Abstract
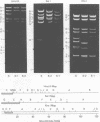
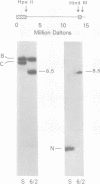
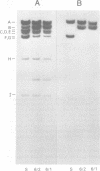
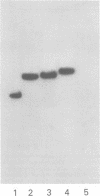
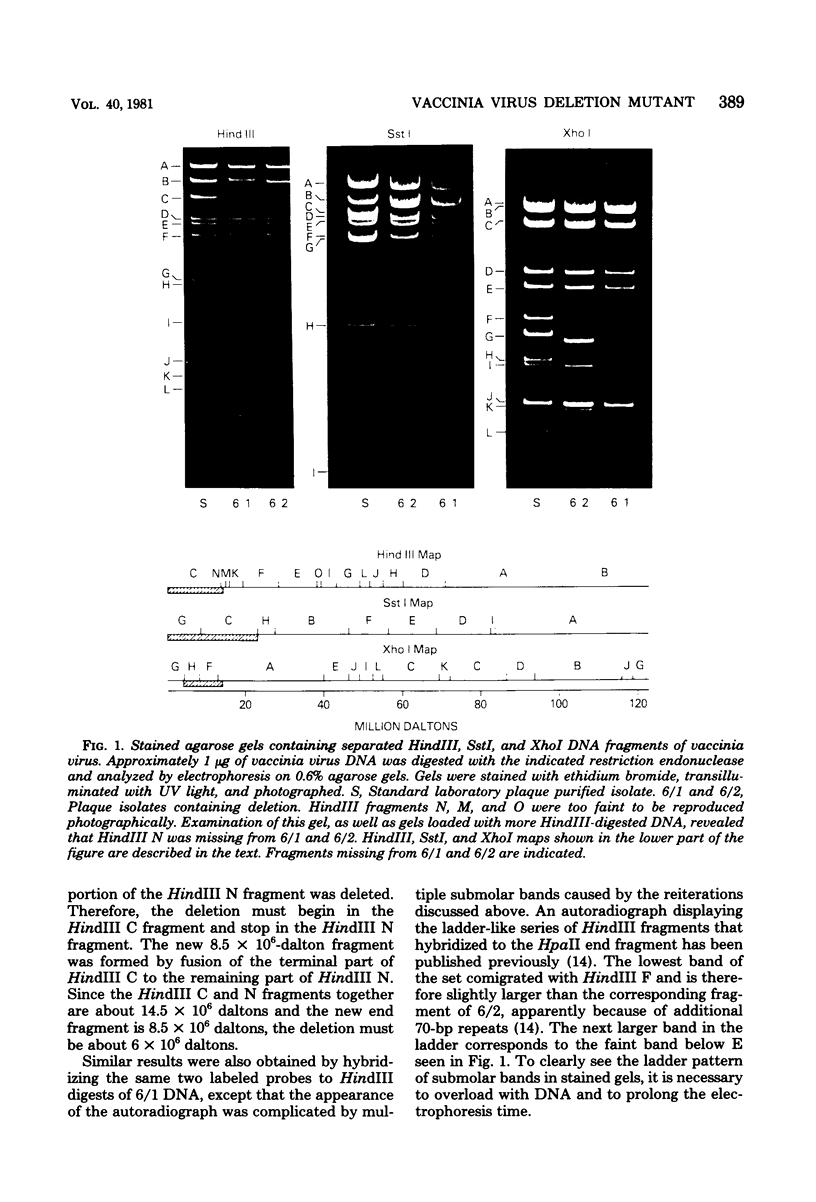
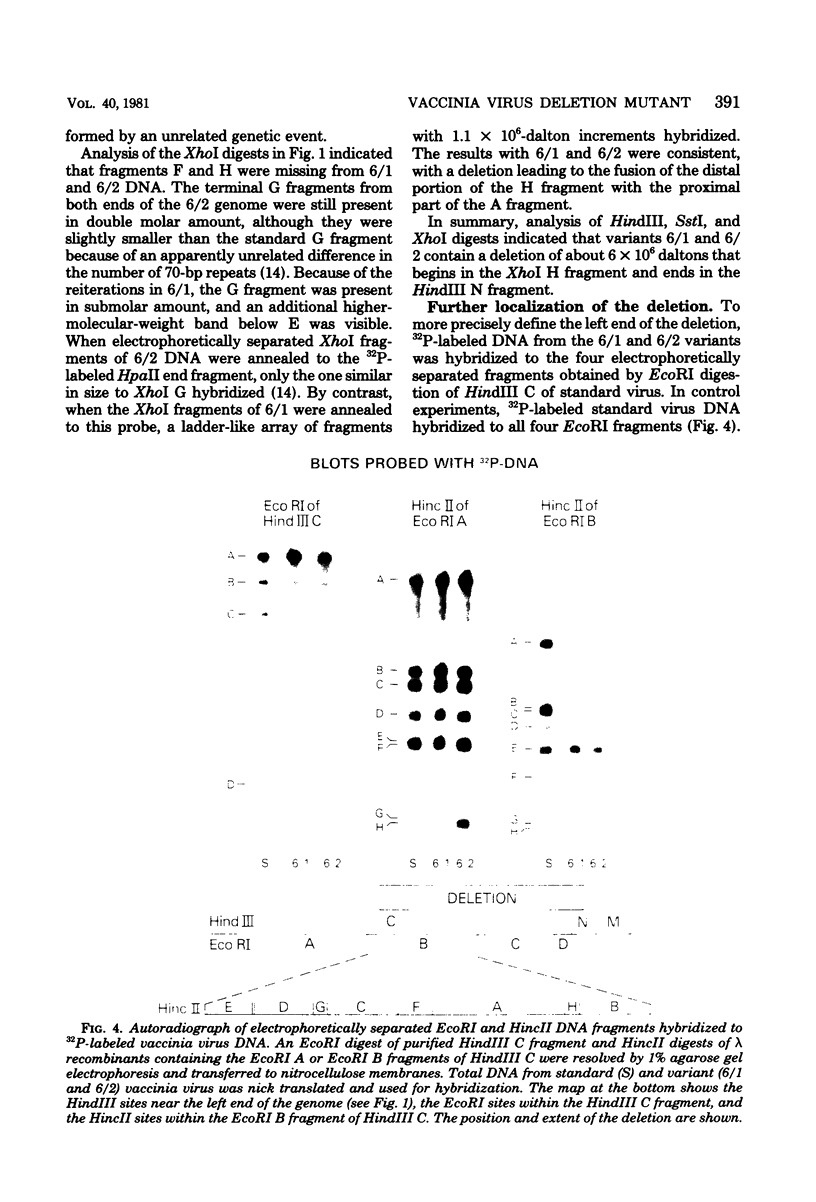
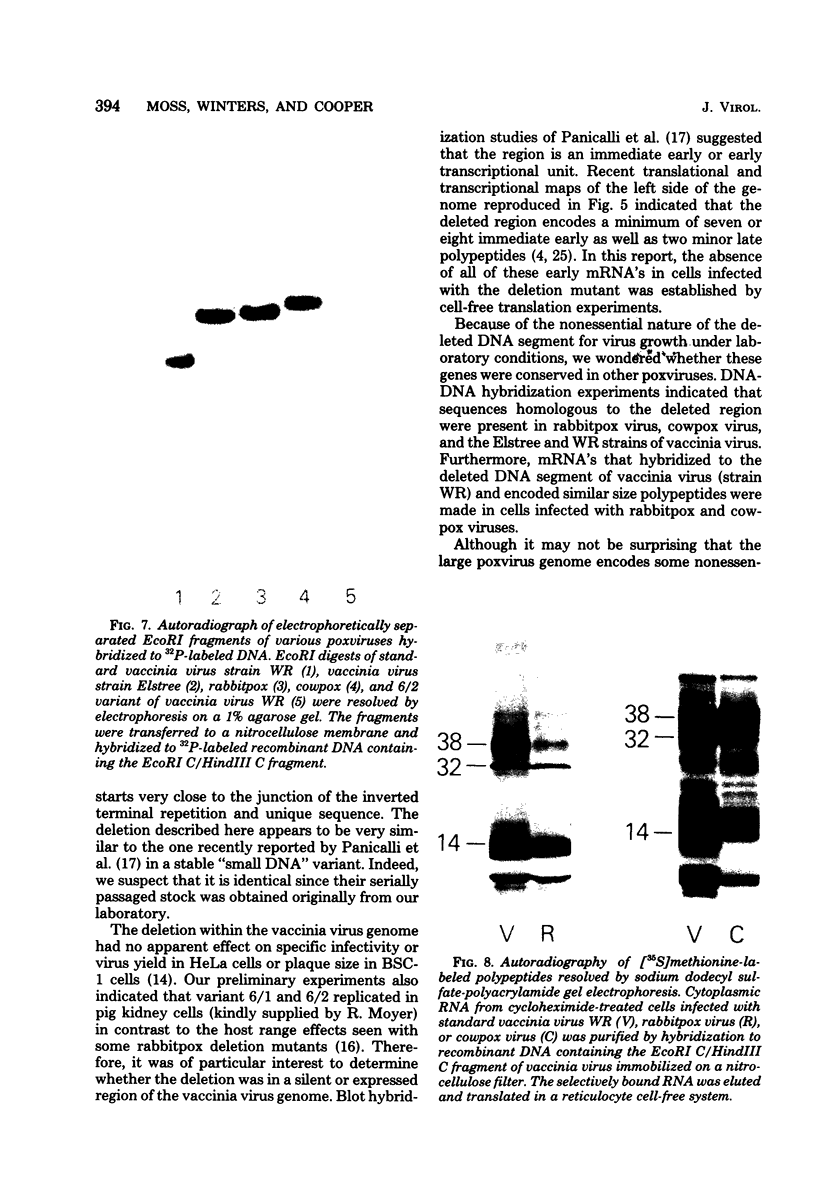
Deletions contained within the genomes of unstable and stable variants of vaccinia virus (strain WR) were analyzed. Restriction endonuclease mapping and hybridization to specific 32P-labeled DNA probes indicated that more than 6 X 10(6) daltons of DNA were deleted from the variants. In each case, the deletion occurred on the left side of the genome and started very close to the junction of the inverted terminal repetition and unique sequence. Both variants also contained a new SstI side on the right side of the genome. Hybridization selection and cell-free translation experiments indicated that these variants lost the ability to synthesize at least eight early mRNA's mapping within the deleted region. Although the deleted DNA was not essential for replication of the WR strain of vaccinia virus under laboratory conditions of infection, it presumably has a defined role under other circumstances. This conclusion was based on the conservation within the Elstree strain of vaccinia, the Utrecht strain of rabbitpox, and the Brighton strain of cowpox virus of sequences homologous to the deleted DNA. Moreover, mRNA's that hybridized to the deleted vaccinia virus DNA segment and encoded similar size polypeptides were made in cells infected with rabbitpox and cowpox viruses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archard L. C., Mackett M. Restriction endonuclease analysis of red cowpox virus and its white pock variant. J Gen Virol. 