Abstract
Whether a freshly isolated immune cell can be equipped with both natural killing and antigen-presenting cell (APC) function has recently become controversial in mice. We sought to probe the existence of a candidate human cell with these properties by searching for cells in healthy subjects that co-express APC surface molecules and NK cell receptors. We have found that CD3−CD14−CD19− mononuclear cells of human blood, spleen, liver, and lymph nodes contain 2 distinct populations of cells that co-express HLA-DR (DR) and CD56. Circulating CD56+ cells expressing high levels of DR were phenotypically and functionally similar to conventional CD56− dendritic cells (DC). Furthermore, we demonstrate here that a separate cohort of CD56+ cells that express low levels of DR are NK cells that possess dual function as potent killers endowed with weak APC function.
Keywords: natural killer cells, dendritic cells, human, antigen-presenting cell
INTRODUCTION
The interplay between innate and adaptive immunity is complex and continually unfolding. NK cells and DC have distinct but complementary roles in regulating the outcome of immune responses. While originally identified as initiators of the adaptive immune response, DC are now also considered crucial mediators of innate immunity with particular influence upon NK cell activation. Reciprocally, NK cells shape the adaptive response by modulating DC [1–3].
Another link between the innate and adaptive immune system has been suggested by reports of a class of murine leukocytes possessing both lytic and APC ability. These interferon-producing killer dendritic cells (IKDC, DX5+CD11cintB220+) [4, 5] and natural killer dendritic cells (NKDC, NK1.1+CD11cintCD3−) [6] have recently been shown to be an activated subset of NK cells [7–9] and their APC capability has been challenged [8]. However, isolation techniques and results of functional APC assays are variable among different laboratories and the issue is further complicated by strain differences in mice. Although a CD11chighDX5+ DC has been identified as a possible APC contaminant of IKDC from Balb/c mice [8], a distinct APC contaminant of NK1.1+CD11c+ preparations in C57BL/6 mice remains elusive.
Nevertheless, circulating human myeloid and plasmacytoid DC lyse tumor targets after type I or II interferon stimulation [10, 11], toll-like receptor 7/8 ligation [12], and Influenza or HIV infection [13, 14]. In addition, unstimulated human peripheral blood DC may lyse select squamous cell tumor targets [15]. Conversely, it is known that following prolonged in vitro activation, human NK cells gain the ability to present antigen to CD4 T cells [16, 17]. However, whether a subset of freshly isolated, steady state human NK cells can possess both natural killing and APC function is uncertain.
MATERIALS AND METHODS
Cell purification and flow cytometry
PBMC were obtained from leukocyte concentrates of healthy donors (New York Blood Center) by density gradient centrifugation over Ficoll-Paque Plus (GE Healthcare). DC and NK cell subsets were enriched by depletion of T cells and B cells using anti-CD3 and anti-CD20 magnetic beads (Miltenyi), purified with a MoFlo cell sorter (DakoCytomation), and used immediately in functional assays. Post-sort purities were routinely 97% or greater. To determine morphology, sort-purified cells were centrifuged at 600 rpm onto glass slides, fixed with 100% methanol before staining with Giemsa, and then photographed at 100x magnification using a Zeiss Axiophot 2 microscope (Carl Zeiss). Cytometric analysis of blood, liver, spleen, and portal lymph node mononuclear cells was performed on a FACSAria (BD Biosciences) with routine collection of one million events. Tissue acquisition was approved by the Institutional Review Board and specimens were obtained from patients undergoing upper abdominal operations for cancer at our institution. Non-diseased spleens and liver tissue greater than 10 cm from tumor were perfused with type IV collagenase (1 mg/ml), morselized, and incubated in collagenase for 30 minutes at 37 degrees before being passed through a 100 µm filter. Lymph nodes were mechanically disrupted and passed through a 100 µm filter. Cell suspensions were washed twice with HBSS and mononuclear cells were collected after Ficoll-Paque density centrifugation. All antibodies were obtained from BD Pharmingen. Antibodies used for cell sorting were FITC-HLA-DR (L243), PE-CD3 (HIT3a), PE-CD14 (M5E2), PE-CD19 (HIB19), and APC-CD56 (B159). Antibodies used for analysis included FITC-CD3 (HIT3a), FITC-CD14 (M5E2), FITC-CD19 (HIB19), FITC-CD19 (FN50), PE-CD205 (MG38), PE-NKp46 (9E2), PE-CD336 (P44-8.1), PE-CD337 (P30-15), PE-CD314 (1D11), PE-Cy7-CD56 (B159), APC-CD11c (HL3), APC-CD40 (5C3), APC-CD83 (HB15e), APC-CD86 (FUN-1), APC-CD161 (DX12), and APC-Cy7-HLA-DR (L243).
