Abstract
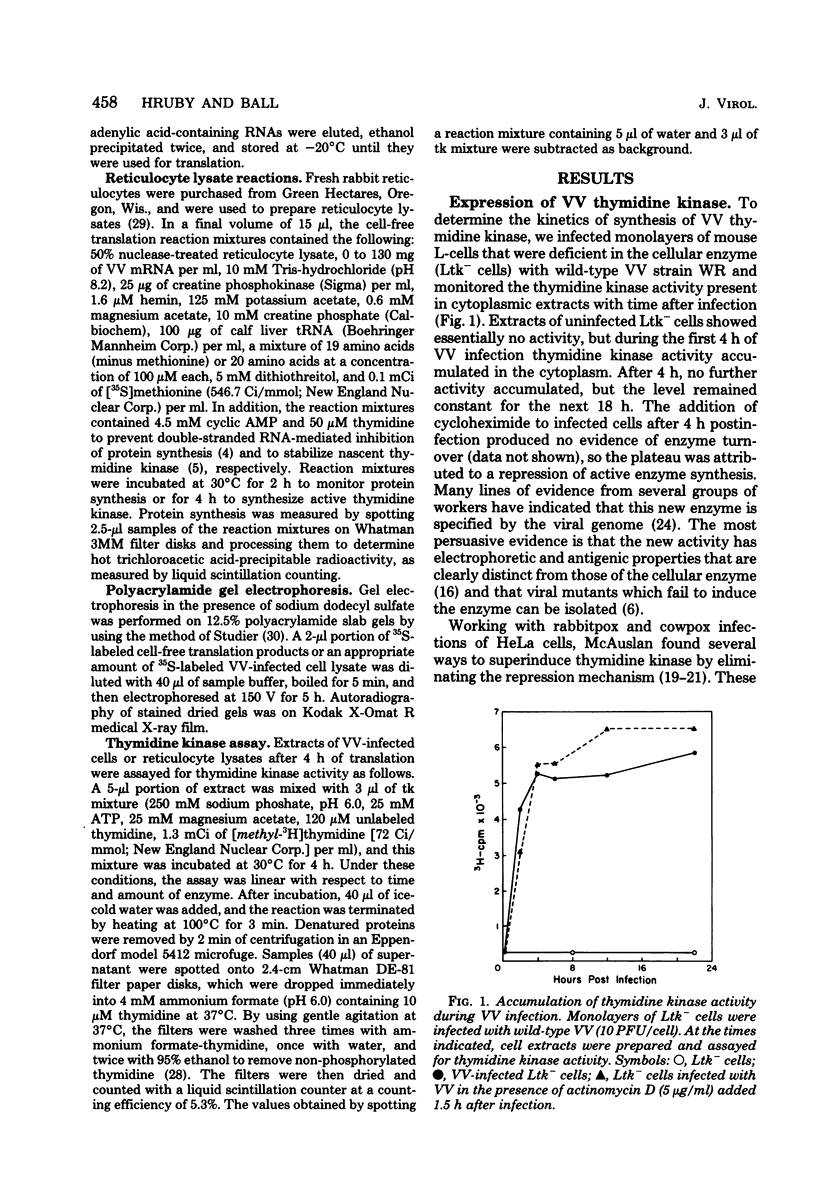
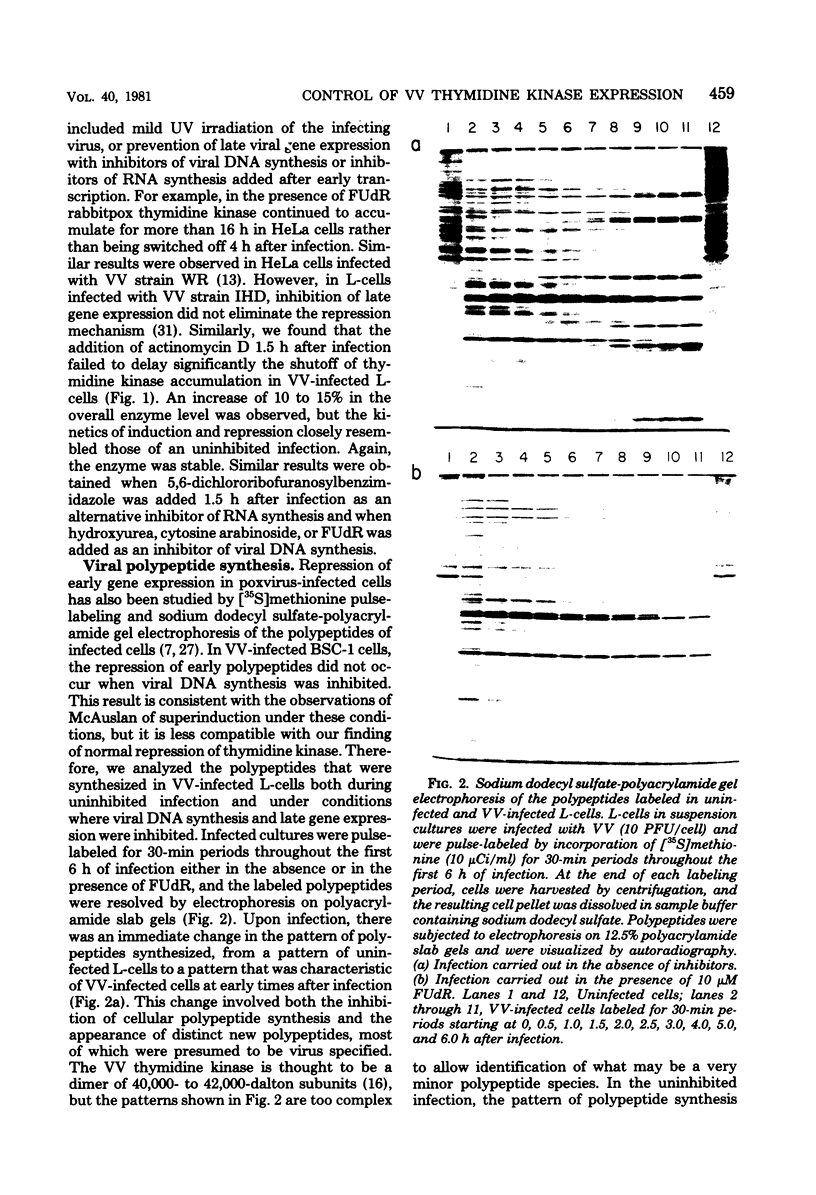
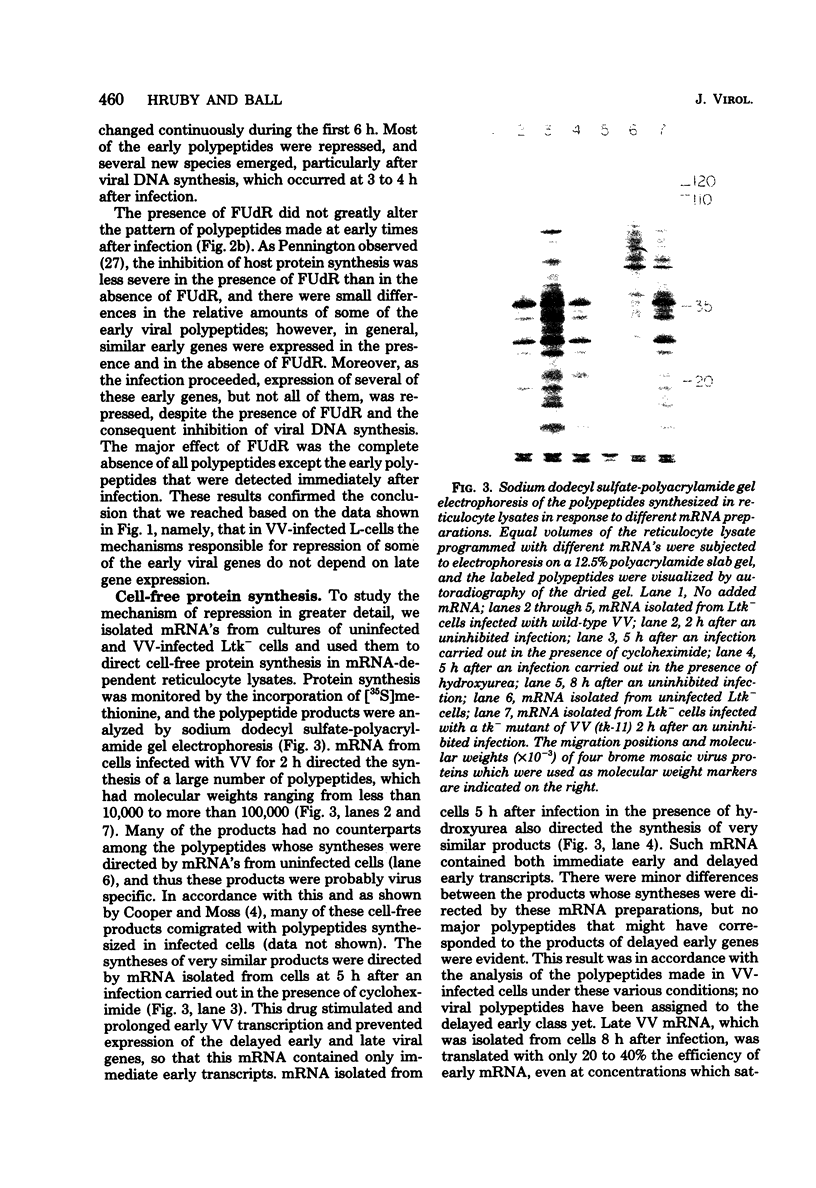
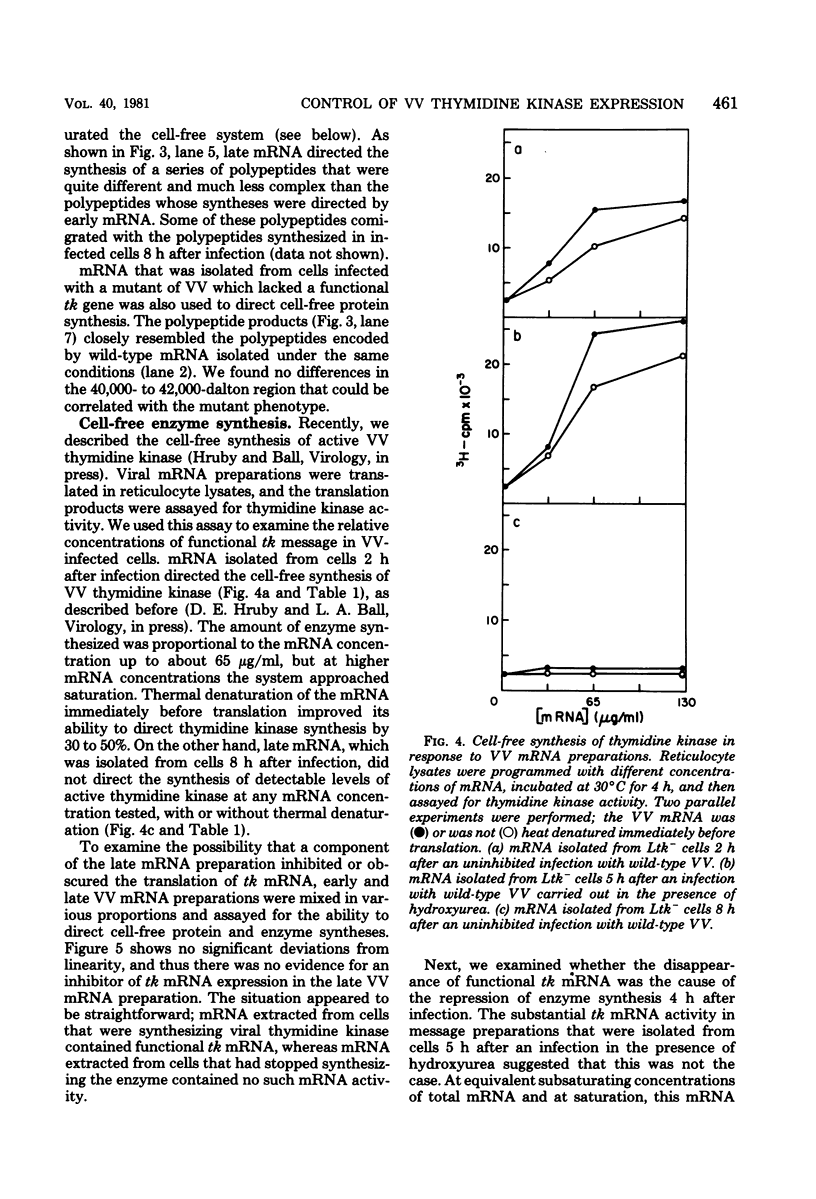
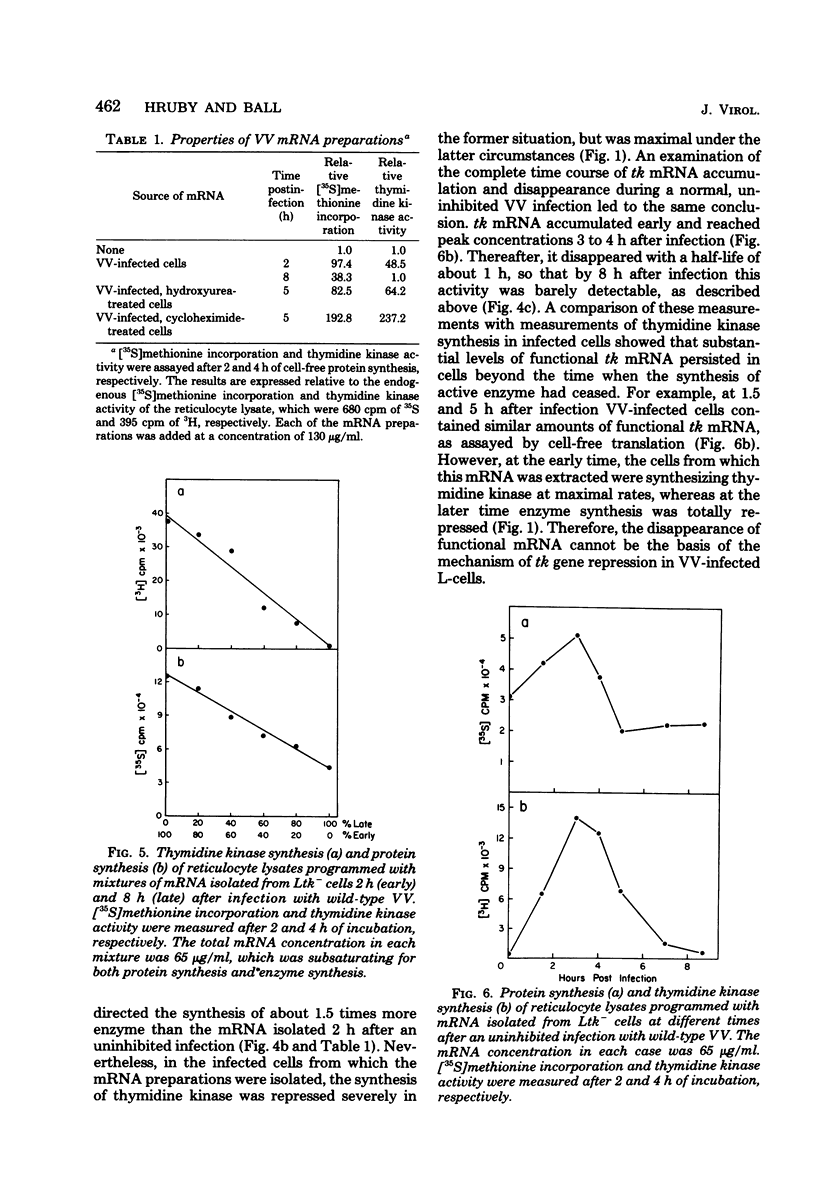
mRNA extracted from vaccinia virus-infected cells early after infection directs cell-free synthesis of enzymatically active viral thymidine kinase (Hruby and Ball, Virology, in press). We used this assay for a specific vaccinia virus mRNA to study the induction and repression of the viral thymidine kinase gene during infection of thymidine kinase-deficient L-cells. As observed previously by other workers, the synthesis of thymidine kinase occurred immediately after infection but was switched off after 4 h later. We observed similar kinetics of accumulation and shutoff under conditions where viral DNA synthesis and late gene expression were inhibited. Cell-free translation of mRNA from infected cells showed that the concentration of functional message for viral thymidine kinase reached a peak 3 to 4 h after infection and then decreased with a half-life of about 1 h. These kinetics indicated that significant levels of thymidine kinase mRNA persisted in cells which had stopped synthesizing the enzyme. Under conditions where late gene expression was inhibited, high concentrations of functional mRNA could be isolated from cells at late times after infection. On the basis