Abstract
Consolidation theory assumes that as time passes, some memories are strengthened and become resistant to change while other memories are weakened and forgotten. Recent demonstrations that implicit, or procedural, memories are retrieved more efficiently after learning and retention are consistent with the idea that these particular memory traces have strengthened with time, and therefore may be accessed faster. However, it is not clear whether the process of explicit memory retrieval also becomes more efficient with time. In two experiments, we explored 1) how much time is required for retrieval of separate explicit and implicit components of hippocampal-dependent visuomotor associative memories after variable retention intervals, and 2) how the explicit and implicit processing times change when the associations are rehearsed after initial retrieval. We found that after learning and retention, explicit and implicit processing times diverged: 1) the time taken to retrieve successfully the explicit component increased relative to a pre-retention baseline but, after spaced rehearsal, decreased, although not to a level significantly below that obtained at the end of learning, and 2) the implicit, or procedural, component processing times continued to gradually decrease after retention, and with continued rehearsal, reached a level significantly below the pre-retention baseline. We conclude that the observed divergence in post-retention reaction times suggests that explicit and implicit memory systems may reorganize differently after learning, and that as a consequence, different amounts of processing time may be required for retrieval of these different memory components.
Keywords: Consolidation, Memory Retrieval, Reaction Times, Conditional Associative Learning, Hippocampus
1. Introduction
Consolidation theory assumes that after encoding, some memories strengthen, become resistant to change, and are made permanent, while others gradually weaken and are forgotten (McGaugh, 2000). The idea of a fixed, consolidated memory trace has become the dominant view in neurobiology: neural connections are formed at encoding, thereby causing the memory trace either to strengthen or weaken during a retention interval. Yet it is not understood how applicable the standard model of consolidation is to different forms of memory, nor how a neurobiological consolidation process would affect the speed of memory recall. The purpose of the present study was to explore changes in the speed with which two different components of memory – one dependent and one not dependent on deliberate recollection of information acquired during a previous session - may be retrieved after learning and retention.
Behavioral evidence for faster post-retention processing comes from studies of procedural learning. These procedural, or implicit, memories, which usually require motor movements or visual judgments, may be executed faster after a single learning session, usually followed by a period of sleep. Speeded performance has been found for motor sequence learning (Karni et al., 1995; Korman et al., 2003; Ungerleider et al., 2002), visual discrimination (Censor et al., 2006; Stickgold et al., 2000a; Stickgold et al., 2000b), and object, letter, and word recognition (Hauptmann and Karni, 2002; Hauptmann et al., 2005; Mitchell and Brown, 1988; van Turennout et al., 2000). While it is not clear whether such speed improvements exist on the first trial of task performance after the retention interval, it is documented that after multiple post-retention trials reaction times for procedural memory tasks is significantly faster than levels obtained during the initial learning session.
Implicit memory has traditionally been defined as facilitation of task performance attributable to information acquired during a previous session (Schacter et al., 1993). It is a type of memory that is able to be recalled without deliberate recollection. It may be contrasted with explicit memory, in which deliberate recollection of information acquired during a previous study period is required. Implicit memory has typically been probed by measuring how fast it takes to perform actions like mirror reading (Ofen-Noy et al., 2003), to complete graphemic word fragments (Graf and Schacter, 1985; Schacter and Graf, 1989), to name visual objects (van Turennout et al., 2003; Wheeldon and Monsell, 1992), or to complete motor sequences (Albouy et al., 2006; Muller et al., 2002; Perez et al., 2007) without subjects explicitly recalling the sequence. By contrast, one way to assess explicit memory is to ask subjects whether they remember particular stimuli from a set presented during a previous session (Musen and Treisman, 1990).
Many everyday tasks contain both implicit and explicit components. In visuomotor associative learning, subjects learn the relationship between a stimulus and an action. Later, they must recall the relationship and perform an action to make a response. For example one may learn that when one sees a red light, a left pedal press is required, but when one sees a green light, a right pedal press is required. During and after learning, whenever one sees a colored light, the time taken to decide to act and the time taken to complete the act of pedal pressing may be measured separately. For example, the time to decide what to do following presentation of a colored light represents the duration needed to retrieve the explicit memory of the relationship between stimulus and action, while the time it takes to make the motor movement of pedal pressing represents the duration needed to carry out the procedural memory of the action, in this case pressing the pedal.
In the present study, we defined explicit memory as the learned association between a picture and a place. The speed of explicit memory retrieval was measured by the time taken to initiate a motor movement in the correct direction of the place after a picture was presented. The validity of this measure is based on the idea that the subject will wait to signal a choice until he or she has decided the correct direction in which the choice should be. The implicit memory was defined as a procedural memory for a learned motor action, specifically the memory of the motor commands needed to move the manipulandum, and thereby the cursor, to the correct place. The speed of the implicit memory retrieval was measured by the time taken to complete a motor movement once it had been initiated. Subject accuracy in recalling the explicit component depends critically on remembering the correct association learned in one of several past episodes, or learning sessions. But subject accuracy in retrieving the implicit component depends on remembering the skill, like riding a bike, which is not dependent on remembering information learned in a specific session. The validity of the implicit measure is based on the idea that learned skills are able to be expressed without deliberate recollection of a past experience. Most importantly, the explicit and implicit components are by definition separable in the present experiments: a subject may incorrectly retrieve the association (explicit component) and then initiate cursor movement in the wrong direction, but may still show speed improvements in completing the movement to the wrong location (implicit component).
The main question addressed by the present study is how processing times for explicit and implicit memory components of a visuomotor associative task change after learning and retention. Answering this question may be important for understanding of how brain systems underlying implicit and explicit memory change during consolidation, the period of time following learning when memories are supposedly made durable. If, after learning and retention, more or less behavioral processing time is required for the explicit or implicit task components, this could reflect additional or fewer neural computations underlying successful memory retrieval. Since existing theories of memory consolidation do not make specific predictions about how reaction times should change following learning, the present study was designed to explore the direction and magnitude of these explicit and implicit processing time changes in two separate experiments.