Functional assays
Lysis assays were performed by culturing sort-purified effectors with 103 Chromium-loaded K562 or Daudi target cells for 4 hours and then measuring supernatant radioactivity (TopCount NXT; PerkinElmer). Some effectors were preincubated with 100 ng/ml concanamycin-A (ICN Biomedical) for 2 hours at 37 degrees, and 100 ng/ml IL-15 (R&D Systems) was added to some wells. Mixed leukocyte reactions (MLRs) were performed by culturing gamma-irradiated effectors with 105 allogeneic T cells (Pan T cell Isolation Kit II, Miltenyi) in complete RPMI 1640 supplemented with 10% human serum (Invitrogen). On day 5, [3H]-thymidine (1 µCi; New England Nuclear) was added to each well and thymidine incorporation was measured after 18 hours (TopCount NXT). To detect activation of memory T cells, effectors were pulsed with the CEF peptide pool (2 µg/ml, CTL Technology) for 1 hour at 37 degrees, and washed 3 times. Two × 104 effectors were co-cultured with 2 × 105 autologous CD8 T cells (CD8 T cell isolation kit, Miltenyi) for 36 hours on an IFN-γ Elispot plate (BD Biosciences). Statistical significance was determined by the student’s t-test using Prism 4.0 statistical software (Graphpad Software).
RESULTS AND DISCUSSION
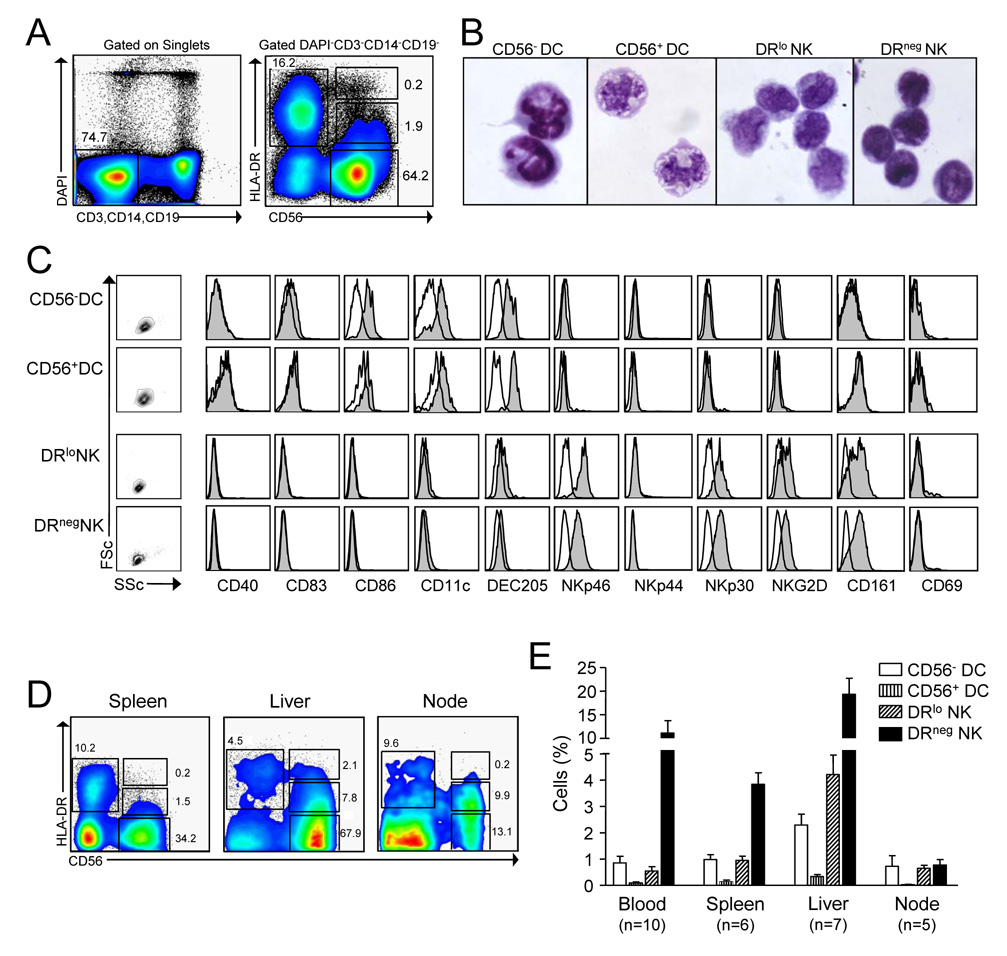
We set out to determine whether a single population of circulating human immune cells co-expressed NK cell receptors and APC surface molecules. Using cell enrichment and high event collection we have identified 2 small populations of cells within the CD3−CD14−CD19− mononuclear cell compartment that express both the NK cell identifier, CD56 and the human MHC II antigen, HLA-DR (Fig. 1A). CD56+ cells expressing high levels of DR (CD56+DC) revealed many similarities to conventional blood DC including large size and vacuolated cytoplasm as well as light scatter properties, the expression of CD86, CD11c, and DEC205, and absence of NK cell receptors (Fig. 1B–C). Meanwhile, CD56+ cells that stained negative for DR (DRneg NK cells) were slightly smaller than CD56+ cells bearing low surface expression of DR (DRlo NK cells) which had a larger proportion of cytoplasm. Both DRneg and DRlo NK cells shared similar expression of the NK cell receptors NKp46, NKp30, NKG2D, and CD161, whereas co-stimulatory molecules and DC identifiers were absent (Fig. 1B–C).
