Abstract
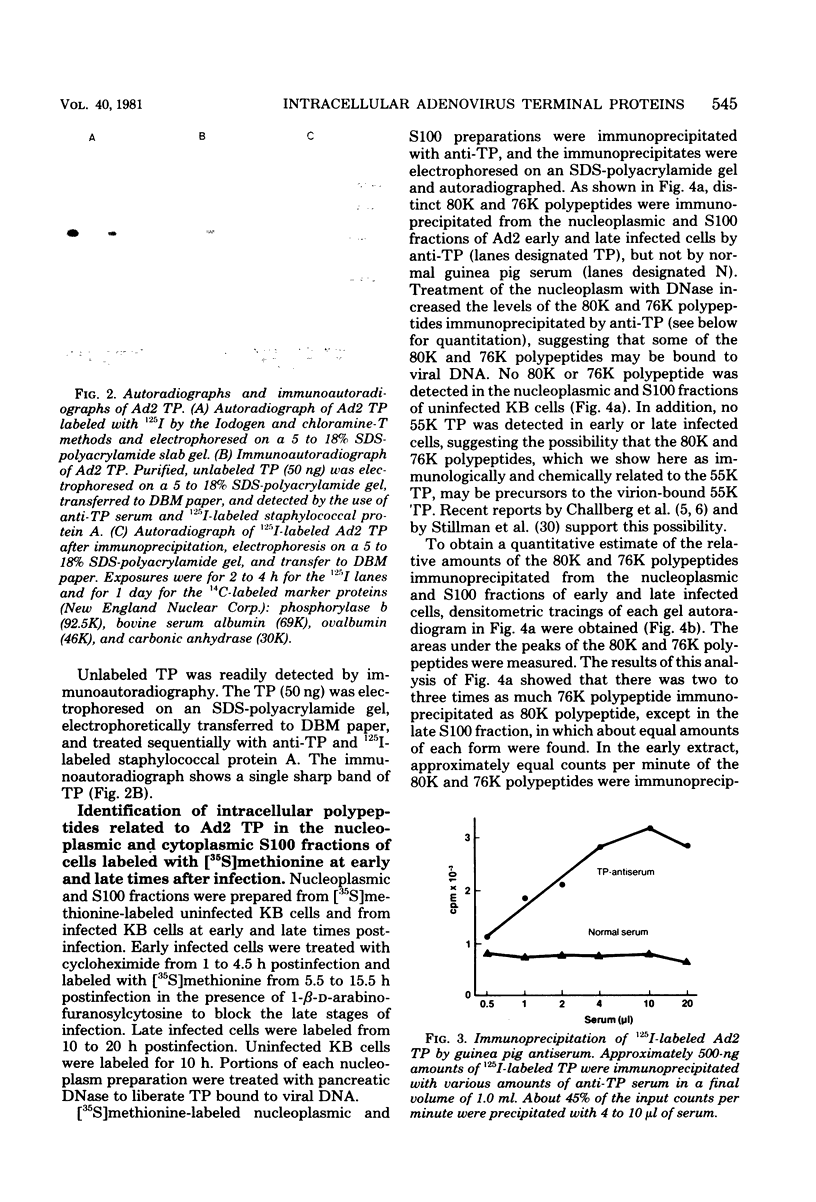
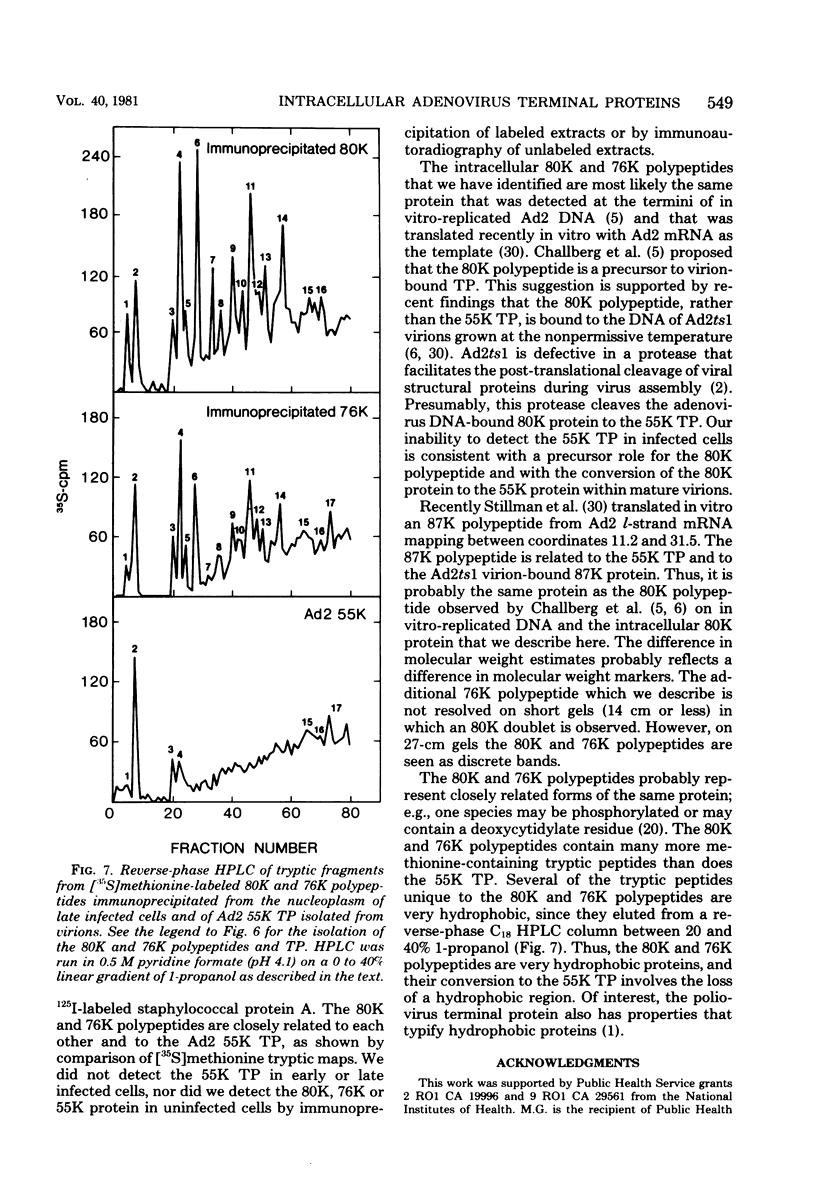
Highly purified adenovirus type 2 terminal protein (TP) with an apparent Mr of 55,000 (55K) was prepared in quantities of 10 to 30 μg from guanidine hydrochloride- or sodium dodecyl sulfate-disrupted virions (60 to 120 mg). Guinea pigs were immunized with 14 to 20 injections of TP in amounts of 1 to 2 μg. Antiserum to TP was used to study the intracellular polypeptides related to adenovirus type 2 TP. By immunoprecipitation with anti-TP serum, we identified 80K and 76K polypeptides in the nucleoplasmic and cytoplasmic S100 fractions of [35S]methionine-labeled cells early and late after infection with Ad2. By immunoautoradiographic analysis which eliminates coprecipitation of unrelated proteins, we identified an 80K polypeptide (probably an 80K-76K doublet) in unlabeled, late infected cells, using anti-TP serum and 125I-labeled staphylococcal protein A. About two- to threefold-higher levels of the 80K and 76K polypeptides were present in the nucleoplasm than in the S100 fraction, and two- to threefold-higher levels were found in late infected cells than in early infected cells (cycloheximide enhanced, arabinofuranosylcytosine treated). We did not detect the 80K or 76K polypeptide in uninfected cells, indicating that these polypeptides are virus coded. Tryptic peptide map analysis showed that the 80K and 76K polypeptides are very closely related and that they share peptides with the DNA-bound 55K TP. Our data provide the first direct demonstration of intracellular 80K and 76K forms of TP. The intracellular 80K and 76K polypeptides are closely related or identical to the 80K polypeptide that Challberg and co-workers (Proc. Natl. Acad. Sci. U.S.A. 77:5105-5109, 1980) detected at the termini of adenovirus DNA synthesized in vitro and to the 87K polypeptide that Stillman and co-workers (Cell 23:497-508, 1981) translated in vitro. We did not detect the 55K TP in early or late infected cells, consistent with the proposal by Challberg and co-workers that the 80K polypeptide is a precursor to the virion-bound TP and that the conversion of the 80K polypeptide to the 55K TP occurs during virus maturation. The 80K and 76K polypeptides have many more methionine-containing tryptic peptides than does the 55K TP, and most of the tryptic peptides unique to the 80K and 76K polypeptides are very hydrophobic. Thus, the conversion of the 80K and 76K polypeptides to the 55K TP may involve the removal of a specific hydrophobic protein region.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V., Baltimore D. Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978 Aug 10;253(15):5263–5266. [PubMed] [Google Scholar]