While previous studies suggest there are post-learning efficiency gains for implicit memories, there is far less evidence that efficiency gains may be realized after learning and retention for explicit memory retrieval. One previous behavioral study has reported slower reaction times after retention for an explicit yes/no recognition paradigm (Reber et al., 1997), but no attempt was made by the authors to separate retrieval from the ensuing completion of the motor program to signal a response, nor is it clear whether performance on such a visual recognition memory tasks depends on an intact hippocampus (Taylor et al., 2007), a brain structure thought critical for encoding explicit memories of episodes and their contexts. Since it is unclear whether visual recognition memory tasks require the hippocampus and visual recognition may not be a good model for studying episodic memory, we used a variant of the conditional visuomotor association task that has been employed extensively in primate behavioral, lesion, and neural recording studies, and is considered a simple model for human episodic memory (Brasted et al., 2002; Brasted et al., 2003; Murray and Wise, 1996). An additional finding from these monkey lesions studies is that after learning associations through extensive training, the hippocampus is no longer required for retrieval of arbitrary visuomotor associations (Brasted et al., 2003), suggesting that, after a period of time during which the associations are not practiced and presumably consolidated, the memory trace may be dependent on a different set or subset of neural systems. It is not clear how much training is needed for retrieval of the associations to become automatic, but it is likely in primates to be on the order of dozens to hundreds of trials per association over multiple days.
Two experiments were included in the present study. The first experiment was designed to answer specifically how the response times for explicit and implicit components of a visuomotor associative task change during learning and after variable retention intervals. The second experiment was designed to determine if the processing time for the explicit component was related to associative retrieval, and to rule out the effects of forgetting on post-retention response time changes by spacing practice of the associations over several sessions. In both experiments, processing times for the explicit and implicit components were measured separately by computing a decision time for the explicit component and an action completion time for the implicit component. During the task, participants used a manipulandum to update the x- and y-axis position of cursor on a computer monitor. After guiding the cursor to a central fixation box, subjects were required to fixate by holding the manipulandum completely still, thereby stopping all cursor movement. Only after fixation was maintained for a pre-determined amount of time did the visual stimulus appear. A decision time, reflecting the time needed to retrieve the explicit associative memory component between picture and spatial location, was computed as the time elapsed from stimulus presentation until the first movement of the manipulandum was detected to move the cursor in direction of correct spatial goal location. The action completion time, reflecting the time needed to affect the implicit memory of the motor program to signal a response, was computed as the time elapsed after the first movement of the manipulandum was detected until the cursor reached the correct spatial goal location.
Assuming the subject waits to make a choice until the memory of the associative relationship is recalled, the decision time reflects the time required for retrieval of the association from memory, while the action completion time reflects the time required to make the fine motor movements necessary to guide the cursor from the central fixation region to the peripheral spatial goal location. Since no additional practice occurred during each retention interval, post-retention changes in the decision and action completion times were expected to reflect changes in neural processing time as a function of the consolidation of the explicit and implicit memory components. Decision and action completion times were measured on each trial during learning and during post-retention testing, thereby allowing assessment of implicit and explicit processing time changes for the same associations within individual subjects.
Following each retention interval, the most significant decision time changes were predicted to occur on the first post-retention trial, since this was the first experimentally-cued retrieval of the memory since the end of learning. Subjects’ accuracy and response times on the first trial were expected to reflect changes in the time needed to retrieve the explicit component of the associative memory for two reasons: 1) the first trial involves recall from long term memory, and since feedback was given on each trial about performance accuracy, subsequent trials could be influenced by practice effects, and 2) after the first post-retention trial, subjects may have used a short-term memory strategy of remembering the answer to a past trial to guide their responses on subsequent trials. However, in the present experiments, several post-retention trials were obtained for each association to determine whether additional practice subsequent to the first long-term retrieval attempt was required to obtain significant changes in decision time relative to levels reached at the end of encoding. Action completion times were predicted to change more gradually based on the previously reviewed behavioral studies of implicit memory which show between-session efficiency gains that appear to evolve over several post-retention trials. To control for the effects of forgetting on decision time changes, multiple post-retention trials, including spaced rehearsal of associations, were added in experiment two. These additional trials allowed for additional practice of the associations so that they would be less likely to be forgotten on subsequent tests of recall.
2. Results
Experiment 1
Learning on Day 1
For the set of all 24 stimulus-associations, participants performed on average significantly above chance (25%) by the second trial of learning (70.31%, SD=15.21), and made on average 95.31% (+/−1.10) accurate responses by the last (13th) trial of Day 1 learning.
Recall on Day 2
Participants’ average accuracy significantly exceeded chance on the first post-retention recall trial of Day 2 (81.25% +/−2.61), and reached (96.88% +/−0.92) by the last (6th) post-retention trial of Day 2.
Post-Retention Changes in Explicit and Implicit Processing Times
Explicit processing time measured by decision times −38.75 to 977.96 ms, t(30)=4.13, p<0.001, black lines in Figure 2) on the first post-retention trial of Day 2 compared to the level reached at the end of Day 1 learning (796.61 ms +/−23.62). Average post-retention decision times for incorrect trials were significantly longer (1377.94 ms +/− 214.75, t(29)=2.78, p<0.01) than on successful retrieval attempts. For subsequent post-retention trials 2 through 6, decision times were faster, but did not reach a level significantly faster than the level obtained at the end of Day 1 learning (751.71 ms +/− 12.37, p=0.09).
Figure 2.

Decision times (black lines) for correct trials increased significantly (* p<0.001) on the first retrieval trial after 24 hours of retention, while action completion times (light gray lines) decreased significantly from pre-retention levels for subsequent rehearsal trials (trials 2–6, p<0.05). Decision times decreased also on trials 2–6, but not to a level significantly different from the end of pre-retention session.
Implicit processing time measured as action completion times for successfully recalled associations did not differ significantly on the first post-retention trial (502.97 ms +/− 25.08) compared to the level reached at the end of Day 1 learning (538.33 ms +/− 35.36), but did become significantly faster than pre-retention levels on post-retention trials 2 through 6 (460.68 ms +/− 24.16, p<0.05, light gray lines in Figure 2). Action completion times on incorrect trials were longer than for successful retrieval trials (531.20 ms +/− 18.43, t(29)=2.69, p=0.01).
Experiment 2
Learning on Day 1
Subjects performed near chance on the first trial of each stimulus-association, but reached accuracy of 96.25% (+− 2.06 S.E.M.) by the 10th and last trial repetition of the learning session; subjects performed close to 100% accuracy on all repetitions of instructed cue trials (98.99% +/− 0.26).
Post-Retention Recall
During the post-retention session 24 hours after learning, all subjects performed at 100% accuracy on instructed cue trials. Subjects recalled as many associations on the first trial after 24 hours of retention as on the last trial of pre-retention learning (diamond in Figure 3a, 94.12% (+/−2.35) vs. 98.13% (+/−1.88), t(31)=1.349, p=0.094) when tested on a subset of 10 of the 30 associations learned the previous day.
Figure 3.

