Abstract
Purpose
to determine whether IP3Rs contribute to the generation of wide long lasting perinuclear Ca2+ release events in canine Purkinje cells.
Methods
Spontaneous Ca2+ release events (elevations of basal [Ca2+] equivalent to F/F0 3.4SD over F0) were imaged using Fluo-4AM and 2D confocal microscope. Only cells free of Ca2+ waves were analyzed. Subsarcolemmal region (SSL) was defined as 5µm from cell edges. Core was the remaining cell.
Results
The majority of events (94%, 0.0035 ± 0.0007 events(ev)/µm2/sec, n=34 cells) were detected within a single frame (typical events, TE). However, a subpopulation (6.0%, 0.00022±0.00005 ev/µm2/sec, n=41 cells: wide long lasting events, WLE) lasted for several frames, showed a greater spatial extent (51.0±3.9 vs. TE 9.0±0.3 µm2, P<0.01) and higher amplitude (F/F0 1.38±0.02 vs. TE 1.20±0.003, P<0.01). WLE event rate was increased by phenylephrine (10µM, P<0.01), inhibited by 2APB and U73122 (P<0.05), and abolished by tetracaine (1mM) and ryanodine (100µM). While SSL WLEs were scattered randomly, Core WLEs (n=69 events) were predominantly distributed longitudinally 18.2±1.6 ìm from the center of nuclei. Immunocytochemistry showed that IP3R1s were located not only at SSL region but also near both ends of nucleus overlapping with RyRs.
Conclusion
In Purkinje cells, wide long lasting Ca2+ release events occur in SSL and in specific perinuclear regions. They are likely due to RyRs and IP3R1s evoked Ca2+ release and may play a role in Ca2+ dependent nuclear processes.
Keywords: Purkinje cells, nucleus, Ca2+ transients, Phenylephrine
INTRODUCTION
In the heart, the ryanodine receptor (RyR) is a major element in the control of the intracellular Ca2+ concentration, whereas the role of the inositol 1,4,5-trisphosphate receptor (IP3R) remains unclear. It has been recently reported in rabbit ventricular myocytes that IP3R is expressed predominantly in the nuclear envelope and as such, may play a role in the gene transcription through Ca2+-dependent signaling pathways (excitation-transcription coupling) [1–3] and/ or response to endothelin [4]. In permeabilized atrial cells, the nucleus appears to be surrounded by its own Ca2+ store and IP3 can induce nuclear Ca2+ release events [5].
Compared to ventricular myocytes, Purkinje cells exhibit a tenfold larger density of IP3Rs [6,7]. A reduction of spontaneous activity in presence of 2APB [8] as well as a good correlation between Ca2+ transients and the cellular distribution of IP3R1 specific antibody [9] support the idea that IP3Rs are involved in Ca2+ activation of Purkinje fibers during both action potential evoked and spontaneous Ca2+ transients. IP3Rs and related local Ca2+ releases have been localized near the sarcolemmal membrane and might constitute the primary event of the sequence leading to large Ca2+ transients and subsequent cell wide Ca2+ waves in Purkinje cells [9]. Cell wide Ca2+ waves in Purkinje cells can lead to non driven electrical activity [10]. However the existence of specific IP3R related Ca2+ release activity in the nuclear region has not been considered. The aim of this study was to investigate the contribution of IP3R to Ca2+ release events in the perinuclear region of canine Purkinje cells.
METHODS
All experiments were performed according to protocols approved by the Columbia University Institutional Animal Care and Use Committee and conform to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication NO. 85-23, revised 1996).
Preparation
Purkinje cells were enzymatically dispersed from the Purkinje fibers of the canine heart (preparations=N=15) as previously described [11,12] and placed in a glass-bottomed perfused chamber on the stage of an inverted microscope and then loaded with 5µM Fluo-4 AM [9]. Fluorescence was measured only in rod-shaped Purkinje cells with typical junctional ends, clear striations and membranes free of blebs. All experiments were completed after cells had been superfused with Tyrodes solution (including 2mM Ca2+, 4mM K+, pH =7.4, 24°C) for at least 15 mins.
Cellular Ca2+ imaging
Ca2+-related variations of Fluo-4 fluorescence were studied using a Yokogawa confocal scanning unit (CSU10, Yokogawa, Japan) attached to a Nikon TE200 microscope equipped with a Nikon x60 Fluorescence objective. The excitation light (wavelength: 488nm) source was a multiline 100 mW argon ion laser. Emitted fluorescence was filtered at 510 nm and sampled at a rate of 17 frames per sec by a CCD camera (ORCA-ER C4742–95, Hamamatsu Photonics KK, Japan). The Ca-related fluorescence was visualized through F/Fo ratio images as following: basal fluorescence (Fo) image was determined as the average of all frames; each fluorescence image was divided pixel-to-pixel by the corresponding Fo image.
