Abstract
Perturbed neuronal Ca2+ homeostasis is implicated in age-related cognitive impairment and Alzheimer’s disease (AD). With advancing age neurons encounter increased oxidative stress and impaired energy metabolism, which compromise the function of proteins that control membrane excitability and subcellular Ca2+ dynamics. Toxic forms of amyloid β-peptide (Aβ) may induce Ca2+ influx into neurons by inducing membrane-associated oxidative stress or by forming an oligomeric pore in the membrane, thereby rendering neurons vulnerable to excitotoxicity and apoptosis. AD-causing mutations in the β-amyloid precursor protein and presenilins may compromise normal of these proteins in the plasma membrane and endoplasmic reticulum, respectively. Emerging knowledge of the actions of Ca2+ upstream and downstream of Aβ provide opportunities to develop novel preventative and therapeutic interventions for AD.
Neuronal Ca2+ Signaling in Healthy Brains and the Adverse Effects of Aging
Neurons use Ca2+ signals to control membrane excitability, to trigger release of a neurotransmitter, to mediate activity-dependent changes in gene expression and modulate neuronal growth, differentiation and transition to apoptosis1. Neuronal Ca2+ signaling involves an intricate interplay between Ca2+ influx across plasma membrane through voltage-gated Ca2+ channels, NMDA receptors and TRP (transient receptor potential) channels, and Ca2+ release from intracellular Ca2+ stores via inositol triphosphate receptor (IP3R) and ryanodine receptor (RyR) channels in the endoplasmic reticulum (ER). Intracellular Ca2+ release via IP3R is triggered by second messenger IP3 which is produced following activation of metabotropic receptors coupled to phospholipase C. Neuronal RyR function primarily as Ca2+-activated Ca2+ channels which further amplify Ca2+ signals originating from other sources. Mitochondria also play an important role in shaping neuronal Ca2+ signaling by utilizing potent mitochondrial Ca2+ uptake mechanisms. Ca2+ uptake into mitochondria plays an important role in neuronal physiology by stimulating mitochondrial metabolism and increasing mitochondrial energy production. Excessive Ca2+ uptake into mitochondria can lead to opening of a permeability transition pore (PTP) and apoptosis2. Owing to its importance for neuronal function, Ca2+ signaling in neurons is tightly compartmentalized and regulated within signaling microdomains which involve, for example, functional coupling between voltage-gated Ca2+ channels and intracellular Ca2+ release channels, or between ER Ca2+ release and Ca2+ uptake into mitochondria.
The major risk factor for Alzheimer’s disease (AD) is advancing age; in the most common sporadic form of AD the individuals first manifest symptoms when they are in their 7th or 8th decades of life. But even those who inherit a disease-causing mutation in the β-amyloid precursor protein (APP) or one of the presenilins (PS1 and PS2) remain asymptomatic into their fourth or fifth decades3. Age-related alterations in specific Ca2+-regulating systems in brain cells have been reported including: elevated intracellular Ca2+ levels; enhanced Ca2+ influx through voltage-dependent Ca2+ channels; impaired ability of mitochondria to buffer or cycle Ca2+; perturbed Ca2+ regulation in ryanodine and IP3-sensitive Ca2+ stores. Gene array and proteomic analyses suggest dysregulation of the expression of an array of Ca2+-handling systems during aging4–7. Many of the alterations in Ca2+-handling described in normal aging can be reproduced by subjecting neurons to oxidative and metabolic stress in culture or in vivo, suggesting important contributions of these fundamental aging processes to the dysregulation of neuronal Ca2+ regulation in AD described below. Moreover, studies of brain tissue samples obtained from brains of AD patients and animal models of AD have revealed significant alterations in levels of proteins and genes directly involved in neuronal Ca2+ signaling8–10. Many of the latest advances in understanding the roles of perturbed cellular Ca2+ handling in AD pathogenesis are described in the remainder of this article.
Amyloid β-Peptide Promotes Ca2+ Influx and Ca2+-Mediated Excitotoxicity
Amyloid plaques, a histological hallmark of AD, are comprised of extracellular aggregates of the amyloid β-peptide (Aβ) a 40–42 amino acid peptide generated by successive enzymatic cleavages of APP by β- and γ-secretases (Fig. 1). Aβ is believed to be a pivotal mediator of neuronal degeneration and impaired cognitive function in AD3,11. Adverse effects of Aβ on synaptic function and neuronal survival are mediated primarily by soluble protein oligomers12. Aβ interaction with the plasma membrane results in elevated intracellular (cytoplasmic) Ca2+ concentrations ([Ca2+]i) and increased vulnerability of the neurons to excitotoxicity13. Oligomeric forms of Aβ42 cause Ca2+-mediated toxicity in cultured cells14. Degenerative changes occur in neurites associated with Aβ deposits in APP mutant mice, suggesting the involvement of Ca2+-mediated Aβ neurotoxicity in vivo15. In addition to increasing the production of Aβ, amyloidogenic processing of APP may perturb neuronal Ca2+ homeostasis by decreasing the production of a secreted form of APP (sAPPα) that activates K+ channels16, and by generating an APP intracellular domain that affects ER Ca2+ release by regulating the expression of genes involved in Ca2+ homeostasis (Fig. 1)17.
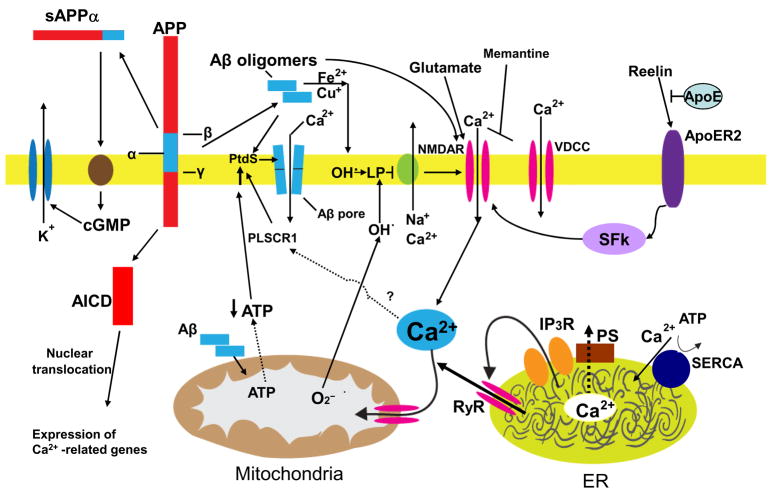
Figure 1.