- Bhatti A. R., Weber J. Protease of adenovirus type 2. Subcellular localization. J Biol Chem. 1979 Dec 25;254(24):12265–12268. [PubMed] [Google Scholar]
- Brackmann K. H., Green M., Wold W. S., Cartas M., Matsuo T., Hashimoto S. Identification and peptide mapping of human adenovirus type 2-induced early polypeptides isolated by two-dimensional gel electrophoresis and immunoprecipitation. J Biol Chem. 1980 Jul 25;255(14):6772–6779. [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Desiderio S. V., Kelly T. J., Jr Adenovirus DNA replication in vitro: characterization of a protein covalently linked to nascent DNA strands. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5105–5109. doi: 10.1073/pnas.77.9.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J., Jr Processing of the adenovirus terminal protein. J Virol. 1981 Apr;38(1):272–277. doi: 10.1128/jvi.38.1.272-277.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs D. H., Robinson A. J., Bodnar J. W., Jones C. J., Pearson G. D. Detection of covalent DNA-protein complexes: the adenovirus DNA-terminal protein complex and HeLa DNA-protein complexes. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):741–753. doi: 10.1101/sqb.1979.043.01.082. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. Similarity of DNAs isolated from tumor-inducing viruses of human and animal origin. Proc Natl Acad Sci U S A. 1963 Jul;50:44–46. doi: 10.1073/pnas.50.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- Green M., Arens M. Q., Yamashita T., Wold W. S., Brackmann K. H. Adenovirus-2 DNA: in vitro replication and analysis of viral early proteins. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):755–767. doi: 10.1101/sqb.1979.043.01.083. [DOI] [PubMed] [Google Scholar]
- Green M., Brackmann K., Wold W. S., Cartas M., Thornton H., Elder J. H. Conserved primary sequences of the DNA terminal proteins of five different human adenovirus groups. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4380–4384. doi: 10.1073/pnas.76.9.4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Mackey J. K., Wold W. S., Rigden P. Thirty-one human adenovirus serotypes (Ad1-Ad31) form five groups (A-E) based upon DNA genome homologies. Virology. 1979 Mar;93(2):481–492. doi: 10.1016/0042-6822(79)90251-4. [DOI] [PubMed] [Google Scholar]