1979 Oct;45(1):51–63. doi: 10.1099/0022-1317-45-1-51. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. In vitro translation of immediate early, early, and late classes of RNA from vaccinia virus-infected cells. Virology. 1979 Jul 30;96(2):368–380. doi: 10.1016/0042-6822(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Wittek R., Moss B. Extension of the transcriptional and translational map of the left end of the vaccinia virus genome to 21 kilobase pairs. J Virol. 1981 Sep;39(3):733–745. doi: 10.1128/jvi.39.3.733-745.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Wittek R., Moss B. Hybridization selection and cell-free translation of mRNA's encoded within the inverted terminal repetition of the vaccinia virus genome. J Virol. 1981 Jan;37(1):284–294. doi: 10.1128/jvi.37.1.284-294.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito J. J., Cabradilla C. D., Nakano J. H., Obijeski J. F. Intragenomic sequence transposition in monkeypox virus. Virology. 1981 Mar;109(2):231–243. doi: 10.1016/0042-6822(81)90495-5. [DOI] [PubMed] [Google Scholar]
- Garon C. F., Barbosa E., Moss B. Visualization of an inverted terminal repetition in vaccinia virus DNA. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4863–4867. doi: 10.1073/pnas.75.10.4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Holowczak J. A. Poxvirus DNA. I. Studies on the structure of the vaccinia genome. Virology. 1976 Jul 1;72(1):121–133. doi: 10.1016/0042-6822(76)90317-2. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. The purification fo four strains of poxvirus. Virology. 1962 Sep;18:9–18. doi: 10.1016/0042-6822(62)90172-1. [DOI] [PubMed] [Google Scholar]