of these results, we conclude that the repression of thymidine kinase expression is mediated at the translational level by one or more early or delayed early viral genes. Repression is accompanied by, but does not depend on, the inactivation or degradation of thymidine kinase mRNA, which is a late gene function.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boone R. F., Parr R. P., Moss B. Intermolecular duplexes formed from polyadenylylated vaccinia virus RNA. J Virol. 1979 Apr;30(1):365–374. doi: 10.1128/jvi.30.1.365-374.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipchase M., Schwendimann F., Wyler R. A map of the late proteins of vaccinia virus. Virology. 1980 Aug;105(1):261–264. doi: 10.1016/0042-6822(80)90176-2. [DOI] [PubMed] [Google Scholar]
- Colby C., Jurale C., Kates J. R. Mechanism of synthesis of vaccinia virus double-stranded ribonucleic acid in vivo and in vitro. J Virol. 1971 Jan;7(1):71–76. doi: 10.1128/jvi.7.1.71-76.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J. A., Moss B. In vitro translation of immediate early, early, and late classes of RNA from vaccinia virus-infected cells. Virology. 1979 Jul 30;96(2):368–380. doi: 10.1016/0042-6822(79)90095-3. [DOI] [PubMed] [Google Scholar]
- Cremer K., Bodemer M., Summers W. C. Characterization of the mRNA for herpes simplex virus thymidine kinase by cell-free synthesis of active enzyme. Nucleic Acids Res. 1978 Jul;5(7):2333–2344. doi: 10.1093/nar/5.7.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. ISOLATION AND PROPERTIES OF VACCINIA MUTANTS DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Feb;22:214–225. doi: 10.1016/0042-6822(64)90006-6. [DOI] [PubMed] [Google Scholar]
- Esteban M., Metz D. H. Early virus protein synthesis in vaccinia virus-infected cells. J Gen Virol. 1973 May;19(2):201–206. doi: 10.1099/0022-1317-19-2-201. [DOI] [PubMed] [Google Scholar]
- Geshelin P., Berns K. I. Characterization and localization of the naturally occurring cross-links in vaccinia virus DNA. J Mol Biol. 1974 Oct 5;88(4):785–796. doi: 10.1016/0022-2836(74)90399-4. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Guarino L. A., Kates J. R. Vaccinia virus replication. I. Requirement for the host-cell nucleus. J Virol. 1979 Feb;29(2):705–715. doi: 10.1128/jvi.29.2.705-715.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Lynn D. L., Kates J. R. Vaccinia virus replication requires active participation of the host cell transcriptional apparatus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1887–1890. doi: 10.1073/pnas.76.4.1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Roberts W. K. Encephalomyocarditis virus RNA: variations in polyadenylic acid content and biological activity. J Virol. 1976 Aug;19(2):325–330. doi: 10.1128/jvi.19.2.325-330.