Figure 1. CD3−CD14−CD19− mononuclear cells contain 2 populations of cells that co-express CD56 and HLA-DR.
(A) The gating strategy used for cell sorting and cytometric analysis is illustrated. PBMC depleted of T cells and B cells were gated by lack of lineage markers (CD3, CD14, and CD19) and DAPI staining. Four populations are depicted based on HLA-DR and CD56 expression: DR+CD56− (conventional DC), DR−CD56+ (DRneg NK cells), DRloCD56+ (DRlo NK cells) and DRhiCD56+ (CD56+DC). (B) These four cell populations were sort-purified from PBMC and stained with Giemsa for light microscopy. (C) Representative phenotype of these 4 cell populations in PBMC from 1 of 10 donors is shown. Open histograms represent isotype controls. (D) Spleen, liver, and lymph node mononuclear cells were analyzed by flow cytometry and representative dot plots are shown. (E) Percentage ± s.e.m. of mononuclear cells for each of the cell types is indicated. N refers to the number of patients analyzed.
To determine the anatomic distribution of these cells in primary and secondary lymphoid organs, we performed cytometric analysis on CD3−CD14−CD19− mononuclear cells of several human anatomic compartments (Fig. 1 D–E). While uncommon in the blood, spleen, and lymph node, we found that DRloNK cells comprised over 4% of liver mononuclear cells and 22% of all liver NK cells. Matched blood and liver samples from four patients confirmed this trend within each individual (not shown). In contrast to a previous report demonstrating that DR expression was higher on inflamed tonsillar NK cells but low on nodal NK cells [18], we discovered that nearly half of the NK cells in the portal lymph node expressed DR. This may reflect the fact that we studied the draining lymph nodes of the liver, which contains a relative abundance of DRlo NK cells.
