Abstract
Background
The increasing incidence of type 2 diabetes mellitus is attributed to increasing weight, reduced physical activity, and poor diet quality. Lifestyle change in patients with pre-diabetes can reduce progression to diabetes but this is difficult to achieve in practice.
Aim
To study the effectiveness of a lifestyle-change intervention for pre-diabetes in general practice.
Design of the study
A feasibility study.
Setting
A medium-sized general practice in Sheffield.
Method
Participants were 33 patients with pre-diabetes. The intervention was a 6-month delayed entry comparison of usual treatment with a lifestyle-change programme: increased exercise and diet change, either reduction in glycaemic load, or reduced-fat diet. The main outcome measures were weight, body mass index (BMI), waist circumference, fasting glucose, lipid profile, and nutrition.
Results
A statistically significant difference was observed between control and intervention groups in three markers for risk of progression to diabetes (weight (P<0.03), BMI (P<0.03), and waist circumference (P<0.001)). No significant differences in fasting glucose or lipid profiles were seen. Aggregated data showed a statistically non-significant improvement in all the measures of metabolic risk of progression to diabetes in the low-glycaemic-load group when compared with a low-fat-diet group (P>0.05). Significant total energy, fat, and carbohydrate intake reduction was achieved and maintained in both groups.
Conclusion
A lifestyle-change intervention feasibility programme for pre-diabetic patients was implemented in general clinical practice. The potential of a low-glycaemic-load diet to be more effective than a low-fat diet in promoting change in the features associated with progression to diabetes is worthy of further investigation.
Keywords: lifestyle, motivation, prediabetic state
INTRODUCTION
The incidence of type 2 diabetes mellitus has been rising steadily in the UK and elsewhere, and is attributed to increasing levels of obesity and decreased physical activity within the population.1 A third factor may be the quality of the diet in terms of the relative proportions of macronutrients (protein, fat, and carbohydrate) and the degree of processing and refinement of the carbohydrates consumed.2
In 1997 the American Diabetic Association modified its criteria for the diagnosis of diabetes and defined two pre-diabetic states: impaired fasting glycaemia (fasting blood glucose 6.1–6.9 mmol/l) and impaired glucose tolerance (2-hour post-load glucose 7.8–11.0 mmol/l), which individually and collectively constitute pre-diabetes.3 It is known that subjects with pre-diabetes are not only at considerably enhanced risk of developing diabetes compared with subjects whose glucose metabolism is normal,4 but also that the risk of developing the cardiovascular pathologies associated with diabetes begins to rise before the threshold for identifying diabetes is crossed.5 Patients with pre-diabetes are identifiable in the primary care setting by measuring their fasting plasma glucose or carrying out an oral glucose tolerance test.6
How this fits in
The incidence of type 2 diabetes mellitus is rising in the UK and elsewhere. Much of this rise is attributable to increasing weight and reduced levels of physical activity in the population. Lifestyle-change programmes in subjects with pre-diabetes can reduce progression to diabetes. This pilot study demonstrated that a diet, behavioural change, and activity programme implemented in UK general practice is feasible for pre-diabetic patients.
The National Service Framework for diabetes suggests that subjects at increased risk of developing diabetes should be offered advice and helped to reduce their risk of progression.7 The key lifestyle changes shown to reduce the risk of progression are weight loss and increased physical activity.2 It is possible that modification of carbohydrate consumption may be another independent variable, which may slow down or avoid progression to diabetes.8 The dietary carbohydrate modifications that show most clinical promise are those that reduce post-prandial glycaemic excursions. Carbohydrate foods vary in their potential for raising plasma glucose levels after ingestion. The glycaemic index ranks carbohydrates and is a measure of the effect on plasma glucose following ingestion of a measured amount of a carbohydrate food item.9 However, these foods are usually eaten in non-standard quantities and in combination with other foods that can modify glucose absorption. This has led to the introduction of the glycaemic load, which is a measure of the actual effect of a mixed meal or series of meals on the sum of post-prandial glucose excursions over a period of time. Reducing the glycaemic index of food choices and the glycaemic load of meals and recipe choices has been shown to reduce insulin resistance and may be an option with therapeutic potential in preventing the progression from pre-diabetes to diabetes.10,11
At least one meta-analysis and several large-scale randomised controlled trials comparing lifestyle intervention with usual management in patients with pre-diabetes have shown that lifestyle modification can prevent progression to diabetes.12,15 Both the US Diabetes Prevention Program and the Finnish Diabetes Prevention Study were able to reduce the progression of pre-diabetes to diabetes by 58% compared to a control group.13,14 Lifestyle change is difficult to achieve in clinical practice; however, incorporation of educational and motivational strategies into the patient encounter can improve the chance of effective change.16,17 Incorporating behavioural modification techniques during clinical encounters, such as motivational practice and the use of small group work, improves the chance of success.18
The purpose of the ISAIAH (Insulin Sensitivity And Its Applications to Health) project is twofold: first to compare a low-fat with a low-glycaemic-load themed programme, and secondly to determine an effective way to achieve lifestyle change in subjects with pre-diabetes to delay or prevent progression to diabetes, and to develop a package of interventions for use within the NHS in the UK. To this end, a pilot study was conducted to test the content, effectiveness, and feasibility of a dietary intervention programme which also included increased physical activity.
