Abstract
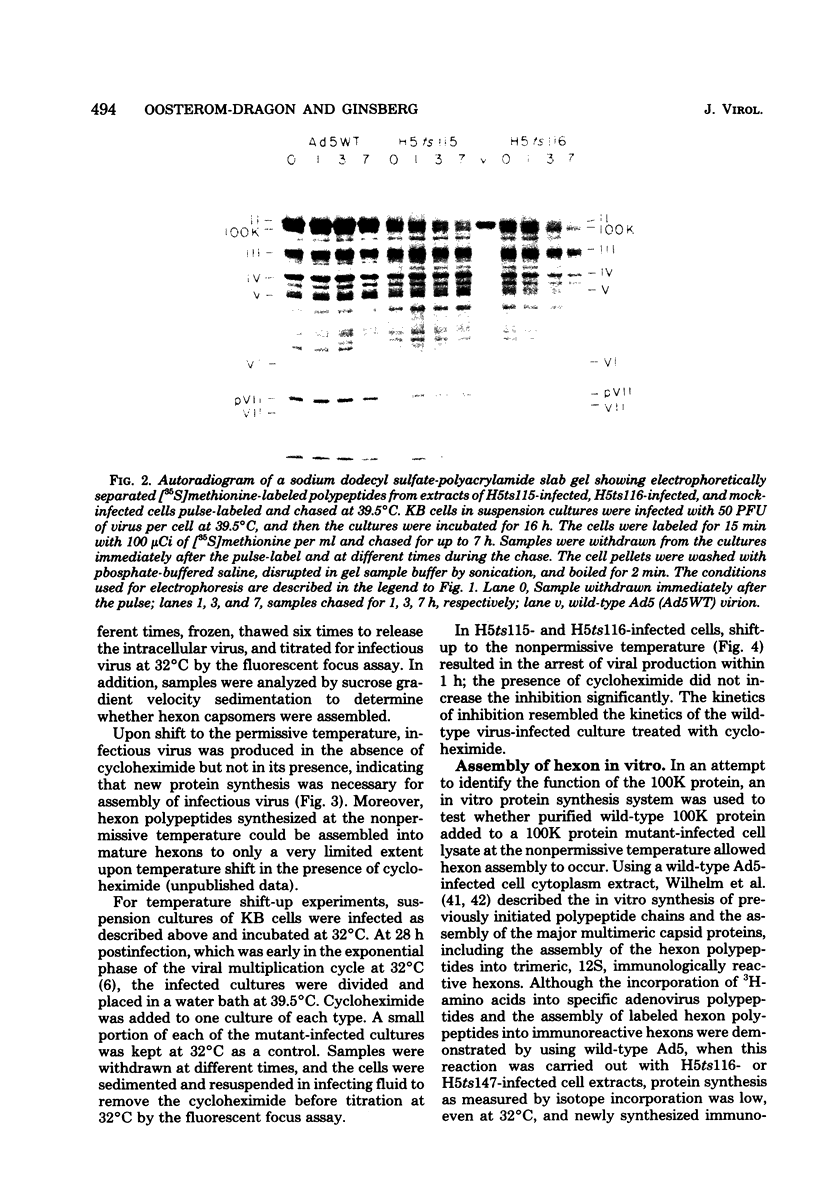
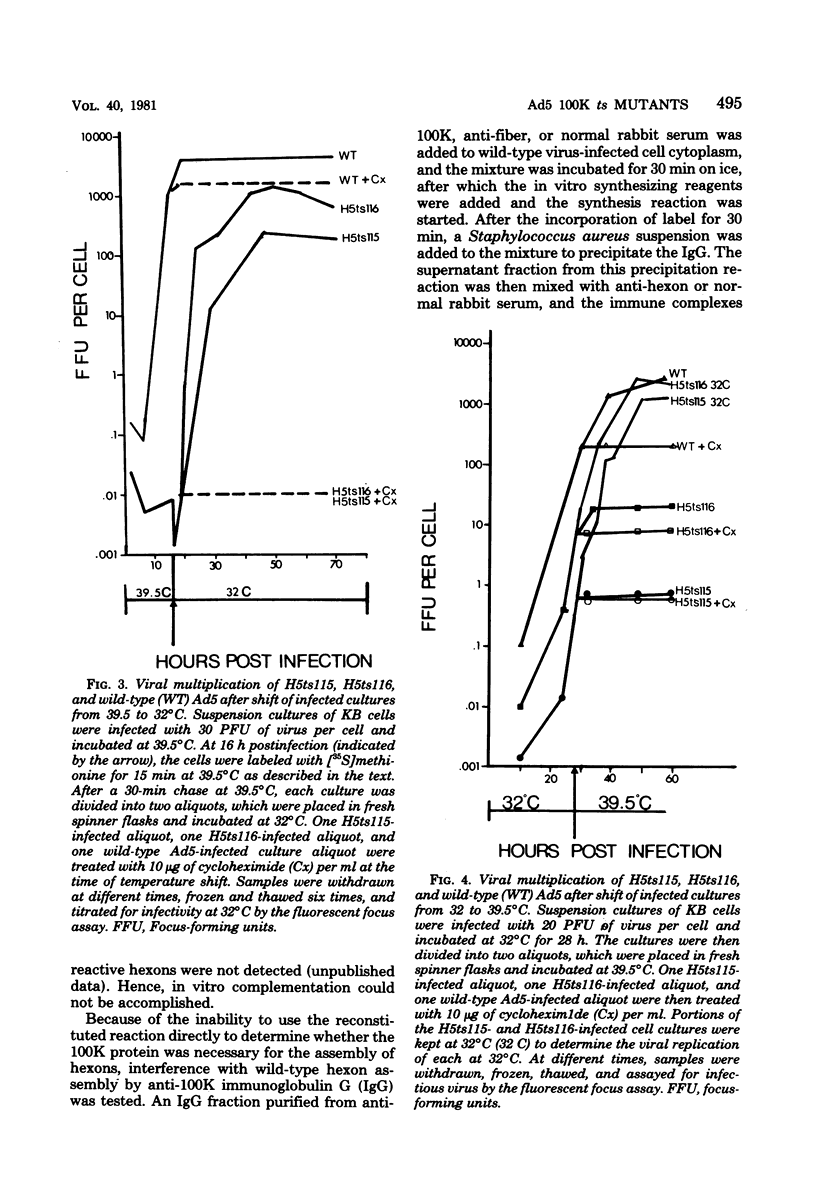
Complementation analysis assigned the mutations of strains H5ts115 and H5ts116, two hexon-minus mutants, to the 100,000-dalton (100K) protein gene. Heterotypic marker rescue (i.e., type 5 adenovirus [Ad5] temperature-sensitive mutants DNA X EcoRI restriction fragments of Ad2 DNA) confirmed the results of previous marker rescue mapping studies, and the heterotypic recombinants yielded unique hybrid (Ad5-Ad2) 100K proteins which were intermediate in size between Ad5 and Ad2 proteins and appeared to be as functionally active as the wild-type 100K protein. Phenotypic characterization of these mutants showed that both the hexon polypeptides and the 100K polypeptides were unstable at the nonpermissive temperature, whereas fiber and penton were not degraded, and that the 100K protein made at 39.5 degrees C could not be utilized after a shift to the permissive temperature (32 degrees C). The role of the 100K protein in the assembly of the hexon trimer was also examined by in vitro protein synthesis. Normally, hexon polypeptides synthesized during an in vitro reaction are assembled into immunoreactive hexons. However, this assembly was inhibited by preincubation of the cell extract with anti-100K immunoglobulin G; neither anti-fiber immunoglobulin G nor normal rabbit immunoglobulin G inhibited hexon assembly. It is postulated that an interaction between the 100K protein and hexon polypeptides is required for effective assembly of hexon trimers.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. W., Baum P. R., Gesteland R. F. Processing of adenovirus 2-induced proteins. J Virol. 1973 Aug;12(2):241–252. doi: 10.1128/jvi.12.2.241-252.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrand J. E. Mapping of adenovirus type 5 temperature-sensitive mutations by marker rescue in enhanced double DNA infections. J Gen Virol. 1978 Dec;41(3):573–586. doi: 10.1099/0022-1317-41-3-573. [DOI] [PubMed] [Google Scholar]
- Axelrod N. Phosphoproteins of adenovirus 2. Virology. 1978 Jun 15;87(2):366–383. doi: 10.1016/0042-6822(78)90141-1. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Chow L. T., Roberts J. M., Lewis J. B., Broker T. R. A map of cytoplasmic RNA transcripts from lytic adenovirus type 2, determined by electron microscopy of RNA:DNA hybrids. Cell. 1977 Aug;11(4):819–836. doi: 10.1016/0092-8674(77)90294-x. [DOI] [PubMed] [Google Scholar]