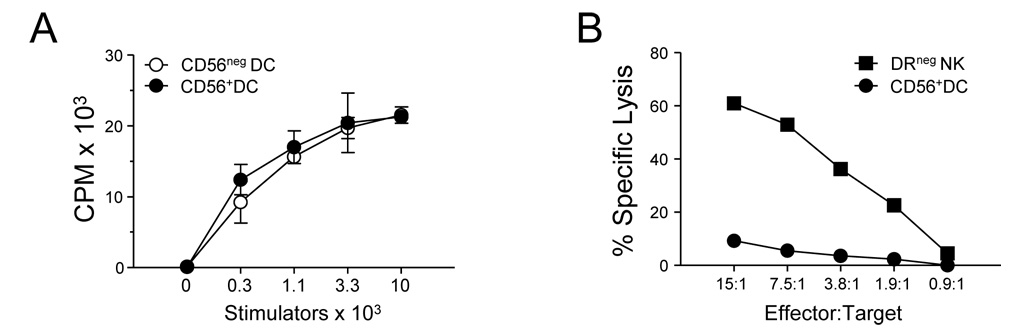
A hallmark of DC function is their ability to cause proliferation of T cells in an allogeneic mixed leukocyte reaction (MLR). In agreement with their phenotypic similarities to conventional DC, sort-purified CD56+ DC were able to cause as much alloproliferation as conventional CD56− DC (Fig. 2A). Furthermore, CD56+ DC lacked the ability to lyse classical NK cell target cells (Fig. 2B). While cells expressing CD56 are generally excluded from human DC preparations [19], our results suggest that 1.2% to 4.2% of circulating DC display CD56.
Figure 2. CD56+ DC stimulate T cells but do not lyse NK cell targets.
(A) Sort-purified circulating CD56+ DC were cultured with allogeneic T cells in an MLR and thymidine incorporation was determined on the sixth day. (B) CD56+ DC were tested in a standard chromium release lysis assay using K562 cells as targets. Data are representative of 3 separate donors.
CD56 (neural cell adhesion molecule, NCAM) is a glycoprotein that is a ligand for fibroblast growth factor 1 that is constitutively expressed on fibroblasts [20]. It is expressed on human but not mouse NK cells and may potentiate the differentiation of NK cells through an interaction with fibroblasts [21]. The significance of CD56 on human DC is unclear but perhaps may play a role in facilitating adhesion of DC to fibroblasts.
To the best of our knowledge, this is the first report of naturally occurring human DC expressing CD56. It has recently been shown that approximately half of monocytes cultured with GMCSF and interferon-alpha will differentiate into DC that express CD56 [22]. Similar to fresh CD56+ DC identified in this study, these cytokine-generated CD56+ DC are potent stimulators of allogeneic T cells and do not display any NK cell receptors. In contrast to fresh CD56+ DC that do not express TRAIL (not shown), cytokine-generated CD56+ DC are capable of some degree (24%) of target cell lysis which was mediated through TRAIL.
While DR is a known indicator of NK cell activation in vitro [23], the significance of DR expression on the small pool of circulating NK cells in healthy subjects is uncertain. HLA-DR positive NK cells have been noted in several pathologic states and their presence in tissue [17] and blood [24], during viral infection and their association with autoimmunity [25, 26] imply that they may have physiologic importance in vivo. Notably, DRlo NK cells in healthy individuals lacked other NK cell activation markers including NKp44 and CD69 (Fig. 1B).
