Luedtke NW, Tor Y. Biopolymers. 2003;70:103–19. doi: 10.1002/bip.10428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details of the synthesis and purity of compounds 1-12, experimental details for gel mobility shift assay, CD, fluorescence polarization and NMR. This material is available free of charge via the Internet at http://pubs.acs.org.