Decision times for successfully retrieved associations increase after retention in both the absence and presence of forgetting for previously learned stimulus sets. A single group of participants (n=17) learned three different sets of 10 arbitrary associations to criterion (>95%). (a) Accuracy was not significantly different when tested on a set after 24 hours of retention (◇ p=0.08), but significant forgetting occurred from the end of learning for sets tested after 1 week and 2 weeks of retention (* p<0.01). (b) Decision times for the first post-retention trial increased significantly after 24 hours, after 1 week, and after 2 weeks of retention relative to the last pre-retention trial. There was no significant difference in accuracy between the sets tested after 1 and 2 weeks of retention, as well as no difference between decision times for sets tested after 1 and 2 weeks.
Subjects recalled a significantly above chance proportion of the subset of 10 associations learned one week earlier (58.24% +/− 5.54, chance=25%), and the subset of 10 associations learned two weeks earlier (50.83% +/− 3.76, chance=25%). Subjects exhibited significant forgetting after 1 week (asterix in Figure 3a, accuracy on first post-retention trial 58.24% +/− 5.54 vs. accuracy on last pre-retention trial 95.63% +/−2.73, t(31)=6.087, p<0.0001), but the extent of forgetting was not significantly different at two weeks compared to 1 week (58.24% +/−5.54 on first trial after 1 week retention vs. 50.83% (+/−3.76 on first trial after 2 weeks retention, t(27)=1.003, p=0.325).
Post-Retention Processing Time Changes for Associative and Instructed Cue Trials
There was no change in instructed cue decision times at 24 hours compared to learning (556.91 ms +/− 13.86 vs. 578.50 ms +/−15.62, t(32)=1.06, p=0.15), but subjects completed instructed cue actions significantly faster (424.00 +/− 18.32 vs. 487.41 +/− 29.87, t(32)=1.87, p<0.05) after 24 hours compared to the pre-retention session.
While stimulus-association action completion times were significantly faster after 24 hours compared to learning (463.07 +/− 11.53 vs. 496.04 +/− 9.88, p<0.05), decision times increased significantly from the level reached on the last trial of learning on the first trial after retention (asterix in Figure 3b, 651.47 +/− 17.89 to 836.88 +/− 34.92) and, despite two more rehearsal trials after initial retrieval, did not decrease below the level reached at the end of learning (669.53 +/− 34.92 vs. 651.47 +/− 17.89).
Decision times for successfully recalled associations increased significantly after both 1 week and 2 weeks compared to pre-retention learning (from 651.47 ms (+/−17.89) to 836.88 ms (+/− 34.92), t(30)=4.627, p<0.0001 after 1 week and from 728.13 ms (+/−36.39) to 1183.35 ms (+/− 56.44), t(30)=6.674, p<0.0001 after two weeks). Decision times for associations successfully remembered after 1 week were significantly greater than decision times for associations successfully remembered after 24 hours (1183.35 ms (+/− 56.44) vs. 836.88 ms (+/− 34.92), t(32)=5.381, p<0.0001), but there was no difference between 1 and 2 week decision times (1183.35 ms (+/−56.44) vs. 1134.71 ms (+/− 50.52), t(27)=0.596, p=0.556).
Decision time changes were examined by plotting pdfs of correct decision times for stimulus-associations at pre-retention learning, and for the same associations answered correctly on the first post-retention trial. A significant rightward shift in the location of the 24 hour post-retention pdf along the time axis (x-axis) was noted (Figure 4 blue vs. gray lines, KS-test=0.29, p<0.0001). The 1- and 2-week post-retention pdfs were shifted even more along the x-axis and differed significantly from the 24 hour post-retention pdf (1-week KS-test=0.54, p<0.0001; 2-week KS-test=0.55, p<0.0001), but exhibited stabilization, as there was no significant difference between 1- and 2- week peak locations (Figure 4 green vs. red lines, KS-test=0.09, p=0.912).
Figure 4.

Decision time changes were examined by computing probability distribution functions (pdf) for decision times on the first successful retrieval trial of each association. The pdf after 24 hours of retention (peak amplitude at 726.9 ms) is skewed to the right and more kurtotic relative to the pdf peak computed from the last correct pre-retention trial (653.65 ms). Skewness and kurtosis increase after 1 week (to 1018.15 ms) and 2 weeks (to 1009.11 ms) of retention relative to both the last pre-retention trial pdf and the 24 hour pdf, but a statistical comparison of the 1 week and 2 week pdfs showed no significant difference, ks test=0.091, p=0.9127).
Throughout the learning and post-retention sessions, there was a consistent large magnitude difference between correct and incorrect retrieval decision times, with incorrect trials overall always taking much longer (Table 1). Changes in decision times within and between sessions also differed between correct and incorrect trials. While decision times for correct trials decreased from the beginning to the end of learning, they increased for incorrect trials (Table 1, day 1 learning start vs. day 1 learning end). Decision times for correct trials increased on the first post-retention retrieval trial relative to the end of day 1 learning, but they decreased for incorrect trials (Table 1, 24 hours, 1 week, and 2 weeks post retention vs. day 1 learning end). Finally, while decision times decreased to levels similar to the end of learning for correct trials, they did not decrease for incorrect trials (Table 1, post-retention rehearsal vs. day 1 learning end).
Table 1.
Average (+/−SEM) Associative Decision Times (ms) on Correct & Incorrect Trials
| Day 1 Learning Start | Day 1 Learning End | 24 Hours Post Retention | 1 Week Post Retention | 2 Weeks Post Retention | Post Retention Rehearsal | |
|---|---|---|---|---|---|---|
| Correct | 1011.44(45.89) | 660.40(17.08) | 836.88(34.92) | 1183.35(56.44) | 1134.71(50.52) | 713.61(28.22) |
| Incorrect | 1265.65(90.79) | 1854.67(242.73) | 1038.39(82.72) | 1381.25(92.35) | 1418.79(69.24) | 1381.50(94.08) |
Processing Time Changes after Spaced Rehearsal
Decision time changes as a function of continued spaced rehearsal were measured in the subset of 12 subjects who participated in the learning session, and in recall sessions 24 hours, 1 week, and 2 weeks later. A two-way ANOVA of correct trial decision times was computed with trial type (associative vs. instructed cue) and trial occurrence (pre-retention, three rehearsal trials 24 hours later, three rehearsal trials 1-week later, and three rehearsal trials 2-weeks later) as the two factors. The analysis included correctly answered stimulus-association trials on a per-subject basis if the subject answered each stimulus-association correctly at the end of learning, and then also answered that association correctly at each interval of post-retention rehearsal. This performance criterion included 86.67% (+/−4.33) of the associations learned during the Day 1 session.