- Mackett M., Archard L. C. Conservation and variation in Orthopoxvirus genome structure. J Gen Virol. 1979 Dec;45(3):683–701. doi: 10.1099/0022-1317-45-3-683. [DOI] [PubMed] [Google Scholar]
- McCarron R. J., Cabrera C. V., Esteban M., McAllister W. T., Holowczak J. A. Structure of vaccinia DNA: analysis of the viral genome by restriction endonucleases. Virology. 1978 May 1;86(1):88–101. doi: 10.1016/0042-6822(78)90010-7. [DOI] [PubMed] [Google Scholar]
- McFadden G., Dales S. Biogenesis of poxviruses: mirror-image deletions in vaccinia virus DNA. Cell. 1979 Sep;18(1):101–108. doi: 10.1016/0092-8674(79)90358-1. [DOI] [PubMed] [Google Scholar]
- Moss B., Winters E., Cooper N. Instability and reiteration of DNA sequences within the vaccinia virus genome. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1614–1618. doi: 10.1073/pnas.78.3.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyer R. W., Graves R. L., Rothe C. T. The white pock (mu) mutants of rabbit poxvirus. III. Terminal DNA sequence duplication and transposition in rabbit poxvirus. Cell. 1980 Nov;22(2 Pt 2):545–553. doi: 10.1016/0092-8674(80)90364-5. [DOI] [PubMed] [Google Scholar]
- Moyer R. W., Rothe C. T. The white pock mutants of rabbit poxvirus. I. Spontaneous host range mutants contain deletions. Virology. 1980 Apr 15;102(1):119–132. doi: 10.1016/0042-6822(80)90075-6. [DOI] [PubMed] [Google Scholar]
- Panicali D., Davis S. W., Mercer S. R., Paoletti E. Two major DNA variants present in serially propagated stocks of the WR strain of vaccinia virus. J Virol. 1981 Mar;37(3):1000–1010. doi: 10.1128/jvi.37.3.1000-1010.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne L. G. Significance of extracellular enveloped virus in the in vitro and in vivo dissemination of vaccinia. J Gen Virol. 