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isle H. B., Venkatesan S., Moss B. Cell-free translation of early and late mRNAs selected by hybridization to cloned DNA fragments derived from the left 14 million to 72 million daltons of the vaccinia virus genome. Virology. 1981 Jul 15;112(1):306–317. doi: 10.1016/0042-6822(81)90636-x. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J. Enhanced thymidine phosphorylating activity of mouse fibroblasts (strain LM) following vaccinia infection. Biochem Biophys Res Commun. 1962 Jun 19;8:72–75. doi: 10.1016/0006-291x(62)90238-3. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. EFFECTS OF 5-FLUOROURACIL, ACTINOMYCIN D AND MITOMYCIN C ON THE INDUCTION OF THYMIDINE KINASE BY VACCINIA-INFECTED L-CELLS. J Mol Biol. 1963 Nov;7:497–510. doi: 10.1016/s0022-2836(63)80097-2. [DOI] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963 Jan;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- Kit S., Jorgensen G. N., Liav A., Zaslavsky V. Purification of vaccinia virus-induced thymidine kinase activity from [35S]methionine-labeled cells. Virology. 1977 Apr;77(2):661–676. doi: 10.1016/0042-6822(77)90490-1. [DOI] [PubMed] [Google Scholar]
- MCAUSLAN B. R. THE INDUCTION AND REPRESSION OF THYMIDINE KINASE IN THE POXVIRUS-INFECTED HELA CELL. Virology. 1963 Nov;21:383–389. doi: 10.1016/0042-6822(63)90199-5. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Kates J. R. Regulation of virus-induced deoxyribonucleases. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1581–1587. doi: 10.1073/pnas.55.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Kraiselburd E., Davis D., Mann J. Transfer of thymidine kinase to thymidine kinaseless L cells by infection with ultraviolet-irradiated herpes simplex virus. J Virol. 1971 Jun;7(6):813–820. doi: 10.1128/jvi.7.6.813-820.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K. I., Joklik W. K. Hybridization and sedimentation studies on "early" and "late" vaccinia messenger RNA. J Mol Biol. 1967 Aug 14;27(3):395–419. doi: 10.1016/0022-2836(67)90047-2. [DOI] [PubMed] [Google Scholar]
- Pennington T. H. Vaccinia virus polypeptide synthesis: sequential appearance and stability of pre- and post-replicative polypeptides. J Gen Virol. 1974 Dec;25(3):433–444. doi: 10.1099/0022-1317-25-3-433. [DOI] [PubMed] [Google Scholar]
- Preston C. M. Cell-free synthesis of herpes simplex virus-coded pyrimidine deoxyribonucleoside kinase enzyme. J Virol. 1977 Sep;23(3):455–460. doi: 10.1128/jvi.23.3.455-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Zaslavsky V., Yakobson E. Control of thymidine kinase synthesis in IHD vaccinia virus-infected thymidine kinase-deficient LM cells. J Virol. 1975 Jul;16(1):210–213. doi: 10.1128/jvi.16.1.210-213.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]