Abstract
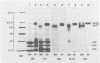
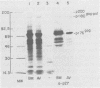
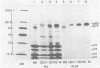
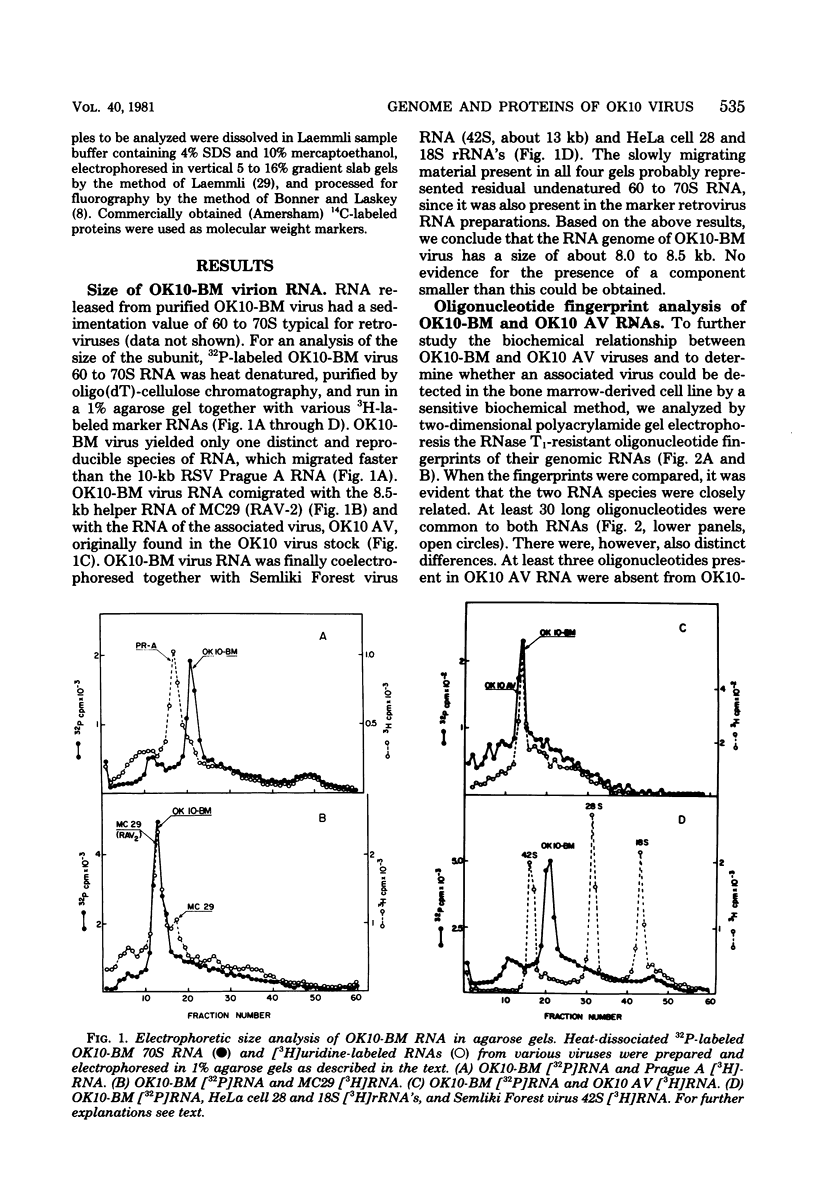
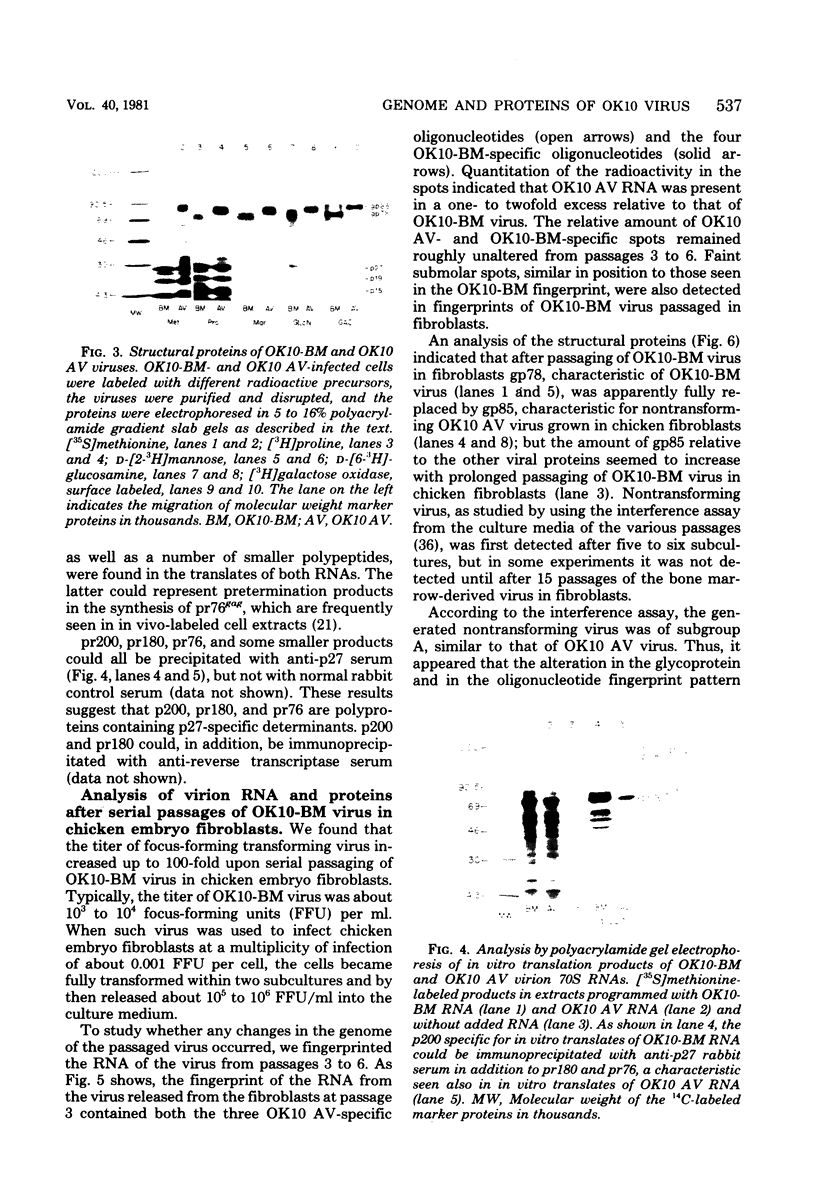
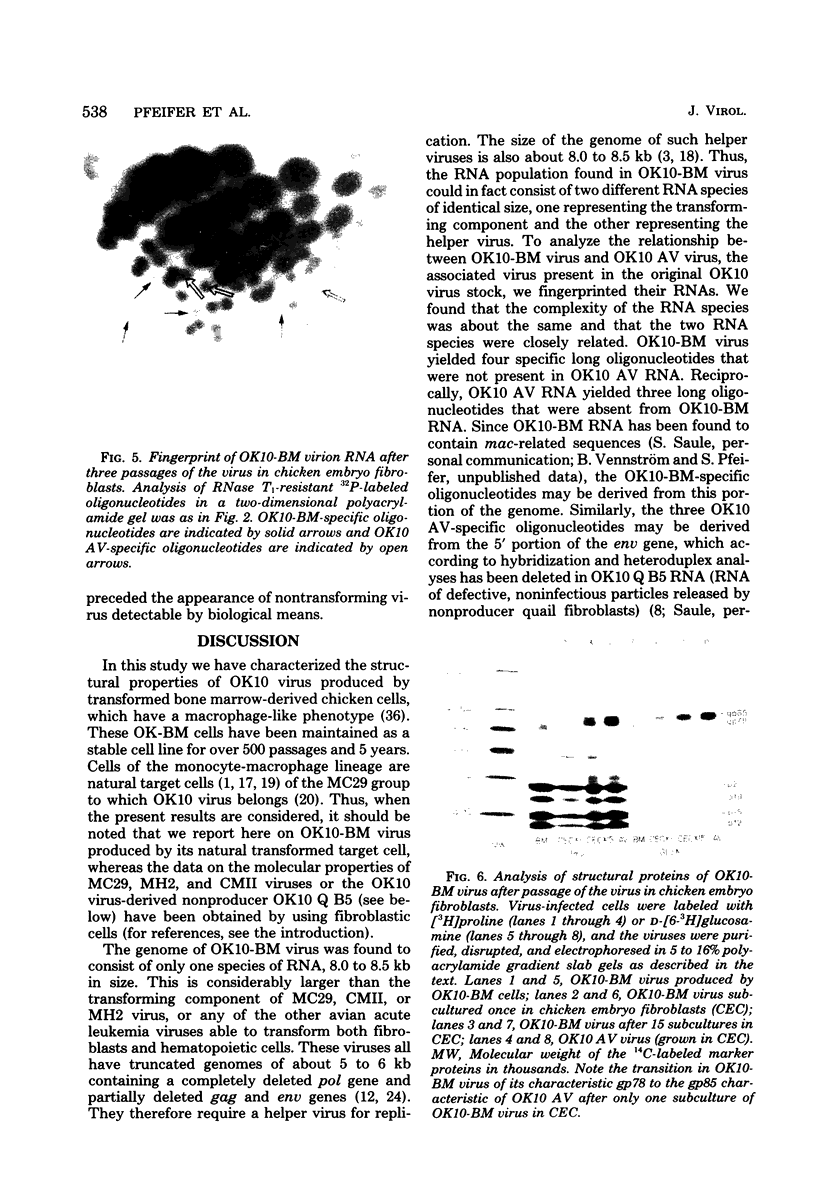
We have analyzed the structure of OK10-BM virus, an avian acute leukemia virus produced by a bone marrow-derived cell line of macrophage origin, and compared it with that of OK10 AV, an associated virus originally present in the OK10 virus stock. The RNAs of OK10-BM virus and OK10 AV had the same mobility in agarose gels, corresponding to 8.0 to 8.5 kilobases, a size considerably larger than that of the transforming component (5 to 6 kb) of most other avian acute leukemia viruses. Fingerprint analysis showed a close relationship between OK10-BM virus and OK10 AV RNAs. The polypeptide compositions of OK10-BM and OK10 AV viruses were similar except for the envelope glycoproteins. In analysis by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, the large envelope glycoprotein of OK10-BM virus migrated at Mr = 78,000 (gp78), whereas OK10 AV had the characteristic 85,000-dalton glycoprotein (gp85) of nondefective avian leukemia viruses. gp78 was weakly labeled with methionine, glycine, proline, or mannose, suggesting that purified OK10-BM virus had reduced amounts of the modified envelope glycoprotein. In cell-free rabbit reticulocyte lysates, OK10-BM virion RNA directed the synthesis of a 200,000-dalton polypeptide (p200), a 180,000-dalton polypeptide (pr180), and a 76,000-dalton polypeptide (pr76), whereas OK10 AV RNA gave rise only to pr180 and pr76, suggesting that p200 may represent an OK10-BM-encoded transforming protein. No biochemical evidence for the presence of an associated helper virus was found in the OK10-BM virus population produced by the macrophage cell line. However, when OK10-BM virus was serially passaged in chicken embryo fibroblasts, a virus having structural properties similar to those of OK10 AV (OK10 AV-specific