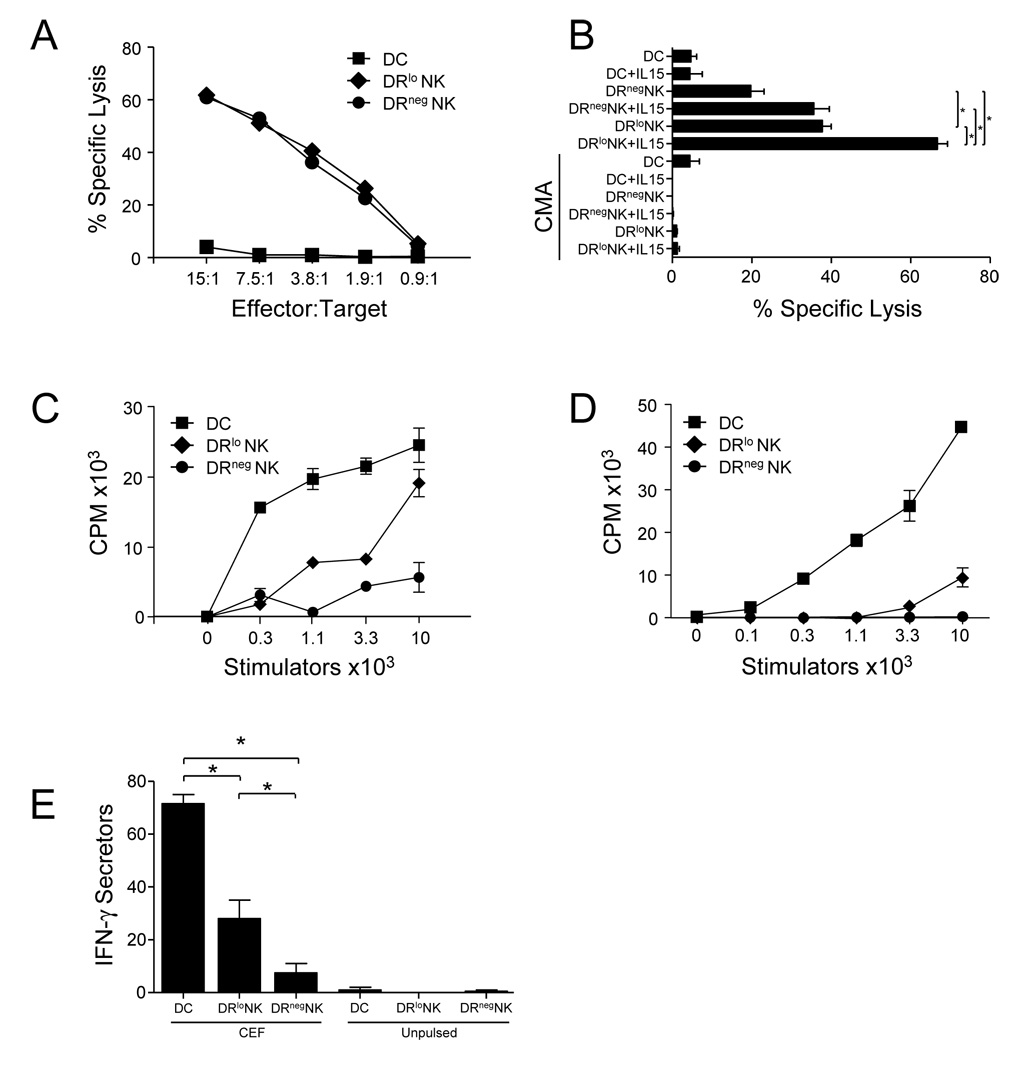
It has been shown that DR+CD16+ cells generated in 5 day MLR cultures are more efficient cytotoxic effectors than their DR−CD16+ counterparts [23]. In contrast, NK cells of HIV patients display higher DR expression than normal controls and the DR+ NK cell fraction has less lytic ability than DR− NK cells from the same patients [24]. We have found that freshly isolated, circulating DRlo NK cells and DRneg NK cells from healthy subjects lysed NK-sensitive K562 targets equally (Fig. 3A). However, DRlo NK cells were more effective than DRneg NK cells at lysing NK-resistant Daudi target (Fig. 3B). The efficiency of Daudi killing by both DRlo and DRneg NK cells was increased by activation with IL-15 (and IL-2, data not shown), but DRlo NK cells remained more potent. Abrogation of lysis by concanamycin-A [27] demonstrated that killing depended upon the granule exocytosis pathway.