Mechanisms that may result in perturbed neuronal Ca2+ homeostasis in Alzheimer’s disease. Sequential cleavages of the β-amyloid precursor protein (APP) by β-secretase (β) and γ-secretase (γ) generates the amyloid β-peptide (Aβ). Aβ forms oligomers which can insert into the plasma membrane and form pores through which Ca2+ passes into the cytoplasm. The interaction of Aβ with the plasma membrane may be facilitated by binding to phosphatidylserine (PtdS); age/AD-related mitochondrial impairment (ATP depletion) may trigger flipping of PtdS from the inner portion of the plasma membrane to the cell surface. The PtdS flipping may also result from Ca2+ influx or release from the endoplasmic reticulum (ER) or mitochondria which can activate a phospholipid scramblase (PLSCR1). Aβ can also interact with Fe2+ and Cu+ to generate hydrogen peroxide and hydroxyl radical (OH.) resulting in membrane lipid peroxidation which generates toxic aldehydes that impair the function of membrane ion-motive ATPases (Na+ and Ca2+ pumps). As a result the membrane becomes depolarized and glutamate receptor channels (NMDAR, N-methyl-D-aspartate receptor) and voltage-dependent Ca2+ channels (VDCC) open and flux toxic amounts of Ca2+ into the cytoplasm. In addition, Aβ acts on mitochondria (either directly or indirectly by causing elevated cytoplasmic Ca2+ levels and oxidative stress) to cause increased free radical (superoxide anion radical; O2−) production, Ca2+ overload and decreased ATP production. Amyloidogenic processing also generates an intracellular APP domain (AICD) which can translocate to the nucleus and modify gene transcription in ways that perturb Ca2+ homeostasis. Studies of the presenilins (PS) have implicated ER Ca2+ mishandling in AD. PS functions as an ER Ca2+ leak channel and FAD PS mutations impair this Ca2+ leak channel function resulting in excessive accumulation of Ca2+ in the ER and, as a consequence, enhances Ca2+ release through ryanodine receptor (RyR) and IP3 receptor (IP3R) channels. There is also evidence that PS can interact directly or indirectly with RyR and SERCA (smooth endoplasmic reticulum Ca2+-ATPase) to alter ER Ca2+ release and uptake. Interaction of the protein reelin with the apolipoprotein E receptor (ApoER2) enhances Ca2+ influx through NMDA receptor channels by a mechanism involving a src family tyrosine kinsase (SFk); ApoE can block this effect of reelin. Finally, amyloidogenic APP processing may prevent α-secretase (α) cleavage of APP which would otherwise generate a secreted form of APP (sAPPα). sAPPα is normally produced in response to synaptic activity and is known to activate a signaling pathway involving cyclic guanosine monophosphate (cGMP) that activates K+ channels, thereby hyperpolarizing the membrane and reducing Ca2+ influx. The mechanisms illustrated here include abundant targets for therapeutic intervention (see also Fig. 3) and one drug that targets the NMDA receptor (memantine) is currently used to treat AD patients.
One mechanism by which Aβ may cause Ca2+ influx is by inserting into the plasma membrane and forming ion-conducting pores18 (Fig. 1). Neurotoxic forms of Aβ are oligomers that share structural and functional homology with pore-forming bacterial toxins and the cytotoxic lymphocyte protein perforin19. Interestingly, the ability of Aβ to associate with membranes and form channels is enhanced by exposure of phosphatidylserine on the cell surface20. Because cell surface exposure of phosphatidylserine is usually indicative of apoptotic or energy-deprived cells, it is possible that age-related mitochondrial impairments may increase surface phosphatydylserine levels in affected neurons and thereby facilitate Aβ-mediated pore formation, Ca2+ influx and cell death (Fig. 1); indeed, neurons with reduced cytosolic ATP levels and elevated surface phosphatidylserine are particulary vulnerable to Aβ toxicity21. The surface exposure of phosphatidylserine may also result from activation of a Ca2+-sensitive phospholipid scrambalase 1 (PLSCR1) which mediates a rapid trans-bilayer reorganization of plasma membrane phospholipids22 (Fig. 1).
A different mechanism by which Aβ perturbs neuronal Ca2+ homeostasis is by inducing membrane lipid peroxidation11. During peptide oligomer formation Aβ generates hydrogen peroxide, a process enhanced by iron (Fe2+) and copper (Cu+)23,24. Hydrogen peroxide is then converted to hydroxyl radical which initiates lipid peroxidation resulting in the generation of toxic lipid aldehydes such a 4-hydroxynonenal which impair the function of ion-motive ATPases, and glutamate and glucose transporters resulting in Ca2+ overload, synaptic dysfunction, neuronal degeneration and cognitive impairment11,25. Particularly striking, is the ability of Aβ to increase the vulnerability of neurons to excitotoxicity mediated by the N-methyl-D-aspartate (NMDA) receptor11,13. Because excessive and sustained Ca2+ elevations induce free radical production (by altering mitochondrial oxidative phosphorylation and activating oxygenases) it is likely that perturbed Ca2+ homeostasis contributes to the increased oxidative stress in neurons in AD, resulting in a self-amplifying cascade of free radical- and Ca2+-mediated degenerative processes (Fig. 1). Lesser amounts of Aβ may also be toxic to neurons. For example, exposure of rat organotypic hippocampal slice cultures to picomolar concentrations of Aβ oligomers caused the loss of dendritic spines and decreased numbers of electrophysiologically active synapses; the spine loss was reversible and required NMDA receptor activity26. Aβ oligomers cause an increase in NMDA receptor activity, which may require direct association between Aβ oligomers and NR1 subunit of the NMDAR. On the other hand, Aβ oligomers may suppress activity of presynaptic P/Q-type voltage-gated Ca2+ channels27. Aβ also blocks the response of α7-containing nicotinic acetylcholine receptors (nAChRs) in hippocampal neurons28 and directly evokes sustained nAChR-mediated increases in presynaptic Ca2+ levels29 suggesting a mechanism for impairment of cholinergic signaling in AD.