METHOD
The trial was conducted in a single general practice with 9200 patients. Over a 6-month period, all patients newly diagnosed within the practice with pre-diabetes, either on fasting plasma glucose or oral glucose tolerance testing criteria, performed as part of their usual medical care, were invited to join a lifestyle-change programme. All had a diagnosis of pre-diabetes, as defined by the American Diabetic Association,3 a body mass index (BMI) <25 (kg/m2), and were willing to engage in a 6-month intervention programme that focused on reducing either the glycaemic load or the fat content of their diet, increasing the amount of exercise they undertook, and motivational guidance. Patients already on other diet programmes were excluded, as were patients on medication that affected glucose metabolism, or who had medical conditions that would have precluded full involvement in all aspects of the programme.
The study was conducted in two 6-month phases: during the first phase of the study patients were randomised (using random number tables) to one of two intervention groups (low-fat diet or low-glycaemic-load diet). Subsequent patients recruited formed a control (delayed entry, phase 2) group and received usual management from their GP or nurse until the start of the second phase, which followed 6 months later, after the first phase programme had been concluded. Control patients were similarly randomised (low fat or low glycaemic load). Baseline biophysical examination (height, weight, BMI, waist circumference) and biochemical assessments (fasting plasma glucose, lipid profile, and triglyceride) were conducted and repeated at weeks 4, 12, and 26. Those patients who had to wait to join the programme (phase 2) had a second baseline examination. The biochemical and biometric measurements were collected by a research healthcare assistant unconnected with the practice. Food diaries were collected at baseline and at weeks 12 and 26.
To reduce ‘contamination of the message’, the education programme was conducted after consultation hours, and practice staff were not involved in its delivery or party to the results as they were generated. Participants were asked to keep the content of the evening meetings and the educational materials they received confidential.
The core content of both lifestyle-change programmes consisted of a series of 90-minute evening meetings split into three equal-duration sessions: nutritional education, aerobic exercise, and group motivational discussion (facilitated by a health professional using the principles of motivational practice).19 The first four evening meetings presented information on the natural history of diabetes, its risk factors and interventions, and lifestyle changes that have been shown to make a difference. Each meeting had a theme, for example ‘week 4, party time’, where the ‘dangers of snacks, festive foods, and eating out’ were discussed (Box 1). The educational content of the meetings was delivered by a nutritional scientist, the motivational group work by a psychologist, and the exercise session by an aerobics instructor. For these meetings to function successfully the group sizes were set at 8–10.
Box 1. A summary of the educational and behavioural aims of the six sessions of the intervention programmes.
The diet programmes included education on nutritional themes as well as education and encouragement in areas where motivation for change could be addressed. In addition, all subjects were encouraged to take part in 30 minutes of aerobic exercise at each session, during which time the instructor informally encouraged incorporation of activity into daily living. The three subject areas (nutritional, behavioural, and aerobic activity) were addressed in a group setting.