- Ensinger M. J., Ginsberg H. S. Selection and preliminary characterization of temperature-sensitive mutants of type 5 adenovirus. J Virol. 1972 Sep;10(3):328–339. doi: 10.1128/jvi.10.3.328-339.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt E., Lutter L., Philipson L. Structural proteins of adenoviruses. XII. Location and neighbor relationship among proteins of adenovirion type 2 as revealed by enzymatic iodination, immunoprecipitation and chemical cross-linking. Virology. 1975 Sep;67(1):197–208. doi: 10.1016/0042-6822(75)90417-1. [DOI] [PubMed] [Google Scholar]
- Everitt E., Sundquist B., Pettersson U., Philipson L. Structural proteins of adenoviruses. X. Isolation and topography of low molecular weight antigens from the virion of adenovirus type 2. Virology. 1973 Mar;52(1):130–147. doi: 10.1016/0042-6822(73)90404-2. [DOI] [PubMed] [Google Scholar]
- Frost E., Williams J. Mapping temperature-sensitive and host-range mutations of adenovirus type 5 by marker rescue. Virology. 1978 Nov;91(1):39–50. doi: 10.1016/0042-6822(78)90353-7. [DOI] [PubMed] [Google Scholar]
- GINSBERG H. S., GOLD E., JORDAN W. S., Jr Tryptose phosphate broth as supplementary factor for maintenance of HeLa cell tissue cultures. Proc Soc Exp Biol Med. 1955 May;89(1):66–71. doi: 10.3181/00379727-89-21718. [DOI] [PubMed] [Google Scholar]
- Goldberg A. L., Dice J. F. Intracellular protein degradation in mammalian and bacterial cells. Annu Rev Biochem. 1974;43(0):835–869. doi: 10.1146/annurev.bi.43.070174.004155. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Anderson C., Sambrook J., Mathews M. B. The physical locations of structural genes in adenovirus DNA. Virology. 1977 Jul 1;80(1):111–126. doi: 10.1016/0042-6822(77)90384-1. [DOI] [PubMed] [Google Scholar]
- Grodzicker T., Williams J., Sharp P., Sambrook J. Physical mapping of temperature-sensitive mutations of adenoviruses. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):439–446. doi: 10.1101/sqb.1974.039.01.056. [DOI] [PubMed] [Google Scholar]
- Jörnvall H., Ohlsson H., Philipson L. An acetylated N-terminus of adenovirus type 2 hexon protein. Biochem Biophys Res Commun. 1974 Jan 23;56(2):304–310. doi: 10.1016/0006-291x(74)90842-0. [DOI] [PubMed] [Google Scholar]
- Kauffman R. S., Ginsberg H. S. Characterization of a temperature-sensitive, hexon transport mutant of type 5 adenovirus. J Virol. 1976 Aug;19(2):643–658. doi: 10.1128/jvi.19.2.643-658.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D., Lodish H. F., Baltimore D. Analysis of the defects of temperature-sensitive mutants of vesicular stomatitis virus: intracellular degradation of specific viral proteins. J Virol. 1977 Mar;21(3):1140–1148. doi: 10.1128/jvi.21.3.1140-1148.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lawrence W. C., Ginsberg H. S. Intracellular uncoating of type 5 adenovirus deoxyribonucleic acid. J Virol. 1967 Oct;1(5):851–867. doi: 10.1128/jvi.1.5.851-867.