Figure 3. Freshly isolated DRlo NK cells are potent killers with weak APC function.
DC and NK cell subsets were sort-purified from PBMC of healthy donors. (A) The lytic ability of conventional (CD56−) blood DC, DRlo NK cells, and DRneg NK cells was compared in standard chromium-release lysis assays using NK cell-sensitive K562 targets. (B) Additional lysis assays were performed using NK cell resistant Daudi targets in the presence or absence of IL-15 and/or concanamycin-A. An effector:target ratio of 30:1 is shown. These data are representative of separate experiments with similar results from 4 independent donors. The relative ability of (CD56−) DC, DRlo NK cells, and DRneg NK cells isolated from blood (C) or liver (D) to stimulate the proliferation of allogeneic T cells was tested in an MLR. These data are representative of separate experiments with similar results from 4 independent donors. (E) Induction of IFN-γ production by antigen-specific memory T cells was determined by ELISPOT. Effector cells were coated with the CEF peptide pool (32 HLA Class I restricted peptides from Cytomegalovirus, Ebstein-bar virus, and Influenza virus with specificities for 15 HLA Class I alleles), washed, and then co-cultured with autologous CD8 T cells. Additional controls included peptide-pulsed and unpulsed effectors alone which produced <3 spots per group. Representative of 3 independent donors. *, p<0.05.
The biosynthesis and expression of MHC Class II molecules have been shown to be essential factors that determine APC capacity [28]. Human NK cells gain the ability to process and present protein antigens to CD4 T cells after prolonged in vitro activation with IL-2 [17]. This is accompanied by variable upregulation of MHC class II expression that correlates to the level of NK cell APC function [16]. Similarly, in vitro activated NK cell cultures have been shown to efficiently co-stimulate anti-CD3 or staphylococcal enterotoxin B-induced proliferation of autologous CD4 T cells in an OX40L-OX40 dependent manner [29].
We have found that DRlo NK cells freshly isolated from blood (Fig. 3C) and liver (Fig. 3D) demonstrate potential for some APC function. In an MLR they were able to cause the proliferation of allogeneic T cells. Similarly, after pulsing DRlo NK cells with peptide, they were able to induce the production of IFN-γ by antigen-specific memory CD8 T cells (Fig. 3E). Uniform expression of NKp46 confirmed the NK cell origin of these cells [30] and conservative gating during sorting and high purity yields made contamination with conventional APC in these assays unlikely.
In summary, we have shown that a minute fraction of circulating DC expresses CD56 and therefore may be present within human NK cell preparations. Conversely, a small proportion of circulating and resident tissue NK cells express HLA-DR. We have found that this rare subset of circulating NK cells in healthy donors possessed robust killing ability and some degree of APC function. The advantage of APC function by a killer lymphocyte is uncertain, although numerous possibilities exist. Possession of such pleiotropic functions by a single cell type raises the possibility that DRlo NK cells bridge innate and adaptive immunity and are capable of accelerating local immune responses. In contrast, the T cell polarizing capability of DRlo NK cells and the outcome of their resulting T cell responses deserve further study. The concentration of DRlo NK cells in the liver, for example, could possibly favor tolerance over immunity.
ACKNOWLEDGMENTS
This work was supported grants from the National Institutes of Health Grants DK068346 (to R.P.D) and AI070658 (to R.P.D).
Abbreviations
- NK cell
Natural killer cell
- DC
dendritic cell
- APC
antigen-presenting cell
- DR
HLA-DR
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors declare no financial or commercial conflict of interest.