| Week number | Title of session | Nutritional aim |
|---|---|---|
| 1 | Our metabolic in heritance | How modern life sits uncomfortably with our evolved metabolism. What is diabetes and pre-diabetes? What's the problem? Can anything be done? |
| 2 | Wise up, and how to wise up | Discussion about the significance of energy and calories. Some meal choices analysed. Low-fat group looked at energy and fat in particular. Reduced-glycaemic-index group looked at glycaemia and insulin response. |
| 3 | Planning and strategy | How to read a food label. Advice on alcohol consumption. Can others help or hinder? |
| 4 | Party time | Dangerous meals: snacks festive foods, eating out and how to cope |
| 12 | General review and 26 | Brief review of course so far. Open discussion of problems encountered. Discussion of groups' positive experiences and sharing of tips and observations |
| All | Motivation sessions | Small group work on motivational decision making, including work on decision balance and cycle of change |
| All | Exercise sessions | 30 minutes of aerobic exercise each week, plus encouragement to do regular exercise between sessions |
The two programmes were identical with the exception of the nutritional advice given. The low-fat groups were given standard healthy-eating and dietary advice based on the UK Food Standard Agency's guidelines for a healthy diet.20 The low-glycaemic-load groups received a diet message based on the Harvard diet pyramid.21 Calorie-control and portion-size advice was given to both diet groups. The low-glycaemic-load group was instructed on the glycaemic index and the glycaemic load (Box 1).
A 4-day food diary was completed by all patients upon entry to the intervention programme phase of the trial, and at 12 and 26 weeks. Instruction on how to complete the diaries was given during the first evening. Completed diaries were analysed by a nutritional scientist and data extracted on the patients' average energy and macronutrient (protein, fat, and carbohydrate) ingestion. Macronutrient intake data were expressed in absolute values and as a percentage of total energy. Fats were subclassified as saturated, monounsaturated, polyunsaturated, and trans fats. Carbohydrates were subclassified as sugars and starches. The nutritional data were shared with the patients as they became available during the programme. The nutritionist provided brief written comments to accompany each food diary analysis.
Biophysical and biochemical data were also fed back to patients as they became available during the programme. Biophysical data (for example, weight, BMI, and waist circumference) were shared with patients at the time the measurements were taken. Biochemical data (lipid, fasting glucose, and triglyceride level) were presented to the patients individually in written form at the next patient contact. The biophysical, biochemical, and nutritional outcome variables were analysed using the analysis of covariance technique. Baseline value, control group, low-glycaemic-load programme, and low-fat-diet programme allocation were the independent variables.
RESULTS
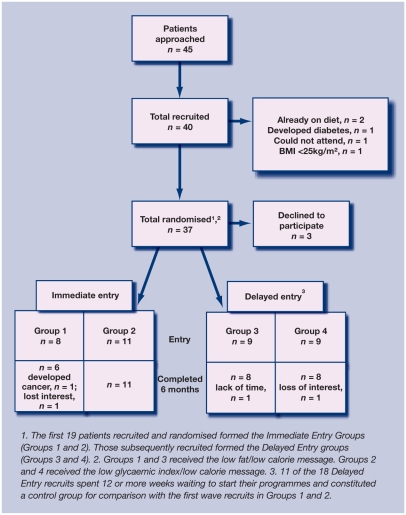
Details of patient recruitment, randomisation and retention, and demographic data are given in Figure 1 and Table 1. Of 45 patients approached, 37 were suitable for randomisation. The first 19 were randomised immediately (phase 1: groups 1, low fat and 2, low glycaemic load), while the subsequent 18 formed the delayed-randomisation (phase 2) group and were subsequently randomised to one of the two dietary intervention programmes (groups 3, low fat and 4, low glycaemic load). Of the delayed-entry group, 11 patients spent 12 or more weeks waiting (mean 21.1 weeks) to enter the study (delayed entry); their data were aggregated and used for comparison with the data from the initial 19 patients.
Figure 1.
Recruitment and retention flow chart.
Table 1.
Baseline demographic data.