The ANOVA was significant for the main effects of retention interval (F(9,220)=7.79, p<0.001), trial type (associative vs. instructed cue, F(1,220)=11.54, p=0.001), and the interaction between retention and type (F(9,220)=4.43, p<0.001). Pairwise comparisons showed significant differences relative to the pre-retention baseline between instructed cue and associative decision times on the first trial after 1 day of retention (p=0.03, Figure 5 trial number 1), on the first trial after rehearsal followed by another 6 days of retention (p<0.001, Figure 5 trial number 4), and on the first trial after more rehearsal and another 6 days of retention (p=0.02, Figure 5 trial number 7). After continued practice following the first trial of each post-retention session, decision times never became significantly faster than pre-retention level (Figure 5, trial numbers 2 & 3, 5 & 6, 8 & 9). This pattern was observed for stimulus-association decision times, not for the instructed cue decision times (Figure 5, blue vs. gray bars), which did not deviate significantly from the pre-retention learning level.
Figure 5.
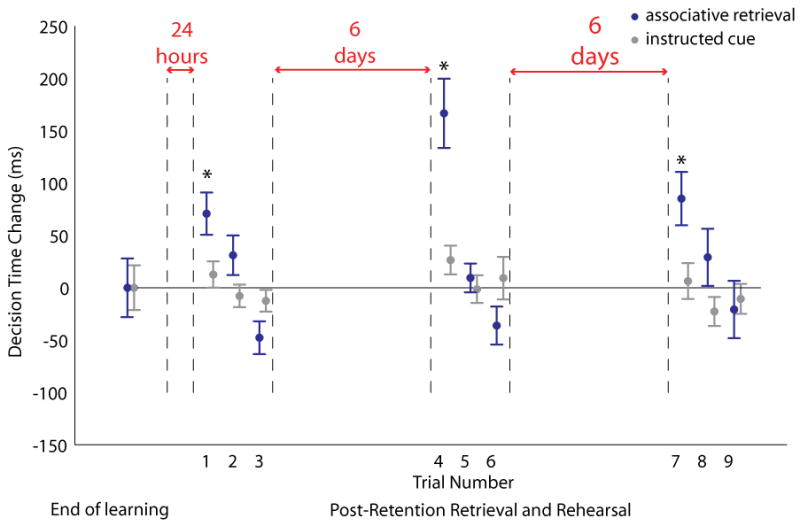
Associative decision time changes after learning, initial post-retention retrieval, and additional post-retention rehearsal. Average (+/− SEM) subject decision times increased for a set of associative stimuli after 24 hours of retention, and after continued practice 1 and 2 weeks later (blue bars), but did not differ significantly from pre-retention levels for instructed cue trials (gray bars). For each subject, decision times were considered only for those trials answered correctly at the end of learning and on each initial retrieval attempt after the retention intervals.
3. Discussion
The present study consisted of two experiments designed to explore changes in behavioral processing times during learning and retrieval of conditional visuomotor associations. The paradigm used in these experiments is a model for real world tasks that require decisions to be made based on long-term associative memories, followed by implementation of skilled actions to affect a response based on the retrieved information.
Two processing speeds were measured from participants during performance of a conditional visuomotor association task. A decision speed was measured as the time to initiate movement of a manipulandum in the correct direction when subjects were presented with a stimulus that was arbitrarily associated with one of four unique goal locations. The decision speed reflected what was termed an explicit processing component, as recall of the correct associative relationship required explicit recollection of information learned in a specific previous episode. Following the decision, subjects completed a skilled motor movement of a manipulandum to place a cursor inside the correct goal location. Completion of this action reflected implicit processing since it involved procedural learning of a motor program, which did not require deliberate recollection of information learned in any particular previous session.
The main finding of the present study is that participants’ explicit and implicit component processing times got faster during the initial learning session, but after long-term retention intervals ranging from 24 hours to two weeks, the direction of the explicit and implicit processing times diverged. The explicit component decision speed increased on the first post-retention trial compared to the level achieved at the end of learning, and with continued practice, never got faster than the level obtained at the end of learning. The implicit component action completion time did get faster after the retention interval compared to the level reached at the end of learning.
The main finding of Experiment 1 is a divergence in explicit and implicit processing times during post-retention associative memory recall. After 24 hours of retention, successful retrieval of a previously learned associative relationship required more time, but less time was needed to complete the action to signal the relationship. The increased explicit processing time parallels the finding of increased post-retention reaction times found by Reber et al (1997) using a yes/no recognition paradigm with retention intervals up to 1 week. The decrease in implicit processing time is consistent with several studies that report faster completion of some types of manual responses after initial training (Cohen et al., 2005; Cohen and Robertson, 2007; Dorris et al., 2000; Song et al., 2007).
While the Experiment 1 results showed that explicit and implicit components at retrieval required different amounts of processing time, suggesting that episodic and procedural memory systems may have reorganized differently during retention, two questions remained regarding the slower decision times: 1) Were the increased decision times in Experiment 1 due to the process of forgetting? And 2) Were the decision time increases related specifically to the process of associative memory retrieval? Experiment 2 was designed to answer these two questions by having subjects overlearn a set of associations across multiple spaced learning sessions, and by incorporating a set of cued instruction trials that did not require associative retrieval, but did require a visually cued decision and movement.
Experiment 2 replicated the main result of Experiment 1: explicit and implicit processing times during retrieval of stimulus-associations diverged after learning and at least 24 hours of retention. Implicit processing duration measured by action completion times was found to be faster after recall of both stimulus-associations and movements that were instructed by arrow instruction cues, suggesting that procedural memories are subject to post-retention efficiency gains. Explicit processing duration measured by decision times was found to be longer on the first post-retention retrieval attempt during associative recall, but not during instructed cue trials, suggesting that successful explicit memory retrieval takes after retention takes more time relative than at encoding.
Further investigation of post-retention decision time increases showed that they occur in the absence of significant forgetting, and that they reach a plateau as the overall amount of information retained stabilizes after 1 week of retention. Attempts to drive post-retention decision times to a level faster than the speed reached during the initial learning session by employing spaced rehearsal were unsuccessful, suggesting that there is a floor to explicit memory retrieval speed which may not be lowered by either consolidation of the memory trace during the retention interval or by continued rehearsal or practice.
The results of Experiment 2 also suggest that remembered and forgotten memories are accessed in two temporally distinct states, one for correctly answered trials and one for incorrectly answered trials. Decision times on incorrect trials always occurred at longer latencies than for correct trials at learning, at initial retrieval after retention, and after more practice. Decision times for correct trials also contained more variance explainable by the experimental manipulation as they were found to co-vary with the progression of learning, the length of the retention interval, the stabilization of the amount of information retained (i.e., leveling off of forgetting rate), and rehearsal after initial post-retention retrieval.