Detection and analysis of Ca2+ events
Ca2+ release events were automatically detected through a series of consecutive F/Fo images using an IDL custom computer-based detection procedure. A Ca2+ event was defined as an elevation of basal [Ca2+] equivalent to F/F0 3.4 SD over F0. The sensitivity of the detection was 95 ± 2 % and the probability that the detection includes a false event was 6 ± 2 percent of total events (n=8, Figure S1 and S2 online supplement). Ca2+ event frequency and maximal event amplitude in different regions of the Purkinje cell were calculated as following: regions of interest (Subsarcolemmal (SSL) and Core ROIs) were first drawn on a cell image (ImageJ) and then superimposed on each of the binary images to determine the subcellular location (SSL or Core) of events (as in Figure S1). The amplitude, spatial extent, and location of events were determined using the custom-made IDL program (IDL6.0, Research Systems). In some cases (eg. Figure 1B) we have displayed Ca2+ events as pseudo linescans (Image J). For more details see Supplement.
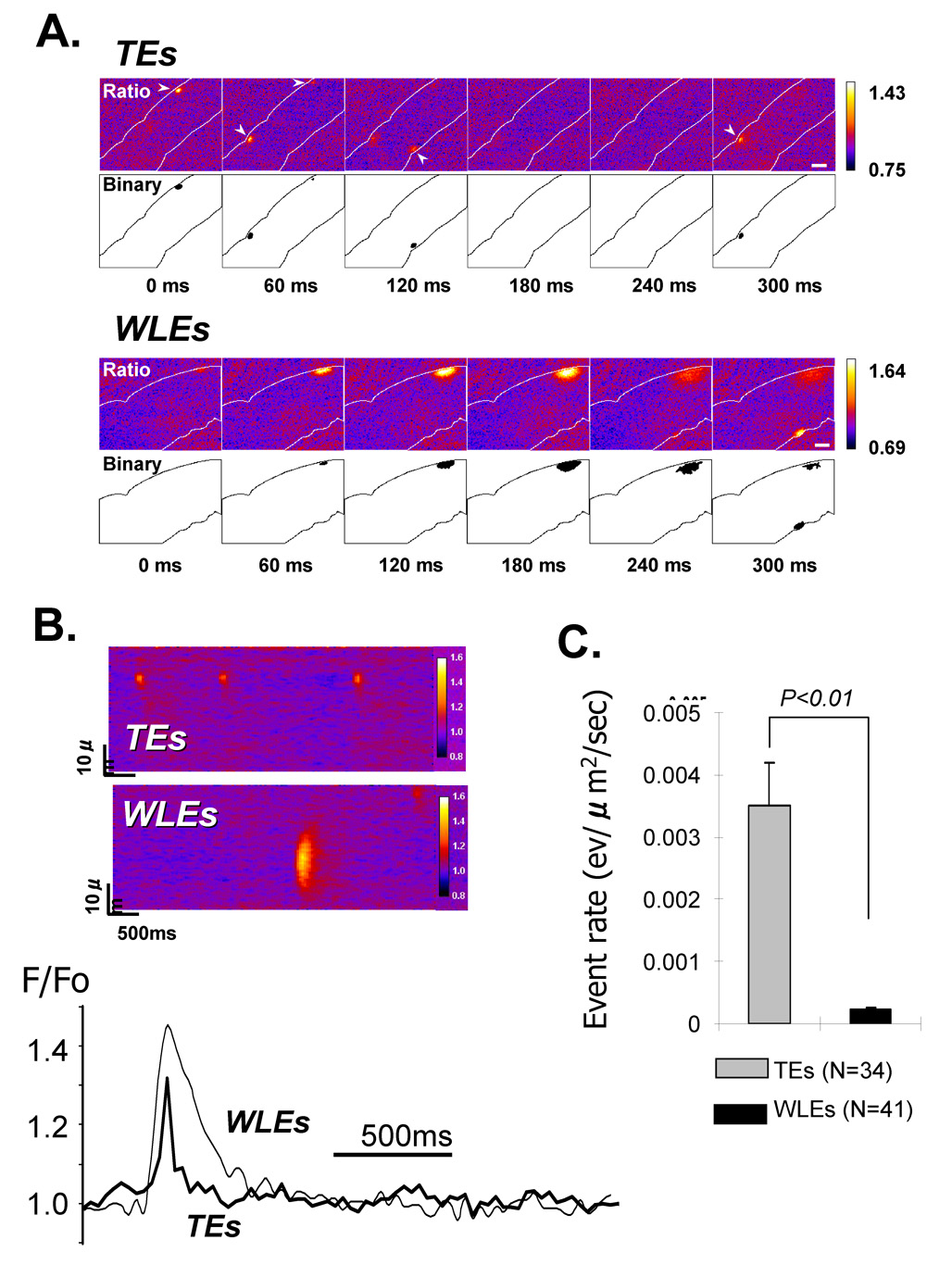
FIGURE 1. Typical (TEs) and Wide long lasting (WLEs) Ca2+ events in intact canine Purkinje cells.