1980 Sep;50(1):89–100. doi: 10.1099/0022-1317-50-1-89. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Barbosa E., Cooper J. A., Garon C. F., Chan H., Moss B. Inverted terminal repetition in vaccinia virus DNA encodes early mRNAs. Nature. 1980 May 1;285(5759):21–25. doi: 10.1038/285021a0. [DOI] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Barbosa E., Moss B. Expression of the vaccinia virus genome: analysis and mapping of mRNAs encoded within the inverted terminal repetition. Cell. 1980 Sep;21(2):487–493. doi: 10.1016/0092-8674(80)90485-7. [DOI] [PubMed] [Google Scholar]
- Wittek R., Cooper J. A., Moss B. Transcriptional and translational mapping of a 6.6-kilobase-pair DNA fragment containing the junction of the terminal repetition and unique sequence at the left end of the vaccinia virus genome. J Virol. 1981 Sep;39(3):722–732. doi: 10.1128/jvi.39.3.722-732.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Menna A., Müller H. K., Schümperli D., Boseley P. G., Wyler R. Inverted terminal repeats in rabbit poxvirus and vaccinia virus DNA. J Virol. 1978 Oct;28(1):171–181. doi: 10.1128/jvi.28.1.171-181.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Menna A., Schümperli D., Stoffel S., Müller H. K., Wyler R. HindIII and Sst I restriction sites mapped on rabbit poxvirus and vaccinia virus DNA. J Virol. 1977 Sep;23(3):669–678. doi: 10.1128/jvi.23.3.669-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Moss B. Tandem repeats within the inverted terminal repetition of vaccinia virus DNA. Cell. 1980 Aug;21(1):277–284. doi: 10.1016/0092-8674(80)90135-x. [DOI] [PubMed] [Google Scholar]