The present study demonstrates a divergence in human explicit and implicit processing speed during memory retrieval. The faster implicit action completion times were predicted based on previous studies of motor and perceptual learning demonstrating post-learning efficiency gains occurring after a period of asleep or awake retention (Rickard, 2007). The slower explicit decision times were not expected, but neither were they predicted as current theories of memory consolidation do not make predictions about how processing directly related to memory retrieval should change after learning and retention. While this and other studies have shown faster implicit processing after learning and retention, the present study also shows that explicit retrieval takes longer after learning and retention. This temporal cost of explicit retrieval exists in the absence of forgetting, and even after continued spaced practice.
Several psychological variables may have caused the increased explicit processing times in the present study. Forgetting may increase the time necessary to retrieve memories (Altmann and Gray, 2002), but in the present experiments explicit processing times increased in the absence of significant forgetting for a set of associative relationships. Retroactive interference (Dewar et al., 2007) may also increase memory retrieval times, but our post-retention sessions were designed such that accuracy and speed for previously learned relationships were assessed before any new associative relationships were learned. In these experiments, memory retrieval may have been effortful rather than automatic (Horton et al., 2001), thereby lengthening response times relative to the end of learning. However, Experiment 2 was designed such that a small set of ten associations was practiced at multiple intervals spaced over several days. The increase in explicit component response times may have been related to the ratio of the number of stimuli to number of unique goal locations. In the present experiments, this ratio was never 1:1 because subjects had to learn that multiple stimuli were associated to each of the four goal locations. The ratio did not change, however, between learning and recall so it is unlikely that a positive ratio at learning and retrieval could have accounted for the increased explicit processing time.
Behavioral processing speeds were measured in the present experiments, and no conclusions may be made about the underlying neurobiological changes that affect these processing times after learning and retention. It is not possible from these results to say whether processing time changes result from a reorganization of separate neural systems, a single neural system, or instead result from molecular changes (i.e., strengthening or weakening of synapses) taking place across the brain. Several studies have documented the neural changes associated with procedural skill learning improvements (Doyon et al., 2002; Karni et al., 1995; Karni et al., 1998; Korman et al., 2003; Linkenkaer-Hansen et al., 2004; Ungerleider et al., 2002 ), and it is possible that the speeded implicit component processing time changes found in our experiments may be related to a gradual change in motor circuitry. It is not clear which neurobiological changes may account for the finding of slower explicit processing times after learning and retention.
The standard model of memory consolidation posits that horizontal connections among cortical areas are strengthened after a single experience or learning session. Strengthening of cortico-cortical or cortico-striatal circuits may underlie the faster action completion times obtained in the present study, but how could such strengthening result in temporally slower decision times? The visuomotor associative task used in the present experiments has been shown to be initially hippocampal-dependent. After learning, the hippocampus has been shown to be unnecessary for accurate retrieval, although it is not clear exactly when or how much practice is needed for the hippocampus to become unnecessary. The standard model of consolidation presumes that the hippocampus is important for learning, and then it orchestrates the strengthening of neocortical connections that eventually support the long-term storage of the memory trace (McClelland et al., 1995; Squire and Alvarez, 1995). After this hippocampal-driven reorganization, one prediction may be that explicit memory retrieval would be faster because fewer synaptic links are required to retrieve the memory, and the ones that remain are stronger. However, the increased explicit processing time suggests a different process may be at work. Two other neurobiological explanations may account for the longer retrieval time.
The finding that the explicit component of memory retrieval takes longer after a retention interval is consistent with a recently reported, empirically-driven theory of remote memory retrieval (Frankland and Bontempi, 2005), which posits an inhibitory influence from frontal cortex to temporarily stop hippocampal processing during remote memory retrieval. If the hippocampus is critical for learning, pausing hippocampal function may be necessary to prevent new learning until feedback about memory integrity is obtained. If the retrieved memory is correct, no new learning is necessary and the same memory trace is maintained, but if feedback confirms that the memory is incorrect, the hippocampus would come back online to guide new learning. Under this scenario, frontal inhibition of the hippocampal system may take place through anatomical pathways documented in the primate (Goldman-Rakic et al., 1984; Morris et al., 1999; Nauta, 1964). There is some functional imaging evidence in humans that seems to support the idea that temporal structures may be inhibited during explicit memory processing (Nyberg et al., 1996), and some recent behavioral evidence to suggest that explicit memory systems can inhibit the expression of efficiency gains in the implicit memory system (Brown and Robertson, 2007). The finding of the present study that implicit processing speed does not show significant speed improvements until after the first retrieval trial, in which decision times show their largest latency increase, is consistent with the idea that explicit memory retrieval involves an inhibitory component.
An alternative neurobiological scenario to explain an increased decision time is that memory retrieval is a process that requires more time because it involves the encoding of a new trace. Support for this idea in humans comes from two human fMRI studies that show increasing hippocampal activation during retrieval of word-pair associations after retention intervals spaced from 10 minutes to 1 month (Bosshardt et al., 2005a; Bosshardt et al., 2005b). These authors suggest that the pattern of increased hippocampal and cortical activation reflects increases in the number of hippocampal-neocortical memory traces formed during memory consolidation, an idea consistent with the multiple trace theory (Moscovitch and Nadel, 1998; Moscovitch et al., 2006; Nadel and Moscovitch, 1997). It not clear from these two fMRI studies whether behavioral reaction times during word-pair retrieval covaried with retention interval duration, nor is it clear how increased neocortical and hippocampal BOLD activation or more rather than fewer cortico-hippocampal traces would affect reaction time. One idea is that the reactivation of a previously “stored” memory reopens the memory for possible modification. The direction of modification could be to strengthen the memory, weaken it, or incorporate new information to make a new memory trace. The process of reopening the memory to modify it may require more time than was necessary at initial encoding. Future high temporal resolution human fMRI and recordings from patients with implanted intracranial EEG electrodes may be used to clarify the relationship between retention interval duration, cortico-hippocampal activation, and the speed of retrieval for stored memories.