A: Two sequences of consecutive serial frames showing respectively TEs (top) and WLEs (bottom); each series of F/Fo images (upper part) is accompanied by the corresponding binary representations (lower part); the computational procedure for the detection of non-propagating events was based on the selection of a F/Fo cutoff value; the equivalent ‘binary’ images were constructed by giving the values of ‘0’ or ‘1’ to pixels depending whether the intensity was below or above the cutoff. Note that the time scale is relative to t=0 of the first frame of the sequence with 60ms per frame; B: Quantitative comparison between TEs and WLEs using pseudo-line scan images: the reconstruction of line scans from 2D images of panel A enables comparisons between local F/Fo profiles of TEs and WLEs such as those shown in the lower panel. C: Compared to TEs, WLEs are less frequent in intact Purkinje cells. N is number of cells.
The 2D confocal approach taken here involves the use of a Nipkow spinning disk that reduces the level of light needed and thus increases the time one can sample the cell without obvious damage. In the case of these experiments, we chose 2 sec sampling of a confocal slice through the Purkinje cell at the level of the nucleus. While this allowed us to sample ALL subcellular sites in that plane at the same time (avg total 2D area of cells 4716±326 µm2, N=30), we may have not detected some events with our frame rate thus our frequency values may be an underestimation. All event rates were divided by area of cell viewed for analysis. Nevertheless what we did record and identify as an event was done with a program that showed a high degree of sensitivity and accuracy (see Figure S2). With most previous LSCM studies one only samples a very short line for brief periods of time. As such, WLEs may have occurred in atrial cells studied with LSCM, but they may have not been recorded since they were out of the view field in another subcellular region. Using our 2D system and overlaying the image of the Purkinje cell we were able to precisely locate WLEs.
In additional studies, we determined the characteristics of Ca2+ events in the absence and presence of pharmacological agents. For these studies, images were collected from groups of cells before (Control) and after 10min superfusion with an agent. We had previously determined that Ca2+ event characteristics in the absence of drug remained stable for more than 30mins (data not shown).
Immunocytochemistry
Freshly isolated Purkinje cells were prepared as in [9] and incubated overnight (at 4°C) with a rabbit polyclonal IP3R1 antibody (PA3-901, Affinity Bioreagents Inc; 1:200) and a mouse monoclonal RyR2 antibody (MA3-916, Affinity Bioreagents Inc; 1:500). Specificity of IP3R1 antibody was tested using the neutralizing peptide (PEP-019, Affinity Bioreagents Inc). The cells were then incubated for 1.5 hours with a mixture of Alexa Fluor 488-conjugated goat anti-rabbit IgG1, and Alexa Fluor 594-conjugated goat anti-mouse IgG1 (Molecular Probes). Cells were re-suspended in Citiflour Mounting Medium (Agar Scientific), plated onto microscope slides and examined using a Zeiss LSM 510 microscope set for dual excitation (100x, oil).
Statistics
Data are expressed as mean ± S.E. Comparisons were made using an unpaired Student t-test or ANOVA. Bonferroni was performed after ANOVA. N means number of cells, while n means number of events used in analysis. The difference was considered significant when P < 0.05.
RESULTS
Two types of non-propagating Ca2+ events
2D confocal imaging revealed two types of spontaneous non-propagating Ca2+ release events in canine Purkinje cells at physiologic [Ca2+]o (2mM) (Figure 1): 1) Most Ca2+ events (94%) had characteristics similar to Ca2+ sparks previously observed in Purkinje cells by laser scanning confocal microscopy [9]; named ‘Typical Events’ (TEs). In the present study, these Ca2+ release events were detected within a single frame (see arrowheads in Figure 1A) and showed amplitude and spatial extent of 1.20±0.003 F/Fo and 9.0±0.3 µm2 respectively; 2) A small fraction (6%) of non-propagating events was composed of local Ca2+ elevations which lasted over several frames (120–360 ms), still remained localized, failing to propagate within confocal plane. These events had a 6 fold greater spatial extent (51.0±3.9 µm2, p<0.01) and slightly larger amplitude (1.38±0.02 F/F0, p<0.01) than TEs; named ‘wide long lasting events’ (WLEs). WLEs occurred at a ~16 fold reduced frequency than TEs (TEs: 0.0035±0.0007 ev/µm2/sec, 1022 events, N=34 cells; WLEs: 0.00022±0.00005 ev/µm2/sec, 150 events, N=41 cells, P<0.01; Figure 2). Both types of Ca2+ events were detected in the cell. However TEs occurred 6 times (0.0015±0.0004 vs 0.0089±0.00011 ev/µm2/sec, p<0.01) and WLEs 4 times (0.00012±0.00004 vs 0.00052±0.00011 ev/µm2/sec, p<0.01) more frequently in the subsarcolemma(SSL) than in the core (Figure 2). For the purposes of this report, we will focus on WLEs.