oligonucleotides and gp85) appeared after three passages. Moreover, nonproducer clones of transformed cells could be readily obtained in OK10-BM virus-infected quail cell cultures. It is thus likely that the bone marrow-derived macrophage cell line produces a transforming virus defective in its env gene and low amounts of an associated helper virus, which upon transfer to fibroblasts is preferentially replicated.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beug H., von Kirchbach A., Döderlein G., Conscience J. F., Graf T. Chicken hematopoietic cells transformed by seven strains of defective avian leukemia viruses display three distinct phenotypes of differentiation. Cell. 1979 Oct;18(2):375–390. doi: 10.1016/0092-8674(79)90057-6. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Bister K., Duesberg P. H. Genetic structure of avian acute leukemia viruses. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):801–822. doi: 10.1101/sqb.1980.044.01.086. [DOI] [PubMed] [Google Scholar]
- Bister K., Löliger H. C., Duesberg P. H. Oligoribonucleotide map and protein of CMII: detection of conserved and nonconserved genetic elements in avian acute leukemia viruses CMII, MC29, and MH2. J Virol. 1979 Oct;32(1):208–219. doi: 10.1128/jvi.32.1.208-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Ramsay G., Hayman M. J., Duesberg P. H. OK10, an avian acute leukemia virus of the MC 29 subgroup with a unique genetic structure. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7142–7146. doi: 10.1073/pnas.77.12.7142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bister K., Vogt P. K. Genetic analysis of the defectiveness in strain MC29 avian leukosis virus. Virology. 1978 Jul 15;88(2):213–221. doi: 10.1016/0042-6822(78)90278-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Avian oncovirus proteins expressed on the surface of infected cells. Virology. 1980 Apr 30;102(2):251–261. doi: 10.1016/0042-6822(80)90092-6. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Moscovici C. Avian acute leukemia virus MC29: conserved and variable RNA sequences and recombination with helper virus. Virology. 1979 Nov;99(1):121–134. doi: 10.1016/0042-6822(79)90043-6. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Bister K., Vogt P. K. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. Avian acute leukemia viruses MC29 and MH2 share specific RNA sequences: evidence for a second class of transforming genes. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1633–1637. doi: 10.1073/pnas.76.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenman R. N., Vogt V. M. The biosynthesis of oncovirus proteins. Biochim Biophys Acta. 1978 Apr 6;473(3-4):187–239. doi: 10.1016/0304-419x(78)90014-8. [DOI] [PubMed] [Google Scholar]
- Gahmberg C. G. Tritium labeling of cell-surface glycoproteins and glycolipids using galactose oxidase. Methods Enzymol. 1978;50:204–206. doi: 10.1016/0076-6879(78)50020-7. [DOI] [PubMed] [Google Scholar]
- Gazzolo L., Moscovici C., Moscovici M. G. Persistence of avian oncoviruses in chicken macrophages. Infect Immun. 1979 Feb;23(2):294–297. doi: 10.1128/iai.23.2.294-297.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzolo L., Moscovici C., Moscovici M. G., Samarut J. Response of hemopoietic cells to avian acute leukemia viruses: effects on the differentiation of the target cells. Cell. 1979 Mar;16(3):627–638. doi: 10.1016/0092-8674(79)90036-9. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H., Hayman M. J. Target cell specificity of defective avian leukemia viruses: hematopoietic target cells for a given virus type can be infected but not transformed by strains of a different type. Proc Natl Acad Sci U S A. 1980 Jan;77(1):389–393. doi: 10.1073/pnas.77.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Oker-Blom N., Todorov T. G., Beug H. Transforming capacities and defectiveness of avian leukemia viruses OK10 and E 26. Virology. 1979 Dec;99(2):431–436. doi: 10.1016/0042-6822(79)90024-2. [DOI] [PubMed] [Google Scholar]