| Intervention programme subjects (groups 1 & 2) versus control subjects | Low-calorie (groups 1&3) versus low-glycaemic-index diet (groups 2 & 4) subjects | |||
|---|---|---|---|---|
| Intervention subjects (groups 1 & 2) n = 8 + 11a | Control subjects n = 11a | Low-calorie subjects (groups 1 & 3) n = 8 + 9a | Low-glycaemic index subjects (groups 2 & 4) n = 11 + 9a | |
| Age, years | 62.3 (50–83) | 67.5 (56–85) | 67.8 (56–85) | 61 (50–83) |
| Sex, % male | 33 | 67 | 48 | 52 |
| Weight, kg | 85.5 (58.4–128.8) | 85.8 (73.1–96.8) | 82.9 (58.4–99.9) | 86.2 (70.3–108.2) |
| BMI, kg/m2b | 29.8 (23.1–43) | 29.5 (22.8–35.5) | 29.5 (23.1–37.8) | 29.8 (23.9–43) |
| Abdominal circumference, cm | 96 (84–121) | 102 (90–109) | 99 (84–114) | 98 (92–121) |
| Entry fasting glucose, mmol/lb | 6.1 (5.3–7.0) | 6.2 (5.8–6.5) | 6.3 (5.3–6.8) | 6.3 (5.0–7.0) |
| Entry total cholesterol, mmol/l | 5.5 (4.0–8.1) | 4.9 (3.5–7.3) | 5.0 (3.1–8.1) | 5.5 (5.0–7.0) |
| Entry triglyceride, mmol/l | 1.4 (0.6–3.7) | 1.3 (0.8–2.2) | 1.5 (0.6–3.7) | 1.2 (0.7–1.9) |
Figures presented in brackets are ranges.
Some subjects entered the trial with a BMI or fasting glucose level outside the entry criteria cut-off. This was because they had been identified with either a higher BMI or fasting glucose on recruitment but had made lifestyle changes before the formal baseline examination. In addition BMI and fasting glucose were lower in some control subjects when they entered the study in groups 3 or 4.
The biochemical and biophysical data are presented in Tables 2 and 3. Table 2 compares data from those subjects assigned to start the programme immediately (irrespective of which dietary programme they were assigned to) (intervention group) with those allocated to the delayed-entry phase. During this time the delayed-entry ‘controls’ received their usual management from their usual clinicians. The results show a trend towards a greater clinical effectiveness of a lifestyle-change programme compared to usual management over a 6-month period in general practice. Indeed, the reduction in waist circumference, BMI, and weight in those patients assigned to the programme was statistically significantly different from that of those assigned to usual management, despite the small numbers involved (Table 2).
Table 2.
A comparison of the change in surrogate markers for the risk of progression to type-2 diabetes over time in patients with pre-diabetes assigned either to usual management or a 6-month diet and lifestyle-change package (analysis of covarience).
| Change from baseline | |||
|---|---|---|---|
| Control group (n = 11)a, mean (SD) | Intervention group, n = 17, mean (SD) | Adjusted differenceb (95% CI), P-value | |
| Weight, kg | −0.30 (1.36) | −2.73 (3.15) | 2.4 (0.3 to 4.6), 0.03 |
| BMI, kg/m2 | −0.10 (0.47) | −0.91 (1.01) | 0.8 (0.1 to 1.5), 0.03 |
| Abdominal circumference, cm | −1.18 (2.27) | −6.01 (3.80) | 5.2 (2.6 to 7.8), <0.01 |
| Fasting glucose, mmol/l | 0.25 (0.67) | −0.02 (0.46) | 0.4 (0.0 to 0.7), 0.049 |
| Total cholesterol, mmol/l | −0.13 (0.55) | −0.33 (0.74) | 0.2 (−0.4 to 0.7), 0.53 |
| Total cholesterol/high-density lipoprotein ratioc | −0.50 (1.09) | −0.27 (0.66) | −0.4 (−1.5 to 0.9), 0.56 |
| Triglycerides, mmol/l | −0.14 (0.55) | −0.24 (0.75) | 0.05 (−0.3 to 0.4), 0.79 |
Average length of control phase 21.1 weeks (range 14-31 weeks).
Diference in the outcomes at 6 months between the two groups, adjusted for baseline.
Controls n = 4, intervention n = 9.
Table 3.
A comparison of the change in markers for the risk of progression from pre-diabetes to type 2 diabetes over time in patients assigned either to a low-fat/low-calorie diet programme with a low-glycaemic-index/low-calorie diet programme over 6 months (analysis of covarience).