FIGURE 2. Characteristics in Typical events(TE) (gray bars) and Wide long lasting events(WLE) (black bars) by subcellular regions (SSL (<5µm from sarcolemma) and Core).
A. Stacked Cell Images with representative Typical Ca2+ events in Core and SSL. Summary bar graphs(right) show the differences between Core and SSL in Event rate (ev/µm2/sec, left), Spatial extent (µm2, middle) and Amplitude(F/Fo, right) for Typical events. n = number of events. TEs: 34 cells; WLEs: 41 cells.
B. Stacked Cell Images with representative Wide long lasting Ca2+ events in Core(lower) and SSL(upper). Summary bar graphs(right) show the differences between Core and SSL in Event rate (ev/µm2/sec, left), Spatial extent(µm2, middle) and Amplitude (F/Fo, right) of WLE events.
WLEs occur in the perinuclear region and occurrence is augmented with IP3R activation
Imaging Ca2+ in 2D revealed that Core WLEs originated specifically near the nuclei (Figure 3). A specific procedure of image analysis was developed and applied to 39 cells to investigate the exact position of WLEs with respect to the nucleus. As shown in Figure 3, Core WLEs occurred predominantly along the longitudinal axis of the cell at both ends of the nucleus. Note none were found in the nucleus or in the nuclear envelope (Figure 3). The average nucleus area was 112um2, the distance was 18.2µm, and the angle was 12.6 degrees.
FIGURE 3. Location of Wide long lasting Ca2+ events in Core plane of Purkinje cells.
A; Typical 2D confocal scan of Purkinje cell through nuclear level. Shown are various measures made to determine subcellular locale of WLEs. B; A plot illustrating the location of Core WLEs in reference to nucleus. Each dot represents a specific data point. Note that WLEs occur along the longitudinal axis of cell and not in core area transverse to nucleus. Total number of normal Purkinje cells/events as indicated.
WLEs were similar to compound sparks(Ca2+ events with multiple peaks (see LSCM data Figure 1B of [9]) described in rabbit portal vein myocytes [13], and canine Purkinje cell aggregates [9]. In these previous studies, arguments presented suggested that IP3R Ca2+ events were initiators (modulators) of compound sparks. To examine whether IP3R could be involved in the WLEs, Purkinje cells were exposed to phenylephrine, an alpha-adrenergic agonist which increases the production of InP3 [14]. 10 min exposure to Phenylephrine(10µM) increased the WLE rate by 97% in the SSL (0.00130±0.00016 vs 0.00066±0.00016 (control) ev/sec/µm2, N=29, p<0.05) and 400% in the Core (0.00045±0.00011 vs 0.00009±0.00004 (control) ev/sec/µm2, N=29, p<0.01) while having no effect on the TE rate(Figure 4A), spatial extent and amplitude, or background fluorescence (Figure 4). WLE augmentations were abolished in presence of U-73122 (2µM), a PLC inhibitor, and 2APB (3µM). U73433 (2µM), an inactive analogue of U-73122, had no effect (Figure 4B). 2APB, in the absence of Phenylephrine, had no significant effect on WLE frequency(data not shown). It has been proposed that compound sparks could result from a sequence involving the initial activation of IP3Rs by InP3 and the subsequent trigger of Ca2+ release from adjacent RyRs [13]. Consistent with this hypothesis, we found that both ryanodine (100µM) and tetracaine (1mM) abolished WLEs in Purkinje cells (Figure 4B). Interestingly, on occasion a WLE gave rise to a Ca2+ wave (Figure 5) which propagated from the perinuclear region into the nucleus.
FIGURE 4. Effects of Pharmacological Agents on WLEs.
Pseudo-linescan images of Ca2+ events in control(absence of any drug), in presence of Phenylephrine (PE) alone(Panels A,B) PE plus U73122, PE plus U73433, PE plus 2APB. Panel A right illustrates the effects of PE alone on Typical event and Wide long lasting event rate in SSL and Core of normal Purkinje cells. Calibration F/Fo Bar below. Panel B Calibration F/Fo Bar to the right. Arrowheads indicate the occurrence of TEs. C. Summary bar graph showing the effects of various agents on the spontaneous WLE Ca2+ event rate (ev/µm2/sec) in Core (right) and SSL (left) of Purkinje cells. The height of each bar indicates average event rate value (+SEM). ( )=the number of cells. *P<0.05 vs Control, **P<0.01 vs Control. ns = no significant difference between Control and agent-treated cells.
FIGURE 5. Perinuclear Ca2+ event initiating a Cell wide Ca2+ wave in a normal Purkinje Cell.