Enter the Presenilins
Presenilins (PS1 and the structurally and functionally related PS2) are integral membrane proteins. The holoprotein form of presenilins is located in the ER. Both PS1 and PS2 holoproteins undergo endoproteolysis in the cytosolic loop between the 6th and 7th transmembrane domains, resulting in the generation of amino-terminal and carboxy-terminal fragments, which remain associated with each other. Cleaved presenilins assemble with nicastrin, Aph-1 and Pen-2, exit the ER and translocate into the Golgi apparatus and eventually to plasma membrane. A mature complex of cleaved presenilins, nicastrin, Aph-1 and Pen-2 possesses aspartyl protease activity and functions as the γ-secretase enzyme that cleaves APP to generate Aβ3,11. Many mutations in presenilins that cause familial (dominantly inherited) AD (FAD) increase the production of the long aggregation-prone form of Aβ (Aβ42) or reduce the production of a short soluble form Aβ40, and therefore one way in which presenilin mutations may perturb neuronal Ca2+ homeostasis is by elevating Aβ42:Aβ40 ratio and activating the Aβ oligomer-mediated mechanisms described above.
Additional roles for presenilins in modulating Ca2+ homeostasis are suggested by data linking two other presenilin substrate cleavage products, the AICD and the Notch intracellular domain (NICD), to Ca2+-mediated neuroplasticity and cell death. AICD may translocate to the nucleus and therein regulate the expression of genes encoding proteins involved in Ca2+ homeostasis17. Notch, a membrane receptor activated by cell surface-associated ligands such as Jagged and Delta, plays fundamental roles in regulating the proliferation and differentiation of neural progenitor cells in the developing and adult brain30. Upon ligand binding, γ-secretase cleaves Notch to release the NICD which translocates to the nucleus where it regulates gene transcription. Recent findings suggest potential roles for Notch and NICD in synaptic plasticity, learning and memory and Ca2+-mediated cell death31.
A γ-secretase-independent connection between presenilins and Ca2+ signaling was initially suggested in Ca2+ imaging experiments with fibroblasts from FAD patients containing PS1-A246E mutation32. It was then shown that cultured neural cells expressing AD PS1 mutations exhibit increased amounts of Ca2+ released from the ER when exposed to ligands that stimulate IP3 production or activation of RyR33,34. Similar results were obtained in Ca2+ imaging experiments with Xenopus oocytes injected with cRNA encoding PS1-M146V and PS2-N141I FAD mutants35, in experiments with synaptosomes and cortical neurons from PS1-M146V mutant mice36,37, and in hippocampal neurons from PS2-N141I transgenic mice38. In vitro and in vivo studies demonstrated that exaggerated ER Ca2+ signaling resulting from FAD mutations in presenilins leads to sensitization of PS-FAD neurons to Aβ and excitotoxic cell death via a Ca2+-dependent mehanism involving excessive Ca2+ release from the ER36–39.
Biochemical and functional interactions have been uncovered between presenilins and several ER Ca2+-regulating proteins including ryanodine receptors40, sorcin41, the myristoylated calcium-binding protein calmyrin42 and calsenilin43. Presenilins may also modulate SERCA Ca2+ pump activity44. Presenilin-2 has been reported to associate with IP3R and to enhance IP3R activity45. Specific effects of FAD mutants PS1-M146V and PS2-N141I on sensitivity of IP3R1 to activation by IP3 have been recently discovered in patch-clamp experiments46. A significant increase in ryanodine receptor expression levels has been reported in brains from PS1 mutant mice47, an alteration that increases as the mice age, providing a potential link between AD pathogenesis and aging.
While the studies described above suggest that FAD mutations in presenilins act by altering a normal function of other Ca2+-regulating proteins, recent findings indicated that presenilins themselves may play a direct role in Ca2+ signaling. It is well established known that the ER membrane is “leaky” for Ca2+, but the exact identity of the putative “Ca2+ leak channel” was previously unknown. Recent results suggest that presenilins function as ER Ca2+ leak channels in cells, and that a balance between SERCA Ca2+ pump activity and presenilin-mediated passive Ca2+ leak determines the steady-state resting ER Ca2+ levels in cells48. The ER Ca2+ leak function of presenilins does not involve γ-secretase activity and is not supported by a cleaved form of presenilins; instead many FAD mutations in presenilins result in “loss of function” for ER Ca2+ leak activity48,49 resulting in excessive Ca2+ accumulation in the ER (Fig. 1). Although most tested FAD mutants in presenilinscompromiseed its ER Ca2+ leak function, the PS1-ΔE9 mutant was unique and appeared to act as a “gain of function” leading to “superleaky” channels48. The “gain of Ca2+ leak function” phenotype of PS1-ΔE9 mutant is consistent with an earlier observation of elevated basal Ca2+ levels in neuronal cells transfected with PS1-ΔE9 expression construct50 Thus, the cells expressing PS1-ΔE9 mutants are expected to be exposed to constitutively elevated cytosolic Ca2+ levels and partially depleted ER. This is in contrast to cells expressing “loss of ER leak function” PS FAD mutants, which are expected to have normal steady-state cytosolic Ca2+ levels and overloaded ER. Interestingly, the PS1-ΔE9 mutation is associated with a unique cotton wool plaques and spastic paraparesis clinical phenotype (CWP/SP), which is not observed for most other FAD PS1 mutations51. It will be very important to determine if other FAD mutations in PS1 associated with CWP/SP phenotype may also be associated with “gain of function” for the ER Ca2+ leak activity. If such a correlation is established, it would support a causal connection between ER Ca2+ dyshomeostasis and Aβ pathology in AD.