- Hayman M. J. Transforming proteins of avian retroviruses. J Gen Virol. 1981 Jan;52(Pt 1):1–14. doi: 10.1099/0022-1317-52-1-1. [DOI] [PubMed] [Google Scholar]
- Hayman M. J. Viral polyproteins in chick embryo fibroblasts infected with avian sarcoma leukosis viruses. Virology. 1978 Mar;85(1):241–252. doi: 10.1016/0042-6822(78)90428-2. [DOI] [PubMed] [Google Scholar]
- Hortling L. Rapid oncogenesis in vivo by chicken retrovirus OK10. Acta Pathol Microbiol Scand B. 1978 Aug;86(4):185–192. [PubMed] [Google Scholar]
- Hu S. S., Duesberg P. H., Lai M. M., Vogt P. K. Avian oncovirus MH2: preferential growth in macrophages and exact size of the genome. Virology. 1979 Jul 15;96(1):302–306. doi: 10.1016/0042-6822(79)90199-5. [DOI] [PubMed] [Google Scholar]
- Hu S. S., Lai M. M., Vogt P. K. Genome of avian myelocytomatosis virus MC29: analysis by heteroduplex mapping. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1265–1268. doi: 10.1073/pnas.76.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki R., Langlois A. J., Chabot J., Beard J. W. Component of strain MC29 avian leukosis virus with the property of defectiveness. J Virol. 1971 Dec;8(6):821–827. doi: 10.1128/jvi.8.6.821-827.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemenz R., Diggelmann H. Extracellular cleavage of the glycoprotein precursor of Rous sarcoma virus. J Virol. 1979 Jan;29(1):285–292. doi: 10.1128/jvi.29.1.285-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G. A rapid slide-agglutination method for typing pneumococci by means of specific antibody adsorbed to protein A-containing staphylococci. J Med Microbiol. 1973 May;6(2):187–190. doi: 10.1099/00222615-6-2-187. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moelling K., Sykora K. W., Dittmar K. E., Scott A., Watson K. F. The isolation of avian viral RNA and polypeptides. J Biol Chem. 1979 May 25;254(10):3738–3742. [PubMed] [Google Scholar]