- Green M., Piña M., Kimes R., Wensink P. C., MacHattie L. A., Thomas C. A., Jr Adenovirus DNA. I. Molecular weight and conformation. Proc Natl Acad Sci U S A. 1967 May;57(5):1302–1309. doi: 10.1073/pnas.57.5.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Wold W. S., Brackmann K. H., Cartas M. A. Identification of families of overlapping polypeptides coded by early "transforming" gene region 1 of human adenovirus type 2. Virology. 1979 Sep;97(2):275–286. doi: 10.1016/0042-6822(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S., Brackmann K. H., Cartas M. A. The 55K protein on the 5' termini of adenovirus type 2 DNA is unrelated to virus-coded candidate transformation proteins (E1-53K, E1-40K-50K) and DNA-binding proteins (E2-42K/47K/73K). J Virol. 1979 Sep;31(3):836–840. doi: 10.1128/jvi.31.3.836-840.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M., Wold W. S. Human adenoviruses: growth, purification, and transfection assay. Methods Enzymol. 1979;58:425–435. doi: 10.1016/s0076-6879(79)58157-9. [DOI] [PubMed] [Google Scholar]
- Harter M. L., Lewis J. B., Anderson C. W. Adenovirus type 2 terminal protein: purification and comparison of tryptic peptides with known adenovirus-coded proteins. J Virol. 1979 Sep;31(3):823–835. doi: 10.1128/jvi.31.3.823-835.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Lechner R. L. The structure of replicating adenovirus DNA molecules: characterization of DNA-protein complexes from infected cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):721–728. doi: 10.1101/sqb.1979.043.01.080. [DOI] [PubMed] [Google Scholar]
- Kimura S., Lewis R. V., Gerber L. D., Brink L., Rubinstein M., Stein S., Udenfriend S. Purification to homogeneity of camel pituitary pro-opiocortin, the common precursor of opioid peptides and corticotropin. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1756–1759. doi: 10.1073/pnas.76.4.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichy J. H., Horwitz M. S., Hurwitz J. Formation of a covalent complex between the 80,000-dalton adenovirus terminal protein and 5'-dCMP in vitro. Proc Natl Acad Sci U S A. 1981 May;78(5):2678–2682. doi: 10.1073/pnas.78.5.2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Surface-specific iodination of membrane proteins of viruses and eucaryotic cells using 1,3,4,6-tetrachloro-3alpha,6alpha-diphenylglycoluril. Biochemistry. 