Calcium and the cytoskeletal pathology in AD
Neurofibrillary tangles, the most overt manifestation of cytoskeletal abnormalities in AD, consist of intracellular fibrillar aggregates of hyperphosphorylated forms of the microtubule-associated protein tau11. Tau is normally located in axons where it maintains microtubules in a polymerized state, but in AD tau dissociates from microtubules resulting in microtubule depolymerization and the accumulation of tau in the cell body. Studies of AD patient brain tissue samples suggest an association between elevated [Ca2+]i and neurofibrillary pathology. For example, neurons prone to neurofibrillary tangle formation are enriched in type II calcium/calmodulin-dependent protein kinase52, and calpains (Ca2+-dependent proteases that cleave cytoskeletal proteins) are elevated in vulnerable neuronal populations early in the disease process53. Overactivation of glutamate receptors in hippocampal neurons can cause Ca2+-mediated changes in tau and microtubules similar to those seen in neurofibrillary tangles72 suggesting a possible cause-effect relationship between aberrant increases in [Ca2+]i and tangle formation (Fig. 2). In addition, Ca2+ can cause AD-like tau phosphorylation and intracellular Aβ accumulation in neurons54. Conversely, tau mutations that cause tangle formation in frontotemporal lobe dementia alter the function of voltage-dependent Ca2+ channels in a manner that increases Ca2+ influx55 and may contribute to the cell death process in this disease.
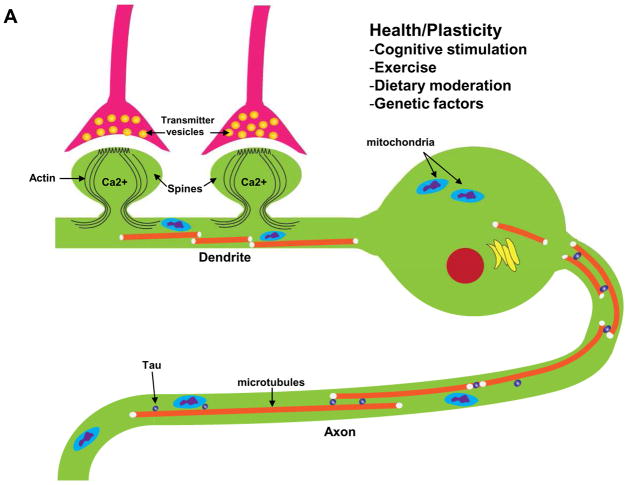
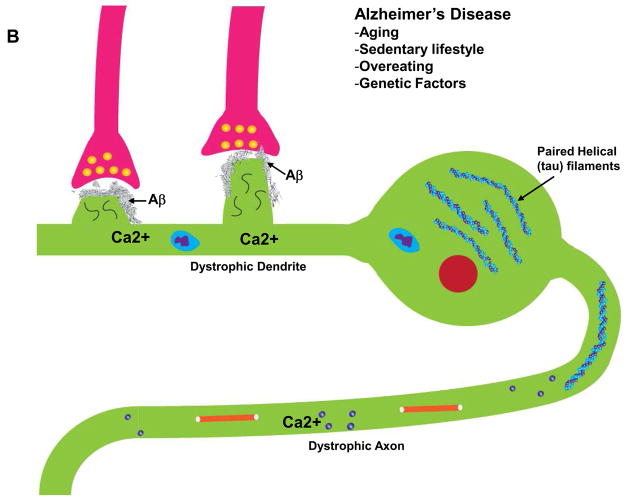
Figure 2.
Interactions of Ca2+ and the neuronal cytoskeleton in AD pathogenesis. A. In healthy neurons the axon contains relatively high amounts of microtubules which are stabilized by the protein tau. Microtubule dynamics in axons play pivotal roles in organellar (mitochondria, for example) and protein transport to presynaptic axon terminals. Dendrites receive synaptic inputs in postsynaptic structures called spines whose shape is controlled by actin filaments and various scaffolding proteins. Ca2+ influx during synaptic activity modifies the dynamics of actin and microtubules in ways that allow the neuron to adapt to environmental demands. B. During the course of AD tau becomes hyperphosphorylated and dissociates from microtubules which then depolymerize. The hyperphosphorylated tau self-aggregates and accumulates in the cell body where it forms paired-helical filaments (neurofibrillary tangles). As a consequence of accumulation of Aβ at synapses, Ca2+ regulation is impaired, the dendritic spines atrophy and the underlying cytoskeletal scaffold is disrupted resulting in synaptic degeneration. In these ways, cytoskeletal abnormalities underlie cognitive impairment in AD.
Calcium Actions Upstream of Amyloidogenesis
The placement of Aβ at the apex of the amyloid cascade hypothesis belies the fact there must be changes that occur during aging and AD that result in increased production and aggregation of Aβ. Evidence suggests that Ca2+ may be such an upstream factor. Environmental factors that inhibit amyloidogenesis (caloric restriction, cognitive stimulation and antioxidants) stabilize neuronal Ca2+ homeostasis, whereas factors that enhance amyloidogenesis disrupt Ca2+ homeostasis. In addition to these kinds of circumstantial evidence, direct evidence that Ca2+ influences APP processing has been reported. Exposure of cultured neurons to Ca2+ ionophores increases their production of Aβ56, as do conditions such as ischemia that cause sustained elevations of [Ca2+]i57. On the other hand, physiological Ca2+ transients (as occur during LTP, for example) increase α-secretase cleavage of APP and may thereby decrease Aβ production58,59.