| Change from baseline (start of intervention) | |||
|---|---|---|---|
| Low-fat diet, n = 13, mean (SD) | Low-glycaemic-index diet, n = 20, mean (SD) | Adjusted differenceb (95% CI), P-value | |
| Weight, kg | −3.21 (2.55) | −3.53 (3.34) | 0.3 (−2.0 to 2.6), 0.81 |
| BMI, kg/m2 | −1.14 (0.88) | −1.15 (1.04) | 0.03 (−0.7 to 0.8), 0.93 |
| Abdominal circumference, cm | −3.16 (3.60) | −5.35 (3.88) | 2.1 (−0.7 to 5.0), 0.14 |
| Fasting glucose, mmol/l | −0.11 (0.64) | −0.13 (0.42) | 0.15 (−0.1 to 0.4), 0.31 |
| Total cholesterol, mmol/l | −0.25 (0.61) | −0.41 (0.75) | 0.08 (−0.4 to 0.6), 0.74 |
| Total cholesterol/high-density lipoprotein ratiob | −0.02 (0.62) | −0.3(30.64) | 0.15 (−0.6 to 0.8), 0.66 |
| Triglycerides, mmol/l | −0.08 (0.86) | −0.20 (0.33) | 0.3 (−0.05 to 0.6), 0.09 |
aDifference in the outcomes at 6 months between the two groups, adjusted for baseline.
Low-fat diet n = 9, low-glycaemic-index diet n = 9.
Table 3 compares the aggregated data from the two low-fat groups (groups 1 and 3) with the two low-glycaemic-load groups (groups 2 and 4) after 6 months. Although analysis of the aggregated data results from the two diet programmes did not reveal a statistically significant difference between the two groups, all seven markers of metabolic health and risk of progression to diabetes showed a trend towards greater improvement in the low-glycaemic-load groups compared with the low-fat-diet groups.
Nutritional data are presented in Appendix 1. The two groups were broadly comparable at baseline. On average the low-glycaemic-load group consumed more calories each day than the low-fat group. The higher calorie intake was accounted for by a slightly higher protein and fat, especially saturated fat at baseline. In addition the low-glycaemic-load group consumed less carbohydrate (both sugars and starches) than the low-fat group.
Both groups reduced total energy intake by week 12 and reduced it further by week 26. Energy restriction was more successful in the low-glycaemic-load group. With the exception of trans fats, both groups managed to reduce all types of fat (and total fat) intake at both week 12 and 26. Both groups managed to reduce carbohydrate ingestion. The low-glycaemic-load group reduced both sugars and starches in absolute terms, although when expressed as a proportion of the reduced total calorific intake there was little change. Their change was achieved within the first 12 weeks and maintained at week 26. The low-fat group reduced their intake of starchy food but not of sugars. The low-fat group's changes only became statistically significant by week 26.
The qualitative data showed that patients commented that they found the programme ‘interesting and informative’. In addition, several commented that they found the group discussions helpful in deciding when and how to make lifestyle changes. The regular feedback of biochemical, biophysical, and especially the annotated nutritional analysis data was greatly valued and appeared to be a strong factor in assisting and maintaining motivation for change. Feedback from the staff delivering the programme was strongly positive. Preparation time for those leading the meetings was modest, although each meeting lasted 1.5 hours. Those involved more peripherally had no complaints and found the programme enjoyable to deliver and well received. As the programme was delivered by non-practice staff, there was no impact on the medical, nursing, or administrative function at the general practice location other than the contribution of the lead clinician.
DISCUSSION
Summary of main findings
This study has shown that it is feasible to deliver a lifestyle-intervention programme in UK general practice that aspires to reduce the progression of pre-diabetes mellitus to diabetes mellitus. Over a 6-month period it was found that patients on the lifestyle-change programme significantly reduced their abdominal circumference and lost more weight than patients given usual management advice for pre-diabetes. A statistically non-significant trend for benefit was seen in the biochemical markers of risk for diabetes (fasting glucose, cholesterol, and triglyceride). No differences were identified between patients randomised to each of the two diet themed programmes (low fat versus low glycaemic load); however, the change in each biochemical and biophysical marker of risk of diabetes was greater in the low-glycaemic-load groups.
Nutritional analysis using 4-day food diaries revealed that both the low-glycaemic-load and low-fat groups reduced caloric intake by 12 weeks, and sustained it until week 26. The low-fat group successfully reduced total fat intake, particularly saturated fats, reduced starch intake but increased slightly the proportion of sugars consumed during the programme. The low-glycaemic-load group reduced total energy intake at both 12 and 26 weeks. They too consumed less fat but the proportions of different fat types did not change. They reduced carbohydrate intake with a marginal reduction in the starch proportion of total energy. The low-glycaemic-load group achieved their changes more quickly than the low-fat group participants.
Informal qualitative feedback from the patients on the programme and the staff delivering it was positive.