1978 Oct 31;17(22):4807–4817. doi: 10.1021/bi00615a031. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R., Padmanabhan R. V. Specific interaction of a protein(s) at or near the termini of adenovirus 2 DNA. Biochem Biophys Res Commun. 1977 Apr 25;75(4):955–964. doi: 10.1016/0006-291x(77)91475-9. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Bellett J. D. A circular DNA-protein complex adenoviruses and its possible role in DNA replication. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):523–531. doi: 10.1101/sqb.1974.039.01.064. [DOI] [PubMed] [Google Scholar]
- Robinson A. J., Younghusband H. B., Bellett A. J. A circula DNA-protein complex from adenoviruses. Virology. 1973 Nov;56(1):54–69. doi: 10.1016/0042-6822(73)90287-0. [DOI] [PubMed] [Google Scholar]
- Rubinstein M., Chen-Kiang S., Stein S., Udenfriend S. Characterization of proteins and peptides by high-performance liquid chromatography and fluorescence monitoring of their tryptic digests. Anal Biochem. 1979 May;95(1):117–121. doi: 10.1016/0003-2697(79)90193-3. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Bellett A. J. An adenovirus protein associated with the ends of replicating DNA molecules. Virology. 1979 Feb;93(1):69–79. doi: 10.1016/0042-6822(79)90276-9. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Bellett A. J. Replication of DNA in adenovirus-infected cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):729–739. doi: 10.1101/sqb.1979.043.01.081. [DOI] [PubMed] [Google Scholar]
- Stillman B. W., Lewis J. B., Chow L. T., Mathews M. B., Smart J. E. Identification of the gene and mRNA for the adenovirus terminal protein precursor. Cell. 1981 Feb;23(2):497–508. doi: 10.1016/0092-8674(81)90145-8. [DOI] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Protein synthesized early after infection is linked to the termini of adenovirus type 2 DNA synthesized in vivo and in vitro. J Virol. 1979 May;30(2):497–507. doi: 10.1128/jvi.30.2.497-507.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wielink P. S., Naaktgeboren N., Sussenbach J. S. Presence of protein at the termini of intracellular adenovirus type 5 DNA. Biochim Biophys Acta. 1979 Jun 20;563(1):89–99. doi: 10.1016/0005-2787(79)90010-8. [DOI] [PubMed] [Google Scholar]