Synapses: the Weakest Link
Studies of patients with mild cognitive impairment and AD suggest that synaptic dysfunction and degeneration may occur relatively early in the disease process, and studies of AD mouse models uniformly support this tenet11. Synaptic terminals are particularly vulnerable to Ca2+-mediated degeneration because they experience repeated bouts of Ca2+ influx and have unusually high energy requirements to support their ion-homeostatic and signaling systems. APP is actively transported to presynaptic terminals and considerable evidence suggests that Aβ is produced and accumulated primarily in synaptic regions60. Aβ can directly disrupt Ca2+ homeostasis in synaptic terminals by causing membrane-associated oxidative stress11. Consistent with a major role for Aβ in synaptic damage in AD are data showing loss of dendritic spines in dendrites associated with Aβ deposits in APP mutant mice61. Aβ also causes down-regulation of expression of calcineurin, a Ca2+-activated phosphatase known to play fundamental roles in synaptic plasticity62. Aβ oligomers caused a rapid decrease in membrane expression of NMDA and EphB2 receptors, followed by abnormal dendritic spine morphology and degeneration of spines63. The latter effects of Aβ are prevented by treatment with an NMDA receptor antagonist suggesting a major role for Ca2+ influx in the dendritic dystrophy. Moreover, Aβ immunotherapy prevented synaptic dysfunction and restores cognitive function in a mouse model of AD64.
Electrophysiological analyses of synaptic activity in hippocampal slices from APP and PS1 mutant mice have revealed abnormalities in several aspects of Ca2+-mediated synaptic function. APP mutant mice exhibit abnormal excitatory neuronal activity and compensatory remodeling of inhibitory circuits in the hippocampus65. Expression of mutant PS1 in cultured hippocampal neurons results in a significant depression of the amplitude of evoked AMPA and NMDA receptor-mediated synaptic currents, and a lower frequency of spontaneous miniature synaptic currents66. Aβ impairs spike-timing-dependent synaptic potentiation at excitatory synapses on neocortical layer 2/3 cortical pyramidal cells in APP mutant mice, which was associated with a decrease in AMPA but not NMDA receptor-mediated currents67. Aβ may also perturb Ca2+ handling in neural stem cells resulting in impaired hippocampal neurogenesis and a compromised ability to form and integrate new neurons from endogenous stem cells68.
PS1 mutations have a local adverse effect on synaptic Ca2+ regulation that may contribute to mitochondrial dysfunction and synaptic degeneration in AD. Thus, synaptosomes from PS1 mutant transgenic mice which exhibit enhanced elevations of cytoplasmic Ca2+ levels following exposure to depolarizing agents, Aβ, and a mitochondrial toxin compared with synaptosomes from nontransgenic mice and mice overexpressing wild-type PS139. Two-photon imaging studies revealed a 10-fold enhancement in RyR-mediated Ca2+ release in spines of PS1-M146V mutant-expressing mice, indicating a major alteration in synaptic ER Ca2+ handling in this AD model (Beth Stutzmann, personal communication). Agents that buffer cytoplasmic Ca2+ or that prevent Ca2+ release from the ER protected synaptosomes against the adverse effect of PS1 mutations.
Polymorphisms in the apolipoprotein E (ApoE) gene affect one’s risk for late onset AD. Of the three isoforms (E2, E3 and E4), E3 is the most common and E2 the least common. The three isoforms differ at residues 112 and 158; E3 has Cys-112 and Arg-158, whereas E4 has arginine in both positions and E2 has cysteine in both positions. Inheritance of the allele for E4 isoform is associated with increased risk and earlier age of onset of the sporadic AD whereas E2 reduces risk69. Several studies indicate a potential link between ApoE and synaptic Ca2+ signaling. As mentioned above, ApoE2, but not ApoE4, can inhibit Aβ association with phosphatydylserine in the membrane, providing a potential explanation for protective effects of ApoE2 in AD. A different line of research demonstrated that application of low levels of ApoE4 to cultured neurons induces NMDAR-mediated Ca2+ influx and causes neuronal toxicity70. In addition, it was recently demonstrated that reelin can activate neuronal NMDAR via a src-family tyrosine kinase (SFK)-mediated mechanism and that reelin association with ApoE receptor 2 (ApoER2) was necessary for activation of NMDAR71. Marked changes in reelin expression levels were observed in brains from AD patients and AD mouse models72,73 further implicating a potential importance of reelin signaling pathway in AD.
Calcium and the Selectivity of Neuronal Vulnerability in AD
Differential production and deposition of Aβ and the resulting disruption of Ca2+ homeostasis is one likely determinant of selective neuronal vulnerability because neurons in brain regions with high Aβ loads (entorhinal cortex, hippocampus, inferior parietal cortex) degenerate, whereas neurons in regions with little or no Aβ accumulation (cerebellum, striatum, motor cortex) typically do not4. However, it is clear that there are additional factors at work because within a vulnerable brain region (in the presence of similar amounts of Aβ) some neurons degenerate in AD, whereas others do not. Populations of neurons that degenerate in AD typically express high levels of NMDA receptors and have relatively low levels of some Ca2+-binding proteins compared to resistant neurons2. Although hippocampal dentate and CA1 neurons each express NMDA receptors, the dentate neurons express high amounts of calbindin, whereas CA1 neurons do not. Experimental findings suggest that calbindin buffers Ca2+ loads and protects neurons against excitotoxicity; in the hippocampus calbindin-positive neurons are relatively preserved in AD patients with moderate plaque and tangle content, but in severe cases the calbindin-positive pyramidal cells are also lost, suggesting the possibility that calbindin protects neurons in the early stages of AD74. Basal forebrain cholinergic neurons may become depleted of calbindin during aging, which may increase their vulnerability to degeneration in AD75. However, in the entorhinal cortex calbindin- and parvalbumin-positive non-principal neurons exhibit degenerative changes early in AD, whereas calretinin- and calbindin-positive pyramidal neurons are relatively preserved76. Changes in the expression of glutamate receptors may also contribute to altered neuronal Ca2+ handling in AD; as AD progresses the levels of NR1/2B subunits in hippocampal neurons decrease, while the NR2A subunit levels remain unchanged77. Other factors that may contribute to selective neuronal vulnerability in AD by perturbing Ca2+ homeostasis are neuron-specific differences in energy metabolism, antioxidant systems and neurotrophic factor support4.