A. Consecutive images showing the initiation of a Cell wide Ca2+ wave from a WLE in the perinuclear region (see upper inset) of an intact Purkinje cell. Middle panels show F/Fo images 1–8 in 2D representation. Right panel shows same images as pseudo 3D. Color bar in middle is calibration of F/Fo. Only on rare occasion did a WLE give rise to a Cell wide Ca2+ wave in normal canine Purkinje cells. B. Linear profiles from two ROIs during the propagation of Ca2+ from perinuclear area to inside nucleus.
Perinuclear localization of IP3R1 and RyR2
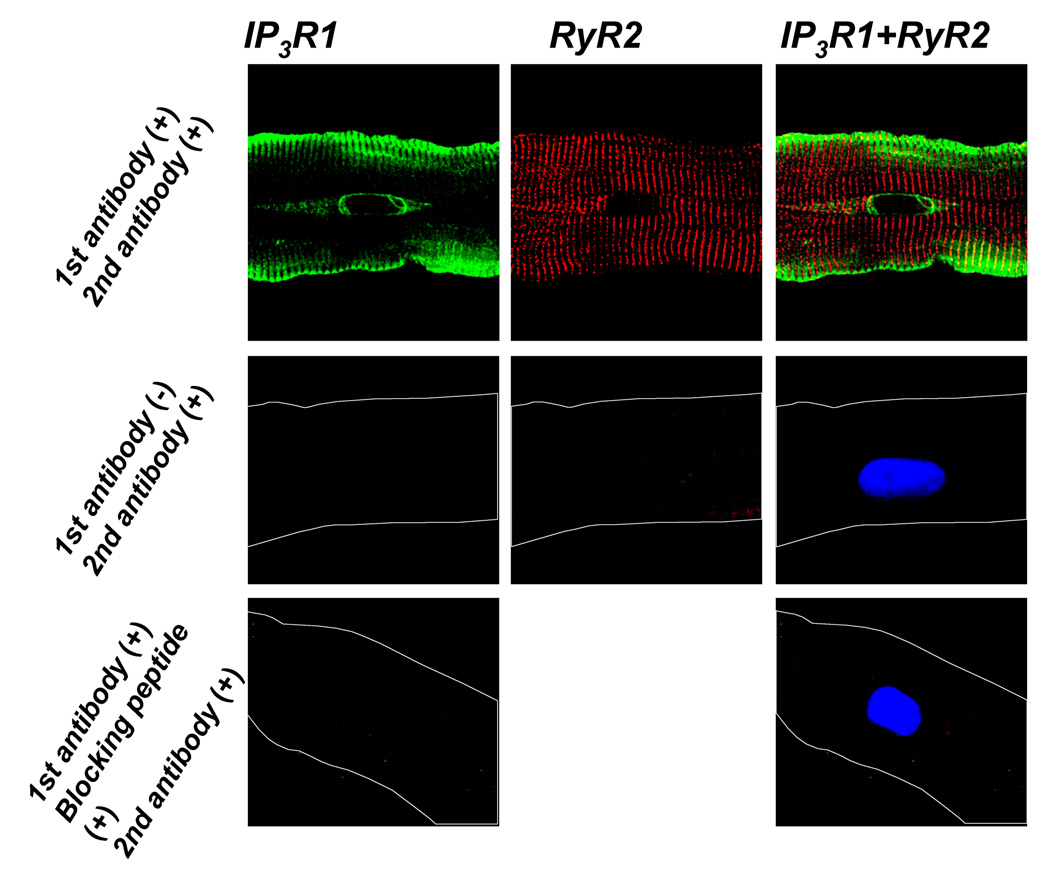
The observation that WLEs occur in the SSL and near the nucleus, and the fact that the frequency of WLEs is sensitive to modulators of IP3R strongly suggests the presence of IP3Rs in the Purkinje perinuclear region. We sought to determine whether IP3R1s are localized in the Core region by using a specific IP3R1 antibody. The origin of this antibody differs from that used in our previous work [9]. Here we found that IP3R1 was expressed in SSL as before [9], but also in the nuclear envelope and at the ends of the nucleus (Figure 6A). In the perinuclear region, the localization of IP3R1s matched remarkably the distribution of WLEs (see Figure 3B). Interestingly, IP3R1s and RyR2s localize at both ends of nucleus (Figure 6C). In fact the well known grid of RyRs overlays the puntate IP3R1 staining at the nuclear poles. Blocking peptides and secondary antibody alone images confirmed the existence of IP3Rs in this region (Figure 6C,D).
FIGURE 6. Co-localization of SR Ca2+ release channels, IP3R1(left) and RyR2(middle), in a normal Purkinje cell.
Overlap images are to the right. Costaining showing that IP3R1 (green) is located in the sarcolemma and perinuclear regions while RyR2 (red) is throughout the cell. As shown secondary antibody alone showed no signal (middle images) and primary IP3R1 antibody plus blocking peptide showed no staining (lower images). Lower images also stained for nucleus (blue,Dapi).