- Oker-Blom N., Hortling L., Kallio A., Nurmiaho E. L., Westermarck H. OK 10 virus, an avian retrovirus resembling the acute leukaemia viruses. J Gen Virol. 1978 Sep;40(3):623–633. doi: 10.1099/0022-1317-40-3-623. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Persson H., Pettersson U., Mathews M. B. Synthesis of a structural adenovirus polypeptide in the absence of viral DNA replication. Virology. 1978 Oct 1;90(1):67–79. doi: 10.1016/0042-6822(78)90334-3. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Hewlett M. J., Baltimore D., Coffin J. M. The genome of Uukuniemi virus consists of three unique RNA segments. Cell. 1977 May;11(1):51–63. doi: 10.1016/0092-8674(77)90316-6. [DOI] [PubMed] [Google Scholar]
- Pettersson R., Käriäinen L., von Bonsdorff C. H., Oker-Blom N. Structural components of Uukuniemi virus, a noncubical tick-borne arbovirus. Virology. 1971 Dec;46(3):721–729. doi: 10.1016/0042-6822(71)90074-2. [DOI] [PubMed] [Google Scholar]
- Pfeifer S., Kallio A., Vaheri A., Pettersson R., Oker-Blom N. Stable bone-marrow-derived cell line producing transforming avian acute leukemia virus OK 10. Int J Cancer. 1980 Feb 15;25(2):235–242. doi: 10.1002/ijc.2910250211. [DOI] [PubMed] [Google Scholar]
- Ramsay G., Hayman M. J. Analysis of cells transformed by defective leukemia virus OK10: production of noninfectious particles and synthesis of Pr76gag and an additional 200,000-dalton protein. Virology. 1980 Oct 15;106(1):71–81. doi: 10.1016/0042-6822(80)90222-6. [DOI] [PubMed] [Google Scholar]
- Roussel M., Saule S., Lagrou C., Rommens C., Beug H., Graf T., Stehelin D. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979 Oct 11;281(5731):452–455. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- Stéhelin D., Saule S., Roussel M., Sergeant A., Lagrou C., Rommens C., Raes M. B. Three new types of viral oncogenes in defective avian leukemia viruses. I. Specific nucleotide sequences of cellular origin correlate with specific transformation. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1215–1223. doi: 10.1101/sqb.1980.044.01.132. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]