Optimizing Neuronal Calcium Homeostasis as a Therapeutic Approach for AD
Because aging is the major risk factor for AD, it follows that interventions that counteract the aging process would protect neurons against Ca2+ dysregulation and AD (Fig. 3). Epidemiological and experimental evidence suggests that exercise, dietary energy restriction and cognitive stimulation may retard aging processes and protect against AD11. Indeed, environmental enrichment78, exercise79 and dietary energy restriction80 suppress the disease process and enhance cognitive performance in mouse models of AD. These beneficial environmental factors may act, in part, by inducing the expression of neurotrophic factors such as BDNF, that stabilize neuronal Ca2+ homeostasis81. Antioxidants and cellular energy-promoting agents might also be expected to stabilize neuronal Ca2+ homeostasis and protect against AD. Because increased Aβ production and accumulation at synapses is of major importance in AD pathogenesis, treatments that reduce Aβ production or enhance its clearance from the brain are being vigorously pursued. One of the most promising anti-Aβ approaches that is currently being tested in patients is immunization with Aβ or treatment with purified Aβ antibodies64. By removing Aβ from the brain, immunization would be expected to prevent or reverse Aβ-induced neuronal Ca2+ dysregulation.
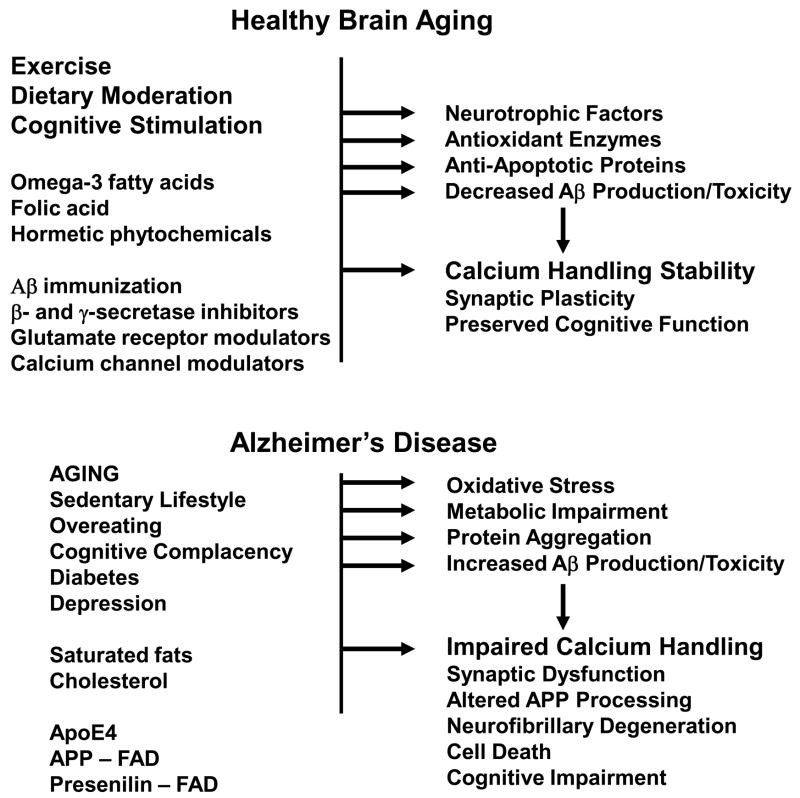
Figure 3.
Plausible mechanisms for healthy brain aging and AD. Healthy brain aging can be promoted by regular exercise, moderation in caloric intake and engaging in intellectually challenging activities. These lifestyle factors may stabilize neuronal Ca2+ homeostasis and counteract aging by inducing the production of neurotrophic factors, cellular antioxidant defense systems and cell survival-promoting proteins. Specific dietary factors may also reduce the risk of AD including omega-3 fatty acids, folic acid and possibly phytochemicals106. By stabilizing neuronal Ca2+ homeostasis the healthy lifestyle and dietary factors can preserve synaptic and cognitive function during aging. Lifestyle factors that may accelerate aging and AD pathogenesis include lack of exercise, overeating and intellectually unchallenging daily activities. The risk of deleloping AD is reduced by the presence of ApoE2 allele and increased by ApoE4. Genetic mutations in presenilins and APP lead to early-onset familial AD. Two disorders that increase the risk for AD are diabetes and depression. Diets high in saturated fats and cholesterol may also adversely affect the brain by increasing oxidative stress and perturbing cell membranes. As a result of the aging process, and genetic and lifestyle factors, neurons suffer from increased oxidative stress, metabolic impairment and the accumulation of aggregated proteins including Aβ and tau. All of the latter alterations perturb neuronal Ca2+ homeostasis resulting in synaptic dysfunction, amyloidogenic APP processing, tau pathology, neuronal death and cognitive dysfunction. Treatments that target Aβ, including immunization and β- and γ-secretase inhibitors may stabilize neuronal Ca2+ homeostasis by preventing the adverse actions of Aβ on membranes and associated Ca2+-regulating proteins (see Fig. 1). Drugs that modulate ion channels involved in Ca2+ influx and release from internal organelles have the potential to counteract neurodegenerative processes in AD.
Drugs that inhibit β- or γ-secretases are another viable approach for reducing Aβ production and associated Ca2+-mediated neurotoxicity82. Drugs that target specific Ca2+-regulating systems (downstream of age- and Aβ-related disruption of Ca2+ homeostasis) provide another approach. Indeed, the only drug thus far shown to slow disease progression in AD patients is the NMDA receptor open channel blocker memantine83. Beneficial effects have also been reported in AD clinical trials of Dimebon84, a drug that has been claimed to stabilize Ca2+ signaling by blocking NMDAR and voltage-gated Ca2+ channels85. As with other major age-related diseases (cardiovascular disease, diabetes and cancers) risk reduction for AD may be achievable by dietary moderation and exercise combined with dietary supplements (omega-3 fatty acids and folic acid, for example). For individuals at high risk for AD (ApoE4 genotype and family history, for example) prophylactic approaches may be prescribed including anti-inflammatory drugs and immunization.