REFERENCES
- 1.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan CW, Crafton E, Fan HN, Flook J, Yoshimura K, Skarica M, D, et al. Interferon-producing killer dendritic cells provide a link between innate and adaptive immunity. Nat Med. 2006;12:207–213. doi: 10.1038/nm1352. [DOI] [PubMed] [Google Scholar]
- 5.Taieb J, Chaput N, Menard C, Apetoh L, Ullrich E, Bonmort M, M, et al. A novel dendritic cell subset involved in tumor immunosurveillance. Nat Med. 2006;12:214–219. doi: 10.1038/nm1356. [DOI] [PubMed] [Google Scholar]
- 6.Pillarisetty VG, Katz SC, Bleier JI, Shah AB, Dematteo RP. Natural killer dendritic cells have both antigen presenting and lytic function and in response to CpG produce IFN-gamma via autocrine IL-12. J Immunol. 2005;174:2612–2618. doi: 10.4049/jimmunol.174.5.2612. [DOI] [PubMed] [Google Scholar]
- 7.Blasius AL, Barchet W, Cella M, Colonna M. Development and function of murine B220+CD11c+NK1.1+ cells identify them as a subset of NK cells. J Exp Med. 2007;204:2561–2568. doi: 10.1084/jem.20070991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caminschi I, Ahmet F, Heger K, Brady J, Nutt SL, Vremec D, S, et al. Putative IKDCs are functionally and developmentally similar to natural killer cells, but not to dendritic cells. J Exp Med. 2007;204:2579–2590. doi: 10.1084/jem.20071351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vosshenrich CA, Lesjean-Pottier S, Hasan M, Richard-Le Goff O, Corcuff E, Mandelboim O, Di Santo JP. CD11cloB220+ interferon-producing killer dendritic cells are activated natural killer cells. J Exp Med. 2007;204:2569–2578. doi: 10.1084/jem.20071451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fanger NA, Maliszewski CR, Schooley K, Griffith TS. Human dendritic cells mediate cellular apoptosis via tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J Exp Med. 1999;190:1155–1164. doi: 10.1084/jem.190.8.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmitz M, Zhao S, Deuse Y, Schakel K, Wehner R, Wohner H, K, et al. Tumoricidal potential of native blood dendritic cells: direct tumor cell killing and activation of NK cell-mediated cytotoxicity. J Immunol. 2005;174:4127–4134. doi: 10.4049/jimmunol.174.7.4127. [DOI] [PubMed] [Google Scholar]
- 12.Stary G, Bangert C, Tauber M, Strohal R, Kopp T, Stingl G. Tumoricidal activity of TLR7/8-activated inflammatory dendritic cells. J Exp Med. 2007;204:1441–1451. doi: 10.1084/jem.20070021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaperot L, Blum A, Manches O, Lui G, Angel J, Molens JP, et al. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J Immunol. 2006;176:248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]
- 14.Hardy AW, Graham DR, Shearer GM, Herbeuval JP. HIV turns plasmacytoid dendritic cells (pDC) into TRAIL-expressing killer pDC and down-regulates HIV coreceptors by Toll-like receptor 7-induced IFN-alpha. Proc Natl Acad Sci U S A. 2007;104:17453–17458. doi: 10.1073/pnas.0707244104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janjic BM, Lu G, Pimenov A, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anticancer effector function of human immature dendritic cells. I. Involvement of an apoptosis-inducing pathway. J Immunol. 2002;168:1823–1830. doi: 10.4049/jimmunol.168.4.1823. [DOI] [PubMed] [Google Scholar]
- 16.Roncarolo MG, Bigler M, Haanen JB, Yssel H, Bacchetta R, de Vries JE, et al. Natural killer cell clones can efficiently process and present protein antigens. J Immunol. 1991;147:781–787. [PubMed] [Google Scholar]