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz J., Horwitz M. S. Synthesis and assembly of adenovirus polypeptides. III. Reversible inhibition of hexon assembly in adenovirus type 5 temperature-sensitive mutants. Virology. 1975 Jul;66(1):10–24. doi: 10.1016/0042-6822(75)90175-0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Anderson C. W., Atkins J. F. Further mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Cell. 1977 Sep;12(1):37–44. doi: 10.1016/0092-8674(77)90183-0. [DOI] [PubMed] [Google Scholar]
- Lewis J. B., Atkins J. F., Anderson C. W., Baum P. R., Gesteland R. F. Mapping of late adenovirus genes by cell-free translation of RNA selected by hybridization to specific DNA fragments. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1344–1348. doi: 10.1073/pnas.72.4.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., Neuwald P. D., Lai S. P., Maizel J. V., Jr, Westphal H. Electron microscopy of late adenovirus type 2 mRNA hybridized to double-stranded viral DNA. J Virol. 1977 Mar;21(3):1010–1018. doi: 10.1128/jvi.21.3.1010-1018.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. S., Ricciardi R. P., Roberts B. E., Paterson B. M., Mathews M. B. Arrangement of messenger RNAs and protein coding sequences in the major late transcription unit of adenovirus 2. J Mol Biol. 1980 Oct 5;142(4):455–488. doi: 10.1016/0022-2836(80)90258-2. [DOI] [PubMed] [Google Scholar]
- Oosterom-Dragon E. A., Ginsberg H. S. Purification and preliminary immunological characterization of the type 5 adenovirus, nonstructural 100,000-dalton protein. J Virol. 1980 Mar;33(3):1203–1207. doi: 10.1128/jvi.33.3.1203-1207.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L. Adenovirus assay by the fluorescent cell-counting procedure. Virology. 1961 Nov;15:263–268. doi: 10.1016/0042-6822(61)90357-9. [DOI] [PubMed] [Google Scholar]
- Paterson B. M., Roberts B. E., Kuff E. L. Structural gene identification and mapping by DNA-mRNA hybrid-arrested cell-free translation. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4370–4374. doi: 10.1073/pnas.74.10.4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell W. C., Skehel J. J., Williams J. F. Characterization of temperature-sensitive mutants of adenovirus type 5: synthesis of polypeptides in infected cells. J Gen Virol. 1974 Aug;24(2):247–259. doi: 10.1099/0022-1317-24-2-247. [DOI] [PubMed] [Google Scholar]
- Sambrook J., Williams J., Sharp P. A., Grodzicker T. Physical mapping of temperature-sensitive mutations of adenoviruses. J Mol Biol. 1975 Sep 25;97(3):369–390. doi: 10.1016/s0022-2836(75)80046-5. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Ginsberg H. S. Antibody to the type 5 adenovirus hexon polypeptide: detection of nascent polypeptides in the cytoplasm of infected KB cells. Intervirology. 