Perinuclear Ca2+ storage
The characteristics of WLEs differ by region (Figure 2). Core WLEs were less frequent, had lower amplitude but comparable spatial extent compared to SSL WLEs. Thus a number of additional Purkinje cells were loaded with Fluo-5N (10µM), which enabled us to monitor Ca2+ in the sarcoplasmic reticulum in specific cell regions. As expected, the Fluo-5N fluorescence was distributed following a ~ 2µm striation pattern which was consistent with the distribution of RyR2s and revealed the ‘sarcomeric’ arrangement of the SR in canine Purkinje cells. Rapid caffeine (10mM) exposure induced a uniform decrease in both the 2µm-periodic signal and the perinuclear signal confirming the presence of large releasable Ca2+ stores in the perinuclear region of the Purkinje cell (data not shown).
DISCUSSION
In canine Purkinje cells, previous investigation of Ca2+ release activity by laser scanning confocal microscopy revealed the presence of various types of spontaneous Ca2+ release events[9]. In addition to Ca2+ sparks, large non-propagating events with very complex spatial and time courses were detected in a restricted region (SSL) extending 5 µm under the sarcolemma. In the present study, the use of a confocal spinning disk technique revealed the presence of similar wide large Ca2+ release events (WLEs) in the perinuclear region.
Our previous computational approach showed that the various spatiotemporal shapes of local Ca2+ events evidenced in Purkinje cells were primarily due to different forms of Ca2+ release channels in the SR [9]. Since large non-propagating Ca2+ events were detected in the SSL where IP3R1s were detected by immunofluorescence, it was proposed that IP3R activation was involved in the production of these large local Ca2+ elevations. Consistent with these data, we report here that production of IP3 due to alpha-adrenergic stimulation notably increased the occurrence of WLEs in both the SSL and perinuclear Core regions with no effect on TEs. In addition, in the perinuclear region, the arrangement of IP3R1 at each end of the nucleus matched remarkably the subcellular sites of the occurrence of Core WLEs suggesting an involvement of IP3Rs in WLE generation. Alternative mechanisms for the alpha adrenergic increase in frequency of WLEs are unlikely. For instance, a phenylephrine IP3 evoked increase in Ca2+ influx (L type Ca current) into these Purkinje cells could increase SR content which in turn could alter WLE frequency. However this scenario is unlikely since the effects on WLEs studied here occurred after 10mins superfusion. If an increase in SR content had occurred, it would have been only transient until a new equilibrium was reached. Second since Phenylephrine has been shown to have both IP3 dependent and independent effects, it may be that IP3R independent effects may relate to the increase in frequency of WLEs. We think this is unlikely since WLE frequency increase induced by Phenylephrine was sensitive to both the PLC inhibitor U73122 and the putative IP3R blocker 2APB(Figure 4). By itself, the IP3R-evoked Ca2+ release is very small in the cardiac myocyte, however, the small Ca2+ release from IP3Rs could then be amplified by surrounding RyRs via CICR. These data differ from the findings in ventricular cells where IP3R2 isoform was found to be confined to the nuclear envelope [15] or even facing the inside of the nucleus [1,16].
Like Purkinje cells, atrial cells also express a high level of IP3Rs and IP3R-dependent Ca2+ release. Endothelin has been shown to enhance typical Ca2+ spark frequency [7,17,18] as well as action potential dependent Ca2+ transients [17,19] via an IP3R dependent mechanism. Recent work using saponin treated atrial cells and their isolated nuclei suggests that IP3 can directly cause a slow rise(mins) in nuclear Ca2+ [5]. In these latter studies, there was no mention of an enhancement of wide long lasting perinuclear events (cytosolic, not nuclear) as we describe here for Purkinje cells. Furthermore, there has not yet been a description as to the precise subcellular location of IP3R dependent Ca2+ releases in Purkinje cells.
The functional nature of WLEs identified here in intact cells could be similar to that of prolonged Ca2+ releases described by Yang and Steel in rat cells [20]. However, there are key differences. First, Purkinje cell WLEs are long lasting yet an event lasting several seconds was never observed while rat prolonged events were sometimes >2 secs in duration. Second, Yang and Steele [20] reported that the rat prolonged Ca2+ release events were inhibited by ryanodine yet not modified by IP3. In Purkinje cells, wide long lasting events are affected by ryanodine as well as by agents known to augment/antagonize IP3 (Figure 4). Finally, subcellular location of IP3R1 receptors in the perinuclear area of WLEs further suggests a role for IP3R in their generation.
Alpha adrenergic stimulation in Purkinje cells; functional effect?
It has been known for a long time that alpha agonist stimulation of normal canine Purkinje fibers can cause an increase in automaticity [21]. Further PLC alone when superfused over normal Purkinje fibers also induces an increase in automaticity. Since both ryanodine and verapamil were found to decrease this PLC-induced automaticity some have suggested a role for an increase in intracellular Ca2+ [22]. In our studies on intact Purkinje cells on rare occasion we observed a WLE giving rise to a cell wide Ca2+ wave(see Figure 5). This cell wide wave could under appropriate circumstances lead to nondriven electrical activity[10]. These results would be consistent with enhanced automaticity in the presence of phenylephrine.