Strengths and the limitations of the study
This project was conducted as a feasibility study for translational research. The study remit was the pragmatic testing of an intervention that might be feasible for clinical use in a primary care setting. Despite the small numbers of patients involved in the study, over a 6-month period patients randomised to the intervention programme showed a trend towards greater improvement in the biochemical, biophysical, and nutritional markers or risk of diabetes than those randomised to usual management. They also found the programme to be interesting, enjoyable, and worth the time they invested in attending.
Contamination of the nutritional messages between the intervention groups and the control/delayed-entry patients was a significant concern. The researchers sought to minimise this by requiring participants to keep the content of, and literature from, the programme confidential. In addition, although members of the practice staff were aware that the study was running, they were, apart from initial recruitment, not involved in the conduct of or party to the results from the study while it was running. Medical and nursing staff were aware of the research questions being addressed but the precise nutritional messages being given were not disclosed to them. During the course of the trial they continued to deliver their usual advice and management in all clinical encounters. Ancillary staff were aware that a study was being conducted, but had no knowledge of the details of the programme. In future studies, a cluster randomisation design will be employed across general practices to reduce such potential confounding factor still further.
Whether the improvement suggested here is ‘real’ or will be maintained over time is not known. A recent long-term follow-up report from the Finnish Diabetes Prevention Study,22 found that patients randomised to a lifestyle-change programme did significantly better than those receiving usual management, and that this improvement was still evident 3 years later. During their initial programme a 43% reduction in relative risk of diabetes was observed in the intervention group. Three years after the conclusion of the programme the relative risk reduction had fallen only slightly to 36% compared with the control patients.
The study was conducted in a single general practice in an affluent city suburb. Such patients might be expected to be more receptive to the educational message of the programme and more motivated to change. A key question raised by the study is how such an intervention would function in practices with differing educational, economic, and ethnic demographics. The programme was conducted and implemented by enthusiasts, and so another key question is whether the methodology of group work using motivational support with progress feedback would be acceptable to the wide range of other practices, especially with current resource constraints. GPs and practice nurses are known to be fearful of being given new unresourced tasks and consider patients to be resistant to change. In addition pre-diabetes is not always seen as a useful clinical entity.23,24
Comparison with existing literature
Type 2 diabetes and pre-diabetes are conditions whose genesis is strongly linked to lifestyle choices and which can be relatively easily identified in general practice.2,6 Lifestyle change has been shown to be effective in the delay or prevention of progression from pre-diabetes to diabetes.12,15 Low-glycaemic-index foods and low-glycaemic-load meals evoke a smaller post-prandial glycaemic response than higher-glycaemic-index/glycaemic-load foods and meals. In patients with insulin resistance, low-glycaemic-index/glycaemic-load diets have been shown to improve insulin sensitivity.25 Insulin resistance is one of the cardinal metabolic abnormalities in pre-diabetes,26 and improvement in peripheral tissue insulin sensitivity has been shown to reverse some of its features.27 The present feasibility study is consistent with the established literature, although the statistical significance of the findings needs validating with larger studies because the numbers of patients included in this study were small.
Implications for future research and clinical practice
Translating research trial interventions into effective programmes for use in regular clinical practice is a challenge that needs to be addressed in the UK.28 The ISAIAH project has been developed to implement the findings of research shown to delay or prevent the onset of pre-diabetes and to determine the feasibility of delivering it as an effective package for use in the primary care setting.
The education and motivational work components of the programme were conducted and delivered by nutritional scientists and psychologists. Given the limitation of resources, in terms of staff and costs in UK primary care, it is envisaged that the programme could be delivered by suitably trained nurses or healthcare assistants, and this will be developed in future studies.
Most of the patients recruited to this study had pre-diabetes defined by a raised fasting plasma glucose. A 2-hour oral glucose tolerance test would identify some patients with normal fasting glucose but impaired glucose tolerance. It is not clear what the implications may be for screening for pre-diabetes and diabetes with a fasting glucose compared with a glucose tolerance test.