In conclusion, we have demonstrated a divergence in two behavioral processing times, one for decision and one for action, during performance of a visuomotor conditional association task containing an explicit episodic component and an implicit procedural component. The significance of the present study is that it provides a framework for investigating how a period of consolidation – the interval between learning and retrieval – affects the speed of memory retrieval. The results show that separate behavioral processing times to 1) retrieve an associative memory, and 2) then act upon the retrieved relationship change differently following learning and retention. While this is not the first study to measure explicit memory retrieval time (Reber et al., 1997), or to document differences in explicit and implicit memory processing (Bitan and Karni, 2004; Hupbach et al., 2006; McBride and Dosher, 1997), it does provides new behavioral evidence to motivate future neurobiological investigations of consolidation. Specifically, the present results suggest that future experiments should be designed to investigate neural changes occurring during the time required for memory retrieval separately from the time required to complete the actions based on the retrieved memories. Retrieval-specific changes would be expected to occur within a post-stimulus time window that is longer relative to pre-retention learning, while action-specific changes would be expected to occur within a post-retrieval time window that is shorter relative to pre-retention learning. Investigation of the neural correlates of our behavioral results could provide a clearer picture of how the brain supports long-term storage of memories and actions.
4. Experimental Procedure
Experiment 1
Participants & Design
A total of 16 subjects (mean age 18.9, s.d. 2.3, 11 females, 1 left handed) participated in Experiment 1. Subjects were recruited from the University of Arizona Department of Psychology undergraduate research pool, and received extra credit points. Written consent was obtained from each in accordance with a protocol approved by the University of Arizona Human Subjects Protection Program. All 16 subjects completed a learning session in the afternoon of Day 1. After learning, 16 subjects were randomly assigned to return for a recall testing session 24 hours later on Day 2.
During the learning session, subjects were given the opportunity to learn 24 arbitrary scene-location associations by trial-and-error. Feedback about the accuracy of responses was given after every trial in both experiments. Each stimulus-association was repeated 13 times in a randomized block design. During the recall session 24 hours later, subjects were tested on the same 24 scene-associations with each association presented 6 times in a randomized order different from the order at learning.
Task & Procedure
Participants were seated at a table and manipulated a joystick with their dominant hand. A CRT monitor (40cm wide by 30 cm high screen) was placed at the end of the table so that during task performance the participant’s eyes were 71 cm from the plane of the monitor and its screen occupied 29 horizontal by 23 vertical degrees visual angle (27 horizontal by 26 vertical pixels per 1 degree visual angle on the 800-by-600 pixel screen display). The task presentation software was written in C++ using Microsoft Visual Studio. NET, the Microsoft Win32 API, a hardware accelerated graphics language (OpenGL 1.2), and the Microsoft DirectX development toolkit for presenting audio and obtaining joystick input with real-time precision. The task was displayed using an NVIDIA GeForce2 MX/MX400 graphics card, and the graphics engine for the visual stimulus presentation was programmed with a double-buffering technique in which each frame was drawn in a back buffer as fast as possible and then swapped to the front buffer at the monitor refresh rate of 60 Hz. The x-axis and y-axis position of the joystick was sampled every 16.6 ms synchronized with the monitor refresh rate.
A single trial of the conditional association task consisted of five components: 1) prefixation, 2) fixation, 3) stimulus-on, 4) stimulus-off, and 5) choice-feedback (Figure 1a). The pre-fixation component started with a triangular cursor appearing on the screen in one of eight locations around the center of the screen (Figure 1b) determined randomly on each trial. During pre-fixation, subjects were given 4500 ms to move the cursor (whose movement was linearly mapped to joystick movement) into a fixation box appearing in the center of the screen. If 4500 ms elapsed and the cursor had not been placed within the box, the pre-fixation period started again. Once the cursor was moved within the center box and cursor movement was stopped completely, a fixation period began (Figure 1c). The fixation period lasted 1000 ms if the cursor was held still, but if the cursor was moved before 1000 ms elapsed, the start time for the fixation period was re-set. If 1000 ms elapsed without cursor movement, the stimulus-on period began and the first frame of the stimulus was presented along with the four choice box locations (Figure 1d). Each stimulus-on period lasted for 500 ms during which the stimulus picture was presented in the center of the screen and the choice locations were presented in the periphery (up, down, left, and right relative to the center box). After the 500 ms stimulus-on period ended, a 4500 ms stimulus-off period began during which the stimulus picture disappeared but choice box locations remained on the screen (Figure 1e). Participants were required to move the cursor into one of the choice boxes before the 4500 ms stimulus-off period ended, or the trial would start over with the pre-fixation period. If subjects made a choice within the 5000 ms stimulus-on and stimulus-off time window, a 1000 ms choice-feedback period began (Figure 1f and Figure 1g) during which subjects received visual and auditory feedback about the accuracy of their choice. A correct choice was followed by presentation of “Correct!” paired with the sound of a cash register, and an incorrect choice was followed by “Wrong! Try Again” paired with the sound of glass breaking.
Figure 1.
Timeline of a single association trial (a), and example screenshots during each component of the task (b–g).
Each of the 24 stimuli was arbitrarily mapped to one of the four choice boxes, which was designated as the correct target choice. The arbitrary mapping between the stimulus and its correct choice location remained the same throughout learning and subsequent post-retention testing sessions, and the mappings were determined so that within the experimental session the number of stimuli mapped to the up, down, left, and right choice locations was equal.
Materials
Color photographs of complex scenes were used as instruction stimuli. Stimuli were 300-by-200 (horizontal-by-vertical) pixel image bitmaps that appeared in the center of the display and subtended a visual angle of 11.1 horizontal by 7.7 vertical degrees. Scene images were acquired from publicly available stock photo libraries. All image cropping, re-sizing, intensity normalization, and color conversion was done with IrfanView 3.98.
Data Analysis
Accuracy at the end of each trial was defined by whether or not the cursor was placed within the correct choice box to complete a trial. The decision time was defined as the time from stimulus onset until the first detectable movement of the joystick. More specifically, stimulus presentation did not begin until after the subject fixated by holding the joystick completely motionless (defined as 0 units of deviation relative to the joystick center for both the x-axis and y-axis) for 1000 ms. As soon as the 1000 ms fixation requirement was met, the stimulus presentation began. The x-axis and y-axis position were sampled continuously throughout fixation and stimulus presentation every 16.6 ms, and the first detectable deviation in either the x-axis or y-axis joystick position after stimulus presentation was recorded as the decision time. The action completion time was defined as the time elapsed from the decision time until the cursor was placed within a choice box. The completion time was velocity-dependent, meaning that faster motor responses resulted in faster completion times. The mapping between joystick movement and cursor movement was linear (i.e., faster joystick movement equaled faster cursor movement), and was held constant between learning and recall sessions. Trial-by-trial changes in performance were assessed by computing the mean and standard error of each subject’s accuracy for the set of associations, while changes in decision and action completion times were assessed by computing means and standard errors of each subject’s reaction times for correctly and incorrectly answered trials. Repeated measures ANOVAs were used to identify session (learning vs. recall) by trial (first vs. last) effects, and unpaired t-tests were used to make planned comparisons of average decision times between the last trial of the learning session and the first trial of the recall session, and action completion times between pre- and post- retention sessions.