- 17.Hanna J, Gonen-Gross T, Fitchett J, Rowe T, Daniels M, Arnon TI, R, et al. Novel APC-like properties of human NK cells directly regulate T cell activation. J Clin Invest. 2004;114:1612–1623. doi: 10.1172/JCI22787. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18.Ferlazzo G, Thomas D, Lin SL, Goodman K, Morandi B, Muller WA, et al. The abundant NK cells in human secondary lymphoid tissues require activation to express killer cell Ig-like receptors and become cytolytic. J Immunol. 2004;172:1455–1462. doi: 10.4049/jimmunol.172.3.1455. [DOI] [PubMed] [Google Scholar]
- 19.MacDonald KP, Munster DJ, Clark GJ, Dzionek A, Schmitz J, Hart DN. Characterization of human blood dendritic cell subsets. Blood. 2002;100:4512–4520. doi: 10.1182/blood-2001-11-0097. [DOI] [PubMed] [Google Scholar]
- 20.Root LL, Shipley GD. Normal human fibroblasts produce membrane-bound and soluble isoforms of FGFR-1. Mol Cell Biol Res Commun. 2002;3:87–97. doi: 10.1006/mcbr.2000.0199. [DOI] [PubMed] [Google Scholar]
- 21.Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179:89–94. doi: 10.4049/jimmunol.179.1.89. [DOI] [PubMed] [Google Scholar]
- 22.Papewalis C, Jacobs B, Wuttke M, Ullrich E, Baehring T, Fenk R, S H, et al. IFN-alpha skews monocytes into CD56+-expressing dendritic cells with potent functional activities in vitro and in vivo. J Immunol. 2008;180:1462–1470. doi: 10.4049/jimmunol.180.3.1462. [DOI] [PubMed] [Google Scholar]
- 23.Phillips JH, Le AM, Lanier LL. Natural killer cells activated in a human mixed lymphocyte response culture identified by expression of Leu-11 and class II histocompatibility antigens. J Exp Med. 1984;159:993–1008. doi: 10.1084/jem.159.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fogli M, Costa P, Murdaca G, Setti M, Mingari MC, Moretta L, A, et al. Significant NK cell activation associated with decreased cytolytic function in peripheral blood of HIV-1-infected patients. Eur J Immunol. 2004;34:2313–2321. doi: 10.1002/eji.200425251. [DOI] [PubMed] [Google Scholar]
- 25.Aranami T, Miyake S, Yamamura T. Differential expression of CD11c by peripheral blood NK cells reflects temporal activity of multiple sclerosis. J Immunol. 2006;177:5659–5667. doi: 10.4049/jimmunol.177.8.5659. [DOI] [PubMed] [Google Scholar]
- 26.Yano N, Endoh M, Nomoto Y, Sakai H, Rifai A. Increase of HLA-DR-positive natural killer cells in peripheral blood from patients with IgA nephropathy. Hum Immunol. 1996;49:64–70. doi: 10.1016/0198-8859(96)00057-2. [DOI] [PubMed] [Google Scholar]
- 27.Kataoka T, Shinohara N, Takayama H, Takaku K, Kondo S, Yonehara S, et al. Concanamycin A, a powerful tool for characterization and estimation of contribution of perforin- and Fas-based lytic pathways in cell-mediated cytotoxicity. J Immunol. 1996;156:3678–3686. [PubMed] [Google Scholar]
- 28.Pure E, Inaba K, Crowley MT, Tardelli L, Witmer-Pack MD, Ruberti G, et al. Antigen processing by epidermal Langerhans cells correlates with the level of biosynthesis of major histocompatibility complex class II molecules and expression of invariant chain. J Exp Med. 1990;172:1459–1469. doi: 10.1084/jem.172.5.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173:3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 30.Walzer T, Blery M, Chaix J, Fuseri N, Chasson L, Robbins SH, et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc Natl Acad Sci U S A. 2007;104:3384–3389. doi: 10.1073/pnas.0609692104. [DOI] [PMC free article] [PubMed] [Google Scholar]