That lifestyle change can be very effective in preventing type 2 diabetes is not in doubt. Developing a workable, sustainable, and cost-effective strategy for use across the NHS in primary care remains the goal, and more studies are urgently needed to test the clinical effectiveness of such interventions in everyday clinical practice.26
Acknowledgments
Coordination of the programme: Maria Platts; administration: Sharon Hart and Tom Barclay; contributing to the running of the course: Alix Bonnett (nutrition) and Nettie Goldsworthy (aerobics); biophysical assessments: Ann Wright; statistical advice: Professor Michael Campbell and Dr Cherk-Jenn Ng. Thanks also to the staff and patients of The Nethergreen Surgery, Sheffield, UK.
Appendix
Appendix 1.
A comparison of the total energy and macronutrient data obtained from 4-day food diaries collected at baseline, week 12, and week 26 of the programme.
| Low-fat groups | Low-glycaemic-load groups | |||||
|---|---|---|---|---|---|---|
| Average daily intake values | Baseline (%) | Week 12 (%) | Week 26 (%) | Baseline (%) | Week 12 (%) | Week 26 (%) |
| n | 14 | 14 | 11 | 20 | 20 | 17 |
| Energy, kCal | 1980 | 1791 | 1682.3 | 2038 | 1679a | 1649.3 |
| Protein, g | 88 | 88 | 89.7 | 92 | 81 | 82.4 |
| Total fat, g | 73 | 68 | 54.8b | 89c | 68a | 62.8 |
| SFA | 25 | 22 | 16.8b | 30 | 23a | 20.5 |
| MUFA | 24 | 23 | 19.0d | 30c | 23a | 20.7 |
| PUFA | 14 | 12 | 10.6 | 15 | 12 | 10.7 |
| Trans | 10 | 11 | 8.4 | 13 | 9a | 11.0 |
| Carbohydrate, g | 235 | 198e | 198.2 | 210 | 174a,c | 167.0 |
| Starch | 127 | 99e | 96.1 | 115 | 96a | 92.0 |
| Sugar | 97 | 92 | 98.0 | 93 | 76a,c | 72.8c |
MUFA = monounsaturated fatty acid. PUFA = polyunsaturated fatty acid. SFA = saturated fatty acid. Trans = trans fatty acid. Statistical analysis compared change within and between the groups over 26 weeks using paired t-test for within-group analyses and independent t-test for between-group analyses.
Week 12 data compared with baseline, P<0.0 01.
Week 12 data compared wit h week 26, P<0.01.
Diference between low-fat and low-glycaemic-load groups, P<0.05.
Week 12 data compared with week 26, P<0.05.
Week 12 data compared with baseline, P<0.05.
Appendix
Additional information can be found in the online version of this article
Funding body
Sheffield Health and Social Research Consortium and Sheffield South West Primary care Trust
Ethical approval
Ethical and governance approval for the study were obtained from the North Sheffield Local Research Ethics committee and the Sheffield Health and Social Research Consortium from whom funding was also received
Competing interests
Chris Barclay has received honoraria from GlaxoSmithKline for lecturing to practice nurses at educational meetings on pre-diabetes
Discuss this article
Contribute and read comments about this article on the Discussion Forum: http://www.rcgp.org.uk/bjgp-discuss
REFERENCES
- 1.Roberts S. Improving diabetesservice — the NSF two years on. London: Department of Health; 2005. Department of Health Diabetes Team. [Google Scholar]
- 2.Hu F, Manson JAE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345(11):790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 3.The American Diabetes Association. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 4.De Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA. 2001;285(16):2109–2113. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 5.Balkau B, Shipley M, Jarrett RJ, et al. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. Diabetes Care. 1998;21(3):360–367. doi: 10.2337/diacare.21.3.360. [DOI] [PubMed] [Google Scholar]
- 6.Greaves CJ, Stead JW, Hattersley AT, et al. A simple pragmatic system for detecting new cases of type 2 diabetes and IFG. Fam Pract. 2004;21(1):57–62. doi: 10.1093/fampra/cmh113. [DOI] [PubMed] [Google Scholar]
- 7.Department of Health. National service framework for diabetes. London: Department of Health; 2001. [Google Scholar]