1974;4(4):226–236. doi: 10.1159/000149967. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Ginsberg H. S. Hexon peptides of type 2, 3, and 5 adenoviruses and their relationship to hexon structure. J Virol. 1975 Apr;15(4):898–905. doi: 10.1128/jvi.15.4.898-905.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Tegtmeyer P., Robb J. A., Widmer C., Ozer H. L. Altered protein metabolism in infection by the late tsB11 mutant of simian virus 40. J Virol. 1974 Oct;14(4):997–1007. doi: 10.1128/jvi.14.4.997-1007.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel J. F., Smith K. O. Fluorescent focus assay of viruses on cell monolayers in plastic Petri plates. Proc Soc Exp Biol Med. 1967 Jul;125(3):892–895. doi: 10.3181/00379727-125-32232. [DOI] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Cytoplasmic synthesis of type 5 adenovirus capsid proteins. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1264–1271. doi: 10.1073/pnas.61.4.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velicer L. F., Ginsberg H. S. Synthesis, transport, and morphogenesis of type adenovirus capsid proteins. J Virol. 1970 Mar;5(3):338–352. doi: 10.1128/jvi.5.3.338-352.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. PRODUCTION OF SPECIFIC NEUTRALIZING ANTIBODY WITH SOLUBLE ANTIGENS OF TYPE 5 ADENOVIRUS. Proc Soc Exp Biol Med. 1963 Oct;114:37–42. doi: 10.3181/00379727-114-28579. [DOI] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. STRUCTURE OF TYPE 5 ADENOVIRUS. I. ANTIGENIC RELATIONSHIP OF VIRUS-STRUCTURAL PROTEINS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:295–306. doi: 10.1084/jem.118.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J. M., Ginsberg H. S. Synthesis in vitro of type 5 adenovirus capsid proteins. J Virol. 1972 Jun;9(6):973–980. doi: 10.1128/jvi.9.6.973-980.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. F. Enhancement of adenovirus plaque formation on HeLa cells by magnesium chloride. J Gen Virol. 1970 Dec;9(3):251–255. doi: 10.1099/0022-1317-9-3-251. [DOI] [PubMed] [Google Scholar]
- Williams J. F., Gharpure M., Ustacelebi S., McDonald S. Isolation of temperature-sensitive mutants of adenovirus type 5. J Gen Virol. 1971 May;11(2):95–101. doi: 10.1099/0022-1317-11-2-95. [DOI] [PubMed] [Google Scholar]
- Williams J., Grodzicker T., Sharp P., Sambrook J. Adenovirus recombination: physical mapping of crossover events. Cell. 1975 Feb;4(2):113–119. doi: 10.1016/0092-8674(75)90117-8. [DOI] [PubMed] [Google Scholar]
- Willians J. F., Young C. S., Austin P. E. Genetic analysis of human adenovirus type 5 in permissive and nonpermissive cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):427–437. doi: 10.1101/sqb.1974.039.01.055. [DOI] [PubMed] [Google Scholar]