Alpha adrenergic stimulation has been reported to affect L type Ca2+ currents but increases, decreases and no changes have been reported (eg.[23]). This suggests that alpha adrenergic stimulation of Ca2+ influx will depend on the dominance of the DAG/PKC pathway [24] and the one initiated by IP3 activation. Such could then contribute to systolic Ca2+ increases. Here we report that under the conditions of our study, phenylephrine causes no change in typical Ca2+ spark events (TEs) in the normal Purkinje cell.
It is more likely that the specific WLE augmentation in perinuclear areas is related to intracellular Ca2+ signaling that is dissociated from normal Purkinje cell EC coupling. Thus we speculate that perinuclear IP3Rs may have a different role from SSL IP3Rs. For some cardiac cells, IP3R is expressed predominantly in the nuclear envelope and may play a role in the gene transcription through Ca2+-dependent signaling pathways (excitation-transcription coupling) [1 3 25 26]. Perinuclear IP3Rs in Purkinje cells may also be related to gene transcription and cellular processes. The locations of perinuclear WLEs and IP3R are close to the nucleus (Figure 3). Thus, it is likely that perinuclear IP3R can mobilize large Ca2+ stores under neurohumoral stimuli in Purkinje cells.
Supplementary Material
Acknowledgments
Supported by grant HL58860 from the National Heart Lung and Blood Institute Bethesda, Maryland; grants 81150(BS), 135573&104907(HtK) from the Canadian Institute of Health Research and AHFMR grant (20040163), Canada (HtK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Wu X, Zhang T, Bossuyt J, Li X, McKinsey TA, Dedman JR, et al. Local InsP3-dependent perinuclear Ca2+ signaling in cardiac myocyte excitation-transcription coupling. J Clin Invest. 2006;116:675–682. doi: 10.1172/JCI27374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac type2 IP3 Receptor: Interaction and modulation by calcium/calmodulin dependent protein kinase II. J Biol Chem. 2005;280:15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 3.Luo D, Yang D, Lan X, Li K, Li X, Chen J, et al. Nuclear Ca2+ sparks and waves mediated by inositol 1,4,5-trisphosphate receptors in neonatal rat cardiomyocytes. Cell Calcium. 2008;43:165–174. doi: 10.1016/j.ceca.2007.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Domeier TL, Zima AV, Maxwell JT, Huke S, Mignery GA, Blatter LA. IP3 receptor-dependent Ca2+ release modulates excitation-contraction coupling in rabbit ventricular myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H596–H604. doi: 10.1152/ajpheart.01155.2007. [DOI] [PubMed] [Google Scholar]
- 5.Zima AV, Bare DJ, Mignery GA, Blatter LA. IP3-dependent nuclear Ca signaling in the mammalian heart. J Physiology. 2007;584:601–611. doi: 10.1113/jphysiol.2007.140731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorza L, Schiaffino S, Volpe P. Inositol 1,4,5-triphosphate receptor in heart: evidence for its concentration in Purkinje myocytes of the conduction system. J Cell Biol. 1993;121:345–352. doi: 10.1083/jcb.121.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lipp P, Laine M, Tovey SC, Burrell KM, Berridge MJ, Li W, et al. Functional InsP3 receptors that may modulate excitation-contraction coupling in the heart. Current Bio. 2000;10:939–942. doi: 10.1016/s0960-9822(00)00624-2. [DOI] [PubMed] [Google Scholar]
- 8.Boyden PA, Dun W, Barbhaiya C, Ter Keurs HEDJ. 2APB- and JTV519(K201) sensitive micro Ca2+ waves in arrhythmogenic Purkinje cells that survive in infarcted canine heart. Heart Rhythm. 2004;1:218–226. doi: 10.1016/j.hrthm.2004.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stuyvers BD, Dun W, Matkovich SJ, Sorrentino V, Boyden PA, Ter Keurs HEDJ. Ca2+ sparks and Ca2+ waves in Purkinje Cells: A Triple Layered System of Activation. Circ Res. 2005;97:35–43. doi: 10.1161/01.RES.0000173375.26489.fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyden PA, Pu J, Pinto JMB, Ter Keurs HEDJ. Ca2+ Transients and Ca2+ waves in Purkinje Cells. Role in action potential initiation. Circ Res. 2000;86:448–455. doi: 10.1161/01.res.86.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyden PA, Albala A, Dresdner K. Electrophysiology and ultrastructure of canine subendocardial Purkinje cells isolated from control and 24 hour infarcted hearts. Circ Res. 1989;65:955–970. doi: 10.1161/01.res.65.4.955. [DOI] [PubMed] [Google Scholar]