- 8.Ludwig DS. The glycaemic index: physiological mechanisms relating to obesity, diabetes and cardiovascular disease. JAMA. 2002;287(18):2414–2423. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 9.Jenkins DJA, Ghafari H, Wolever TMS, et al. Relationship between rate of digestion of foods and post-prandial glycemia. Diabetologia. 1982;22(6):450–455. doi: 10.1007/BF00282589. [DOI] [PubMed] [Google Scholar]
- 10.Schulze M, Liu S, Rimm EB, et al. Glycemic index, glycaemic load, and dietary fibre intake and incidence of type 2 diabetes in younger and middle-aged women. Am J Clin Nutr. 2004;80(2):348–356. doi: 10.1093/ajcn/80.2.348. [DOI] [PubMed] [Google Scholar]
- 11.Willett W, Manson J, Liu S. Glycemic index, glycemic load, and the risk of type 2 diabetes. Am J Clin Nutr. 2002;76(suppl):274S–80S. doi: 10.1093/ajcn/76/1.274S. [DOI] [PubMed] [Google Scholar]
- 12.Norris SL, Zhang X, Avenell A, et al. Long term effectiveness of weight-loss interventions in adults with Pre-diabetes. Am J Prev Med. 2005;28(1):126–139. doi: 10.1016/j.amepre.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Knowler WC, Barrett-Connor E, Fowler SE, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 15.Pan X-R, Li G-W, Wang J-X, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance: the Da Qing IGT diabetes study. Diabetes Care. 1997;20(4):537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 16.Yamaoka K, Tango T. Efficacy of lifestyle education to prevent type 2 diabetes: a meta-analysis of randomized controlled trials. Diabetes Care. 2005;28(11):2780–2786. doi: 10.2337/diacare.28.11.2780. [DOI] [PubMed] [Google Scholar]
- 17.Rubak S, Sandbaek A, Lauritzen T, et al. An education and training course in motivational interviewing influence: GPs' professional behaviour — ADDITION Denmark. Br J Gen Pract. 2006;56(527):429–436. [PMC free article] [PubMed] [Google Scholar]
- 18.Vasilaki EI, Hosier SG, Cox WM. The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol Alcohol. 2006;41(3):328–335. doi: 10.1093/alcalc/agl016. [DOI] [PubMed] [Google Scholar]
- 19.Motivate Healthy Habits. The concept of motivational practice. http://motivatehealthyhabits.com/htm1/for-professionals/mp-concept-paper.html (accessed 11 Jul 2008)
- 20.Food Standards Agency. London: Food Standards Agency; 2000. Healthy diet. http://www.eatwell.gov.uk/healthydiet/eighttipssection/8tips/ (accessed 12 May 2008) [Google Scholar]
- 21.Harvard School of Public Health The nutrition source: healthy eating pyramid. http://www.hsph.harvard.edu/nutritionsource/pyramids.html (accessed 12 May 2008)
- 22.Lindstrom J, Ilanne-Parikka P, Peltonen M, et al. Sustained reduction in the incidence of type 2 diabetes by lifestyle intervention: follow-up of the Finnish Diabetes Prevention Study. Lancet. 2006;368(9548):1673–1679. doi: 10.1016/S0140-6736(06)69701-8. [DOI] [PubMed] [Google Scholar]
- 23.Williams R, Rapport F, Elwyn G, et al. The prevention of type 2 diabetes: general practitioner and practice nurse opinions. Br J Gen Pract. 2004;54(504):531–535. [PMC free article] [PubMed] [Google Scholar]
- 24.Wylie G, Hungin APS, Neely J. Impaired glucose tolerance: qualitative and quantitative study of general practitioners' knowledge and perception. BMJ. 2002;324(7347):1190–1192. doi: 10.1136/bmj.324.7347.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Frost G, Leeds A, Magara R, Dornhorts A. Insulin sensitivity in women at risk of coronary heart disease and the effect of a low glycaemic diet. Metabolism. 1998;47:1245–1251. doi: 10.1016/s0026-0495(98)90331-6. [DOI] [PubMed] [Google Scholar]
- 26.World Health Organization. Definition, diagnosis and classification of diabetes mellitus and its complications. Report of a WHO consultation. Geneva: WHO; 1999. [Google Scholar]
- 27.Jarvi AE, Karlstrom BE, Granfeldt YE, et al. Improved glycaemic control and lipid profile and normolised fibrinolytic activity on a low glycaemic index diet in type 2 diabetic patients. Diabetes Care. 1999;22(1):10–18. doi: 10.2337/diacare.22.1.10. [DOI] [PubMed] [Google Scholar]
- 28.Heneghan C, Thompson M, Perera R. Prevention of diabetes [editorial] BMJ. 2006;333(7572):764–765. doi: 10.1136/bmj.38996.709340.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]