Experiment 2
Participants & Design
A total of 17 subjects participated in multiple sessions; 12 of these attended four sessions (learning day, recall 24 hours later, recall 1 week later, and recall 2 weeks later), and 5 attended three sessions (learning day, recall 24 hours later, and recall 1 week later).
Task
The task was the same as in Experiment 1, but instructed cue trials were randomly distributed among arbitrary association trials. Instructed cue stimuli consisted of white filled arrows pointing up, down, left, or right displayed on the a black background. Subjects were required to move to the choice location indicated by the arrow as fast as possible.
Data Analysis
The same parametric methods were used as in the analysis of the Experiment 1 data. Additional non-parametric methods were used to visualize the decision time changes in Experiment 2. For the non-parametric analysis, a decision time was stored for each association on the last trial of the learning day, if the trial was correct. For each of the associations answered correctly at the end of the learning day, a decision time was stored on the post-retention first trial, if that first trial was also correct. The pre-retention and post-retention stores consisted of successful decision times measured at separate points in time. A probability density estimate was computed from the sample using a kernel smoothing method (Bowman and Azzalini, 1997), which allows estimation of the underlying probability distribution by considering where each reaction time measurement falls along the time axis, windowing Gaussian kernels modeled as unit normal distributions around each measurement’s abscissa, and summing the kernels to get a smooth estimate of the underlying distribution’s probability density function (pdf). Differences between the pre- and post-retention retrieval time distributions were visualized by superimposing the estimated pdfs on the same set of axes. Statistical comparison of the distributions using the Kolmogorov-Smirnov (KS) test was used to reject the null hypothesis that the sampled decision times were drawn from a similar underlying distribution.
Table 2.
Design of Experiment 2
| Learning (Day 1) | 24 Hours Later (Day 2) | 1 Week Later (Day 3) | 2 Weeks Later (Day 4) |
|---|---|---|---|
| Learn 30 associations(10 reps, 300 trials) | tested on unique subset of 10 associations from Day 1 (3 reps) | tested on unique subset of 10 associations from Day 1 (3 reps) | tested on unique subset of 10 associations from Day 1 (3 reps) |
| intermixed instructed cue trials | intermixed instructed cue trials | rehearsal of the Day 2 subset(3 reps per set) | rehearsal of the Day 2 and Day 3 subsets(3 reps per set) |
| intermixed instructed cue trials | intermixed instructed cue trials | ||
Acknowledgments
This work was submitted in partial fulfillment of a doctoral dissertation by T.M.E. The authors would like to thank Bruce L. McNaughton, Mary Peterson, Nathan Insel, and Betty Glisky for helpful discussions and suggestions. The experiments were supported by an NIH grant to B.L.M (MH046823) and an SBSRI Dissertation Research Grant to T.M.E.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albouy G, et al. Implicit oculomotor sequence learning in humans: Time course of offline processing. Brain Res. 2006;1090:163–71. doi: 10.1016/j.brainres.2006.03.076. [DOI] [PubMed] [Google Scholar]
- Altmann EM, Gray WD. Forgetting to remember: the functional relationship of decay and interference. Psychol Sci. 2002;13:27–33. doi: 10.1111/1467-9280.00405. [DOI] [PubMed] [Google Scholar]
- Bitan T, Karni A. Procedural and declarative knowledge of word recognition and letter decoding in reading an artificial script. Brain Res Cogn Brain Res. 2004;19:229–43. doi: 10.1016/j.cogbrainres.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Bosshardt S, et al. One month of human memory consolidation enhances retrieval-related hippocampal activity. Hippocampus. 2005a;15:1026–40. doi: 10.1002/hipo.20105. [DOI] [PubMed] [Google Scholar]
- Bosshardt S, et al. Effects of memory consolidation on human hippocampal activity during retrieval. Cortex. 2005b;41:486–98. doi: 10.1016/s0010-9452(08)70189-8. [DOI] [PubMed] [Google Scholar]
- Bowman A, Azzalini A. Applied Smoothing Techniques for Data Analysis. Oxford University Press; 1997. [Google Scholar]
- Brasted PJ, et al. Fornix transection impairs conditional visuomotor learning in tasks involving nonspatially differentiated responses. J Neurophysiol. 2002;87:631–3. doi: 10.1152/jn.00656.2001. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, et al. Role of the hippocampal system in associative learning beyond the spatial domain. Brain. 2003;126:1202–23. doi: 10.1093/brain/awg103. [DOI] [PubMed] [Google Scholar]
- Brown RM, Robertson EM. Inducing motor skill improvements with a declarative task. Nat Neurosci. 2007;10:148–9. doi: 10.1038/nn1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Censor N, et al. A link between perceptual learning, adaptation and sleep. Vision Res. 2006;46:4071–4. doi: 10.1016/j.visres.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Cohen DA, et al. Off-line learning of motor skill memory: a double dissociation of goal and movement. Proc Natl Acad Sci U S A. 2005;102:18237–41. doi: 10.1073/pnas.0506072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Robertson EM. Motor sequence consolidation: constrained by critical time windows or competing components. Exp Brain Res. 2007;177:440–6. doi: 10.1007/s00221-006-0701-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar MT, et al. Forgetting due to retroactive interference: a fusion of Muller and Pilzecker’s (1900) early insights into everyday forgetting and recent research on anterograde amnesia. Cortex. 2007;43:616–34. doi: 10.1016/s0010-9452(08)70492-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorris MC, et al. Immediate neural plasticity shapes motor performance. J Neurosci. 2000;20:RC52. doi: 10.1523/JNEUROSCI.20-01-j0005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, et al. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci U S A. 2002;99:1017–22. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. The organization of recent and remote memories. Nat Rev Neurosci. 2005;6:119–30. doi: 10.1038/nrn1607. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, et al. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12:719–43. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Graf P, Schacter DL. Implicit and explicit memory for new associations in normal and amnesic subjects. J Exp Psychol Learn Mem Cogn. 1985;11:501–18. doi: 10.1037//0278-7393.11.3.501. [DOI] [PubMed] [Google Scholar]
- Hauptmann B, Karni A. From primed to learn: the saturation of repetition priming and the induction of long-term memory. Brain Res Cogn Brain Res. 2002;13:313–22. doi: 10.1016/s0926-6410(01)00124-0. [DOI] [PubMed] [Google Scholar]
