Abstract
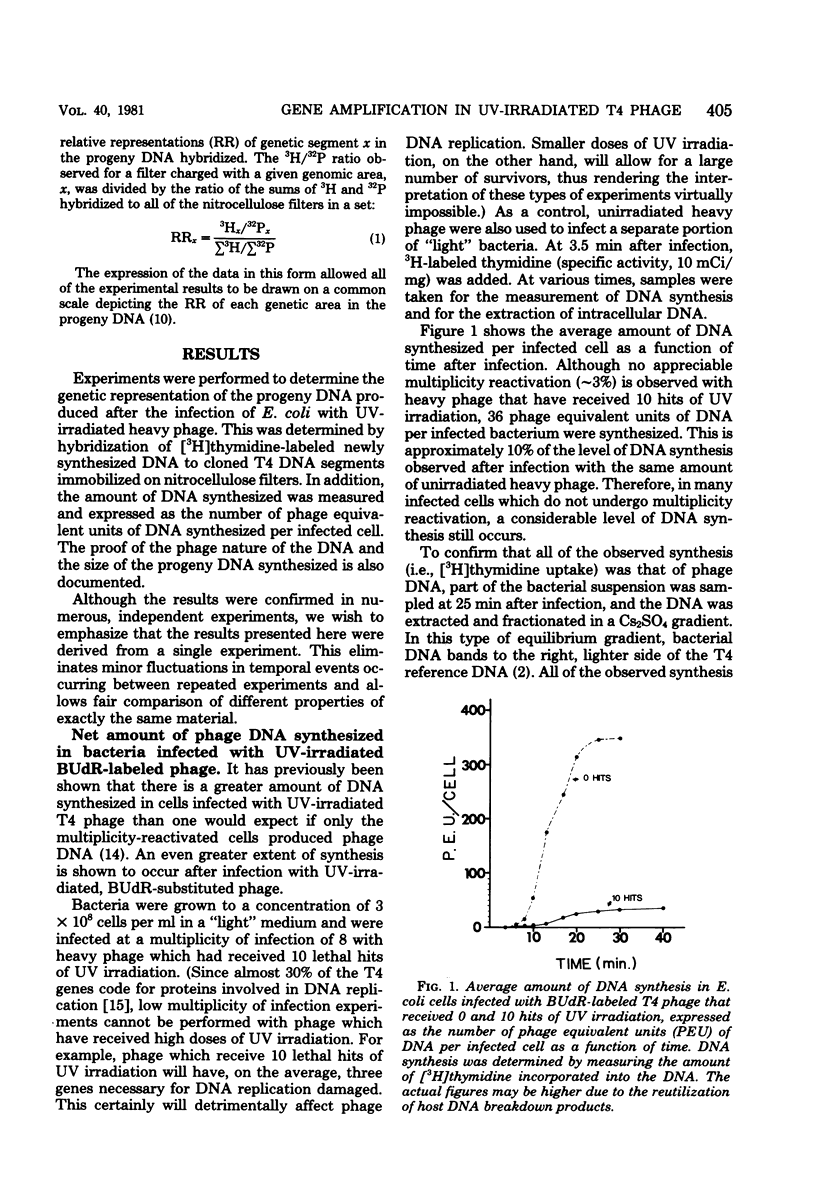
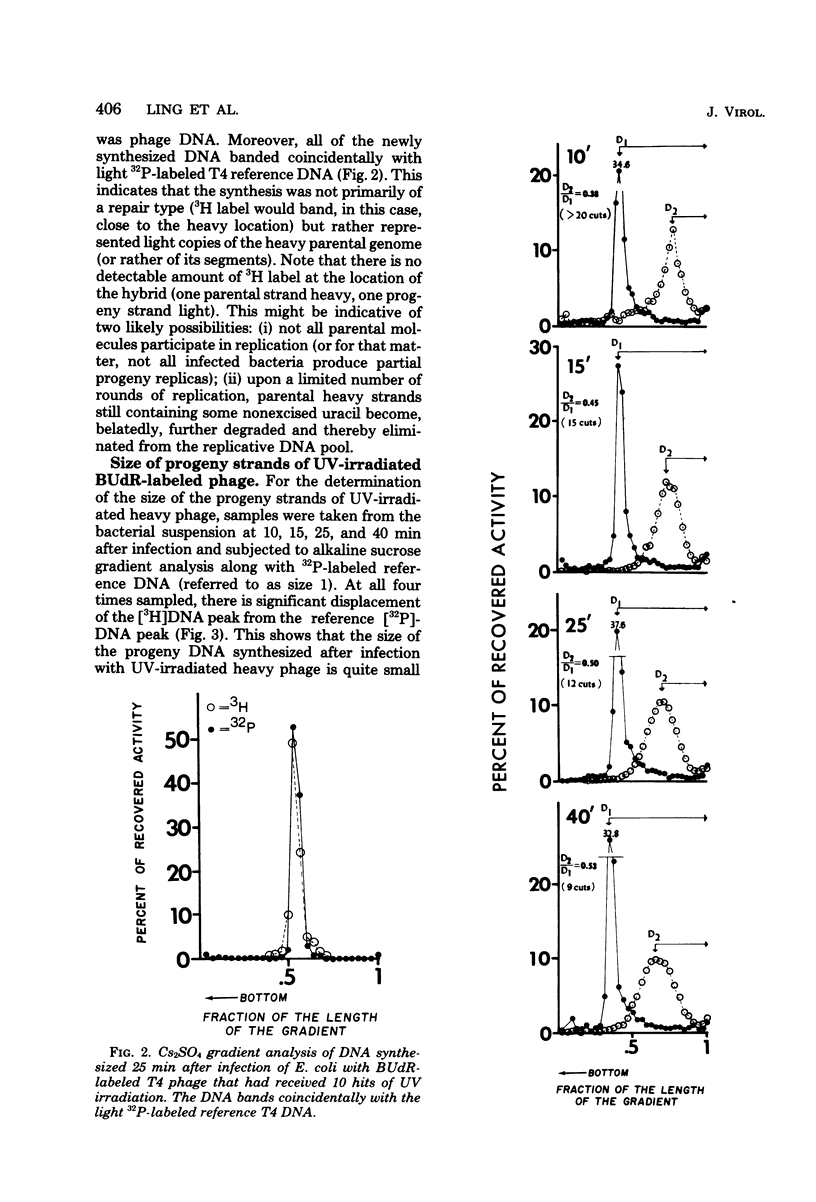
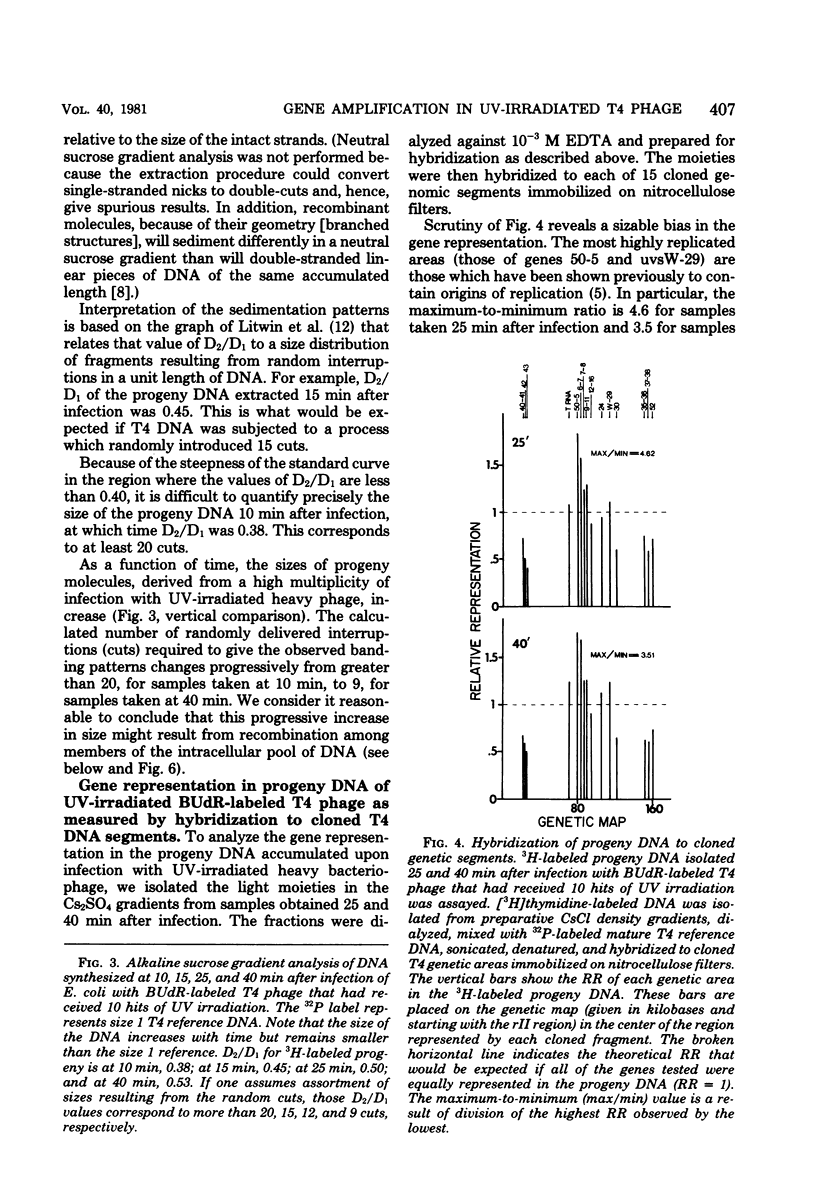
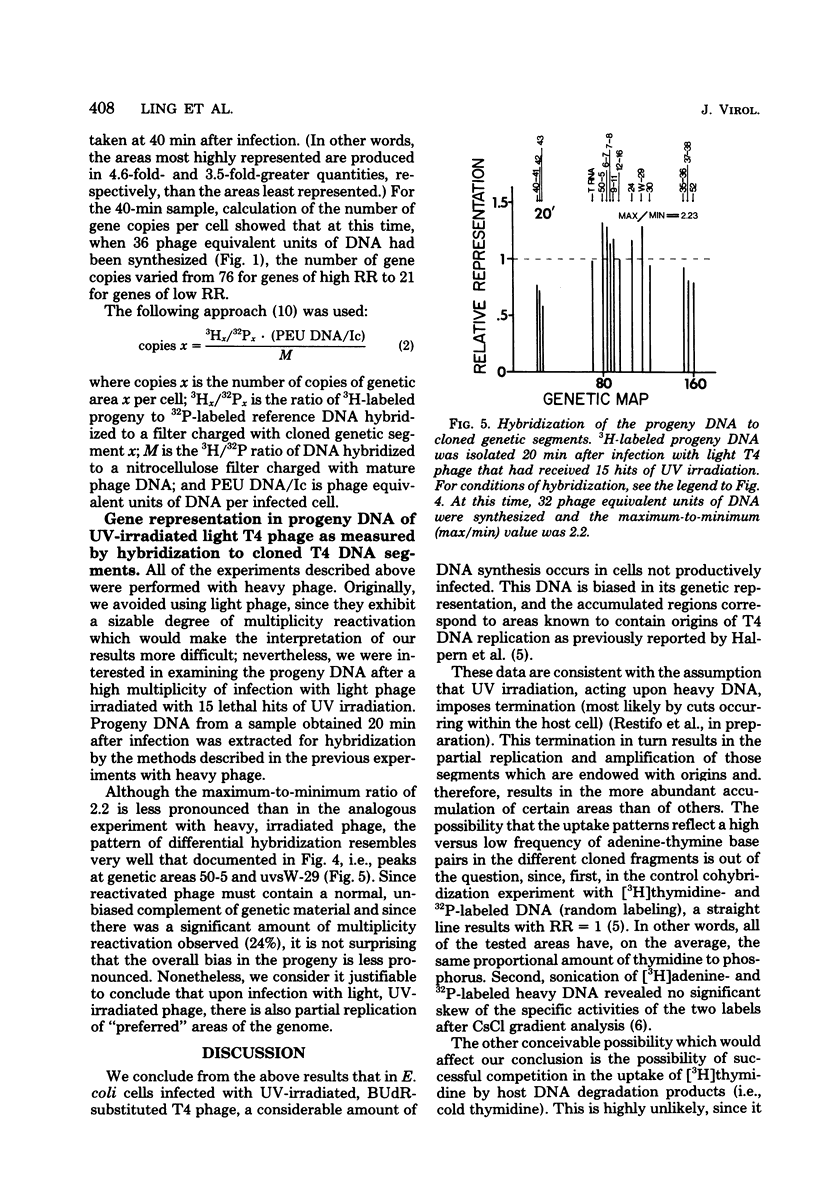
Upon infection of Escherichia coli with bromodeoxyuridine-labeled t4 phage that had received 10 lethal hits of UV irradiation, a sizable amount of phage DNA was synthesized (approximately 36 phage equivalent units of DNA per infected bacterium), although very little multiplicity reactivation occurs. This progeny DNA was isolated and analyzed. This DNA was biased in its genetic representation, as shown by hybridization to cloned segments of the T4 genome immobilized on nitrocellulose filters. Preferentially amplified areas corresponded to regions containing origins of T4 DNA replication. The size of the progeny DNA increased with time after infection, possibly due to recombination between partial replicas and nonreplicated subunits or due to the gradual overcoming of the UV damage. As the size of the progeny DNA increased, all of the genes were more equally represented, resulting in a decrease in the genetic bias. Amplification of specific genetic areas was also observed upon infection with UV-irradiated, nonbromodeoxyuridine-substituted (light) phage. However, the genetic bias observed in this case was not as great as that observed with bromodeoxyuridine-substituted phage. This is most likely due to the higher efficiency of multiplicity reactivation of the light phage.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K., Kozinski A. W. Nonreplicated DNA and DNA fragments in T4 r- bacteriophage particles: phenotypic mixing of a phage protein. J Virol. 1974 Jun;13(6):1274–1290. doi: 10.1128/jvi.13.6.1274-1290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K. Multiple initiation of bacteriophage T4 DNA replication: delaying effect of bromodeoxyuridine. J Virol. 1973 Aug;12(2):349–359. doi: 10.1128/jvi.12.2.349-359.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Halpern M. E., Mattson T., Kozinski A. W. Origins of phage T4 DNA replication as revealed by hybridization to cloned genes. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6137–6141. doi: 10.1073/pnas.76.12.6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe C. C., Buckley P. J., Carlson K. M., Kozinski A. W. Multiple and specific initiation of T4 DNA replication. J Virol. 1973 Jul;12(1):130–148. doi: 10.1128/jvi.12.1.130-148.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOZINSKI A. W., KOZINSKI P. B. Fragmentary transfer of P32-labeled parental DNA to progeny phage. II. The average size of the transferred parental fragment. Two-cycletransfer. Repair of the polynucleotide chain after fragmentation. Virology. 1963 Jun;20:213–229. doi: 10.1016/0042-6822(63)90109-0. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., SZYBALSKI W. Dispersive transfer of the parental DNA molecule to the progeny of phage phiX-174. Virology. 1959 Oct;9:260–274. doi: 10.1016/0042-6822(59)90119-9. [DOI] [PubMed] [Google Scholar]
- Kozinski A. W., Lin T. H. Early intracellular events in the replication of T4 phage DNA. I. Complex formation of replicative DNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):273–278. doi: 10.1073/pnas.54.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Ling S. K., Hutchinson N., Halpern M. E., Mattson T. Differential amplification of specific areas of phage T4 genome as revealed by hybridization to cloned genetic segments. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5064–5068. doi: 10.1073/pnas.77.9.5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litwin S., Shahn E., Kozinski A. W. Interpretation of sucrose gradient sedimentation pattern of deoxyribonucleic acid fragments resulting from random breaks. J Virol. 1969 Jul;4(1):24–30. doi: 10.1128/jvi.4.1.24-30.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. C., Jr, Kozinski A. W. Early intracellular events in the replication of bacteriophage T4 deoxyribonucleic acid. V. Further studies on the T4 protein-deoxyribonucleic acid complex. J Virol. 1970 Apr;5(4):490–501. doi: 10.1128/jvi.5.4.490-501.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayssiguier C., Kozinski A. W., Doermann A. H. Partial replicas of UV-irradiated bacteriophage T4 genomes and their role in multiplicity reactivation. J Virol. 1980 Aug;35(2):451–465. doi: 10.1128/jvi.35.2.451-465.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. B., Revel H. R. The genome of bacteriophage T4. Bacteriol Rev. 1976 Dec;40(4):847–868. doi: 10.1128/br.40.4.847-868.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young E. T., Mattson T., Selzer G., Van Houwe G., Bolle A., Epstein R. Bacteriophage T4 gene transcription studied by hybridization to cloned restriction fragments. J Mol Biol. 1980 Apr 15;138(3):423–445. doi: 10.1016/s0022-2836(80)80011-8. [DOI] [PubMed] [Google Scholar]