Conclusion and Future Directions
The ability of neurons to regulate the influx, efflux and subcellular compartmentalization of Ca2+ is compromised in AD as the result of age-related oxidative stress and metabolic impairment in combination with disease-related accumulation of Aβ oligomers. Aβ may promote cellular Ca2+ overload by inducing membrane-associated oxidative stress and by forming pores in the membrane. Mutant forms of presenilins that cause many cases of early-onset FAD cause ER Ca2+ overload, apparently by impairing the normal ER Ca2+ leak channel function of the presenilins. Synapses are particularly sensitive to the adverse effects of Aβ and presenilin mutations, and environmental factors and therapeutic agents that promote synaptic Ca2+ homeostasis may be effective in preventing and treating AD. Key remaining questions include: does perturbed Ca2+ occur early in the AD process and contribute to altered APP processing and Aβ production?; is there a cause-and-effect connection between abnormal neuronal Ca2+ signaling and amyloid plaque accumulation in AD brains?; what is a connection between “amyloid toxicity” and “Ca2+ toxicity” in AD?; what are the weakest links in the various cellular Ca2+-regulating systems in AD?; what is a role played by mitochondria in AD pathogenesis?; how, at the molecular and cellular levels, do risk factors for AD impact neuronal Ca2+ homeostasis?; can specific Ca2+-regulating mechanisms be targeted for therapeutic intervention? Answering these and related questions will clarify a possibile role of abnormal Ca2+ signaling in AD pathogenesis and may open a door to development of new classes of therapeutic agents targeting neuronal Ca2+ signaling pathways.
Box 1 Calcium Stability in the Face of Adversity
Neurons possess multiple defenses against the Ca2+-destabilizing forces of aging and AD-specific pathogenic abnormalities (Fig. 3). Activity-dependent neurotrophic factor signaling plays a major role in stabilizing neuronal Ca2+ homeostasis as is evident from the abilities of BDNF, NGF, bFGF and others to protect neurons against excitotoxic, oxidative and metabolic insults relevant to AD81. The neurotrophic factors protect against sustained elevations of [Ca2+]i by modifying the expression of Ca2+-binding proteins, glutamate receptor subunits, antioxidant enzymes and mitochondrial membrane-stabilizing proteins such as Bcl-2. In addition, transcription factors that mediate adaptive stress responses, including NF-κB and Nrf-2 may be activated oxidative and metabolic stress resulting in the up-regulation of antioxidant and phase 2 enzymes. Two organelles in which it is particularly important to maintain Ca2+ regulation are the ER and mitochondria. Evidence suggests that the ER is under stress in neurons affected in AD and may contribute to perturbed cellular Ca2+ homeostasis86. Three proteins that have been shown to stabilize ER Ca2+ homeostasis and protect neurons against insults relevant to AD are Bcl-2 which stabilizes membranes and GRP-78 (glucose-regulated protein 78) which guards against protein misfolding and Herp (homocysteine-inducible ER protein87. Depletion of Herp by RNA interference sensitizes neural cells to apoptosis induced by ER stress, whereas Herp overexpression promotes survival by a mechanism involving stabilization of ER Ca2+ levels, preservation of mitochondrial function and suppression of caspase 3 activation. Mitochondrial Ca2+ handling may be impaired in AD. For example, cyclical fluctuations in mitochondrial membrane potential, which are mediated by Ca2+ and likely represent coupling of membrane potential to ATP production, are reduced in AD cybrid cells88. Mitochondrial uncoupling proteins (UCPs) may play important roles in stabilizing mitochondrial and cellular Ca2+ homeostasis as suggested by studies showing that UCP-4 stabilizes total cellular Ca2+ homeostasis (including ER and plasma membrane systems) which is associated with reduced mitochondrial oxidative stress and resistance of neurons to death89.
Acknowledgments
We would like to thank our colleagues and collaborators for insightful discussions that help us formulate many ideas expressed in this article. In particular, we would like to thank Joachim Herz, Gang Yu and Bart De Strooper for productive collaboration, Frank La Ferla, Beth Stutzmann, and Kevin Foskett for sharing their unpublished results with us, Sam Gandy, Harvey B. Pollard and Zaven Khachaturian for stimulating discussions. We also would like to sincerely apologize to many scientists working in this field whose interesting work we could not cite due to space limitations. We also thank K. C. Alexander for preparing Figures 1 and 2. I.B. is a holder of Carla Cocke Francis Professorship in Alzheimer’s Research and supported by the McKnight Neuroscience of Brain Disorders Award, the Alzheimer’s Association Research Grant IIRG-06-24703, and NINDS grants R01 NS38082 and R01 NS056224. MM is supported by the Intramural Research Program of the National Institute on Aging.