- Hauptmann B, et al. The predictive value of the leveling off of within session performance for procedural memory consolidation. Brain Res Cogn Brain Res. 2005;24:181–9. doi: 10.1016/j.cogbrainres.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Horton KD, et al. Measuring automatic retrieval. J Exp Psychol Learn Mem Cogn. 2001;27:958–66. doi: 10.1037//0278-7393.27.4.958. [DOI] [PubMed] [Google Scholar]
- Hupbach A, et al. The mere exposure effect is sensitive to color information: evidence for color effects in a perceptual implicit memory test. Exp Psychol. 2006;53:233–45. doi: 10.1027/1618-3169.53.3.233. [DOI] [PubMed] [Google Scholar]
- Karni A, et al. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature. 1995;377:155–8. doi: 10.1038/377155a0. [DOI] [PubMed] [Google Scholar]
- Karni A, et al. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci U S A. 1998;95:861–8. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korman M, et al. Multiple shifts in the representation of a motor sequence during the acquisition of skilled performance. Proc Natl Acad Sci U S A. 2003;100:12492–7. doi: 10.1073/pnas.2035019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linkenkaer-Hansen K, et al. Prestimulus oscillations enhance psychophysical performance in humans. J Neurosci. 2004;24:10186–90. doi: 10.1523/JNEUROSCI.2584-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride DM, Dosher BA. A comparison of forgetting in an implicit and explicit memory task. J Exp Psychol Gen. 1997;126:371–92. doi: 10.1037//0096-3445.126.4.371. [DOI] [PubMed] [Google Scholar]
- McClelland JL, et al. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–57. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–51. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- Mitchell DB, Brown AS. Persistent repetition priming in picture naming and its dissociation from recognition memory. J Exp Psychol Learn Mem Cogn. 1988;14:213–22. doi: 10.1037//0278-7393.14.2.213. [DOI] [PubMed] [Google Scholar]
- Morris R, et al. Fiber system linking the mid-dorsolateral frontal cortex with the retrosplenial/presubicular region in the rhesus monkey. J Comp Neurol. 1999;407:183–92. doi: 10.1002/(sici)1096-9861(19990503)407:2<183::aid-cne3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, Nadel L. Consolidation and the hippocampal complex revisited: in defense of the multiple-trace model. Curr Opin Neurobiol. 1998;8:297–300. doi: 10.1016/s0959-4388(98)80155-4. [DOI] [PubMed] [Google Scholar]
- Moscovitch M, et al. The cognitive neuroscience of remote episodic, semantic and spatial memory. Curr Opin Neurobiol. 2006;16:179–90. doi: 10.1016/j.conb.2006.03.013. [DOI] [PubMed] [Google Scholar]
- Muller RA, et al. Functional MRI of motor sequence acquisition: effects of learning stage and performance. Brain Res Cogn Brain Res. 2002;14:277–93. doi: 10.1016/s0926-6410(02)00131-3. [DOI] [PubMed] [Google Scholar]
- Murray EA, Wise SP. Role of the hippocampus plus subjacent cortex but not amygdala in visuomotor conditional learning in rhesus monkeys. Behav Neurosci. 1996;110:1261–70. doi: 10.1037//0735-7044.110.6.1261. [DOI] [PubMed] [Google Scholar]
- Musen G, Treisman A. Implicit and explicit memory for visual patterns. J Exp Psychol Learn Mem Cogn. 1990;16:127–37. doi: 10.1037//0278-7393.16.1.127. [DOI] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–27. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Nauta W. Some efferent connections of the prefrontal cortex in the monkey. In: Warren J, Akert K, editors. The frontal granular cortex and behaviour. McGraw Hill; New York: 1964. pp. 397–409. [Google Scholar]
- Nyberg L, et al. Network analysis of positron emission tomography regional cerebral blood flow data: ensemble inhibition during episodic memory retrieval. J Neurosci. 1996;16:3753–9. doi: 10.1523/JNEUROSCI.16-11-03753.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ofen-Noy N, et al. Skill learning in mirror reading: how repetition determines acquisition. Brain Res Cogn Brain Res. 2003;17:507–21. doi: 10.1016/s0926-6410(03)00166-6. [DOI] [PubMed] [Google Scholar]
- Perez MA, et al. Neurophysiological mechanisms involved in transfer of procedural knowledge. J Neurosci. 2007;27:1045–53. doi: 10.1523/JNEUROSCI.4128-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, et al. Reaction time distributions across normal forgetting: searching for markers of memory consolidation. Learn Mem. 1997;4:284–90. doi: 10.1101/lm.4.3.284. [DOI] [PubMed] [Google Scholar]
- Rickard TC. Forgetting and learning potentiation: dual consequences of between-session delays in cognitive skill learning. J Exp Psychol Learn Mem Cogn. 2007;33:297–304. doi: 10.1037/0278-7393.33.2.297. [DOI] [PubMed] [Google Scholar]
- Schacter DL, et al. Implicit memory: a selective review. Annu Rev Neurosci. 1993;16:159–82. doi: 10.1146/annurev.ne.16.030193.001111. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Graf P. Modality specificity of implicit memory for new associations. J Exp Psychol Learn Mem Cogn. 1989;15:3–12. doi: 10.1037//0278-7393.15.1.3. [DOI] [PubMed] [Google Scholar]
- Song S, et al. Sleep does not benefit probabilistic motor sequence learning. J Neurosci. 2007;27:12475–83. doi: 10.1523/JNEUROSCI.2062-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Alvarez P. Retrograde amnesia and memory consolidation: a neurobiological perspective. Curr Opin Neurobiol. 1995;5:169–77. doi: 10.1016/0959-4388(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Stickgold R, et al. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000a;3:1237–8. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- Stickgold R, et al. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000b;12:246–54. doi: 10.1162/089892900562075. [DOI] [PubMed] [Google Scholar]
- Taylor KJ, et al. Recognition memory for faces and scenes in amnesia: dissociable roles of medial temporal lobe structures. Neuropsychologia. 2007;45:2428–38. doi: 10.1016/j.neuropsychologia.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, et al. Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem. 2002;78:553–64. doi: 10.1006/nlme.2002.4091. [DOI] [PubMed] [Google Scholar]
- van Turennout M, et al. Modulation of neural activity during object naming: effects of time and practice. Cereb Cortex. 2003;13:381–91. doi: 10.1093/cercor/13.4.381. [DOI] [PubMed] [Google Scholar]
- van Turennout M, et al. Long-lasting cortical plasticity in the object naming system. Nat Neurosci. 2000;3:1329–34. doi: 10.1038/81873. [DOI] [PubMed] [Google Scholar]
- Wheeldon LR, Monsell S. The locus of repetition priming of spoken word production. Q J Exp Psychol A. 1992;44:723–61. doi: 10.1080/14640749208401307. [DOI] [PubMed] [Google Scholar]