- 12.Boyden PA, Barbhaiya C, Lee T, Ter Keurs HEDJ. Nonuniform Ca2+ Transients in Arrhythmogenic Purkinje Cells that survive in the infarcted canine heart. Cardiovasc Res. 2003;57:681–693. doi: 10.1016/s0008-6363(02)00725-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP(3) receptors as a factor shaping spontaneous Ca(2+)-release events in rabbit portal vein myocytes. J Physiol. 2002;543(3):743–762. doi: 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Remus TP, Zima AV, Bossuyt J, Bare DJ, Martin JL, Blatter LA, et al. Biosensors to Measure Inositol 1,4,5-Trisphosphate Concentration in Living Cells with Spatiotemporal Resolution. J Biol Chem. 2006;281:608–616. doi: 10.1074/jbc.M509645200. [DOI] [PubMed] [Google Scholar]
- 15.Bare DJ, Kettlun CS, Liang M, Bers DM, Mignery GA. Cardiac Type 2 Inositol 1,4,5-Trisphosphate Receptor: INTERACTION AND MODULATION BY CALCIUM/CALMODULIN-DEPENDENT PROTEIN KINASE II. J Biol Chem. 2005;280:15912–15920. doi: 10.1074/jbc.M414212200. [DOI] [PubMed] [Google Scholar]
- 16.Proven A, Roderick HL, Conway SJ, Berridge MJ, Horton JK, Capper SJ, et al. Inositol 1,4,5-trisphosphate supports the arrhythmogenic action of endothelin-1 on ventricular cardiac myocytes. J Cell Sci. 2006;119:3363–3375. doi: 10.1242/jcs.03073. [DOI] [PubMed] [Google Scholar]
- 17.Zima AV, Blatter LA. Inositol-1,4,5-trisphosphate-dependent Ca2+ signaling inc at atrial excitation-contraction coupling and arrhythmias. J Physiol. 2004;555:607–615. doi: 10.1113/jphysiol.2003.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mackenzie L, Bootman MD, Laine M, Berridge MJ, Thuring J, Holmes A, et al. The role of inositol 1,4,5-trisphosphate receptors in Ca(2+) signaling and the generation of arrhythmias in rat atrial myocytes. J Physiol. 2002;541:395–409. doi: 10.1113/jphysiol.2001.013411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li X, Zima AV, Sheikh F, Blatter LA, Chen J. Endothelin 1 induced arrhythmogenic Ca2+ Signaling is abolished in atrial myocytes of Inositol 1,4,5 Trisphosphate (IP3) receptor type 2-deficient mice. Circ Res. 2005;96:1274–1281. doi: 10.1161/01.RES.0000172556.05576.4c. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Steele DS. Characteristics of Prolonged Ca2+ Release Events Associated With the Nuclei in Adult Cardiac Myocytes. Circ Res. 2005;96:82–90. doi: 10.1161/01.RES.0000151841.63705.01. [DOI] [PubMed] [Google Scholar]
- 21.Rosen MR, Steinberg SF, Chow Y, Bilezikian JP, Danilo P. Role of a pertussis toxin-sensitive protein in the modulation of canine Purkinje fiber automaticity. Circ Res. 1988;62:315–323. doi: 10.1161/01.res.62.2.315. [DOI] [PubMed] [Google Scholar]
- 22.Viamonte VM, Steinberg SF, Chow YK, Legato MJ, Robinson RB, Rosen MR. Phospholipase C modulates automaticity of canine cardiac Purkinje fibers. J Pharmacol Exp Ther. 1990;252:886–893. [PubMed] [Google Scholar]
- 23.Hartmann HA, Mazzocca NJ, Kleiman RB, Houser SR. Effects of phenylephrine on calcium current and contractility of feline ventricular myocytes. Am J Physiol. 1988;255:H1173–H1180. doi: 10.1152/ajpheart.1988.255.5.H1173. [DOI] [PubMed] [Google Scholar]
- 24.Tseng G-N, Boyden PA. Different effects of intracellular Ca2+ and a phorbol ester on the T and L types Ca2+ currents in ventricular and Purkinje cells. Am J Physiol. 1991;261:H364–H379. doi: 10.1152/ajpheart.1991.261.2.H364. [DOI] [PubMed] [Google Scholar]
- 25.Bootman MD, Harzheim D, Smyrnias I, Conway SJ, Roderick HL. Temporal changes in atrial EC-coupling during prolonged stimulation with endothelin-1. Cell Calcium. 2007;42:489–501. doi: 10.1016/j.ceca.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Kockskamper J, Seidlmayer L, Walther S, Hellenkamp K, Maier LS, Pieske B. Endothelin-1 enhances nuclear Ca2+ transients in atrial myocytes through Ins(1,4,5)P3-dependent Ca2+ release from perinuclear Ca2+ stores. J Cell Sci. 2008;121:186–195. doi: 10.1242/jcs.021386. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.