References
- 1.Berridge MJ, Bootman MD, Lipp P. Calcium - a life and death signal. Nature. 1998;395:645–648. doi: 10.1038/27094. [DOI] [PubMed] [Google Scholar]
- 2.Spat A, Szanda G, Csordas G, Hajnoczky G. High- and low-calcium-dependent mechanisms of mitochondrial calcium signalling. Cell Calcium. 2008 doi: 10.1016/j.ceca.2007.11.015. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy J. A hundred years of Alzheimer’s disease research. Neuron. 2006;52:3–13. doi: 10.1016/j.neuron.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 4.Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murchison D, Griffith WH. Calcium buffering systems and calcium signaling in aged rat basal forebrain neurons. Aging Cell. 2007;6:297–305. doi: 10.1111/j.1474-9726.2007.00293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown MR, Geddes JW, Sullivan PG. Brain region-specific, age-related, alterations in mitochondrial responses to elevated calcium. J Bioenerg Biomembr. 2004;36:401–406. doi: 10.1023/B:JOBB.0000041775.10388.23. [DOI] [PubMed] [Google Scholar]
- 7.Toescu EC, Verkhratsky A, Landfield PW. Ca2+ regulation and gene expression in normal brain aging. Trends Neurosci. 2004;27:614–620. doi: 10.1016/j.tins.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Poon HF, et al. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol Aging. 2006;27:1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Blalock EM, et al. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emilsson L, Saetre P, Jazin E. Alzheimer’s disease: mRNA expression profiles of multiple patients show alterations of genes involved with calcium signaling. Neurobiol Dis. 2006;21:618–625. doi: 10.1016/j.nbd.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 13.Mattson MP, et al. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J Neurosci. 1992;12:376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 15.Meyer-Luehmann M, et al. Rapid appearance and local toxicity of amyloid-beta plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Furukawa K, et al. Activation of K+ channels and suppression of neuronal activity by secreted beta-amyloid-precursor protein. Nature. 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- 17.Leissring MA, et al. A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP. Proc Natl Acad Sci USA. 2002;99:4697–4702. doi: 10.1073/pnas.072033799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arispe N, et al. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci USA. 1993;90:567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshiike Y, et al. Pore-forming proteins share structural and functional homology with amyloid oligomers. Neuromolecular Med. 2007;9:270–275. doi: 10.1007/s12017-007-0003-6. [DOI] [PubMed] [Google Scholar]
- 20.Lee G, Pollard HB, Arispe N. Annexin 5 and apolipoprotein E2 protect against Alzheimer’s amyloid-beta-peptide cytotoxicity by competitive inhibition at a common phosphatidylserine interaction site. Peptides. 2002;23:1249–63. doi: 10.1016/s0196-9781(02)00060-8. [DOI] [PubMed] [Google Scholar]
- 21.Simakova O, Arispe NJ. The cell-selective neurotoxicity of the Alzheimer’s Abeta peptide is determined by surface phosphatidylserine and cytosolic ATP levels. Membrane binding is required for Abeta toxicity. J Neurosci. 2007;27:13719–13729. doi: 10.1523/JNEUROSCI.3006-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahu SK, Gummadi SN, Manoj N, Aradhyam GK. Phospholipid scramblases: an overview. Arch Biochem Biophys. 2007;462:103–14. doi: 10.1016/j.abb.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Hensley K, et al. A model for beta-amyloid aggregation and neurotoxicity based on free radical generation by the peptide: relevance to Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:3270–3274. doi: 10.1073/pnas.91.8.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang X, et al. The A beta peptide of Alzheimer’s disease directly produces hydrogen peroxide through metal ion reduction. Biochemistry. 1999;38:7609–7616. doi: 10.1021/bi990438f. [DOI] [PubMed] [Google Scholar]
- 25.Mark RJ, et al. Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation. J Neurosci. 1997;17:1046–1054. doi: 10.1523/JNEUROSCI.17-03-01046.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shankar GM, et al. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nimmrich V, et al. Amyloid beta oligomers (A beta(1–42) globulomer) suppress spontaneous synaptic activity by inhibition of P/Q-type calcium currents. J Neurosci. 2008;28:788–97. doi: 10.1523/JNEUROSCI.4771-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin Q, Kawai H, Berg DK. beta -Amyloid peptide blocks the response of alpha 7-containing nicotinic receptors on hippocampal neurons. Proc Natl Acad Sci USA. 2001;98:4734–4749. doi: 10.1073/pnas.081553598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dougherty JJ, Wu J, Nichols RA. Beta-amyloid regulation of presynaptic nicotinic receptors in rat hippocampus and neocortex. J Neurosci. 2003;23:6740–6747. doi: 10.1523/JNEUROSCI.23-17-06740.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker NE. Notch signaling in the nervous system. Pieces still missing from the puzzle. Bioessays. 2000;22:264–273. doi: 10.1002/(SICI)1521-1878(200003)22:3<264::AID-BIES8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, et al. Involvement of Notch signaling in hippocampal synaptic plasticity. Proc Natl Acad Sci USA. 2004;101:9458–9462. doi: 10.1073/pnas.0308126101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ito E, et al. Internal Ca2+ mobilization is altered in fibroblasts from patients with Alzheimer disease. Proc Natl Acad Sci USA. 1994;91:534–538. doi: 10.1073/pnas.91.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo Q, et al. Alzheimer’s PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid β-peptide. NeuroReport. 1996;8:379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 34.Guo Q, et al. Alzheimer’s presenilin mutation sensitizes neural cells to apoptosis induced by trophic factor withdrawal and amyloid beta-peptide: involvement of calcium and oxyradicals. J Neurosci. 1997;17:4212–4222. doi: 10.1523/JNEUROSCI.17-11-04212.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leissring MA, et al. Alzheimer’s presenilin-1 mutation potentiates inositol 1,4,5-trisphosphate-mediated calcium signaling in Xenopus oocytes. J Neurochem. 1999;72:1061–1068. doi: 10.1046/j.1471-4159.1999.0721061.x. [DOI] [PubMed] [Google Scholar]
- 36.Guo Q. Increased vulnerability of hippocampal neurons to excitotoxic necrosis in presenilin-1 mutant knock-in mice. Nat Med. 1999;5:101–106. doi: 10.1038/4789. [DOI] [PubMed] [Google Scholar]
- 37.Stutzmann GE, Caccamo A, LaFerla FM, Parker I. Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci. 2004;24:508–513. doi: 10.1523/JNEUROSCI.4386-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schneider I, et al. Mutant presenilins disturb neuronal calcium homeostasis in the brain of transgenic mice, decreasing the threshold for excitotoxicity and facilitating long-term potentiation. J Biol Chem. 2001;276:11539–11544. doi: 10.1074/jbc.M010977200. [DOI] [PubMed] [Google Scholar]
- 39.Begley JG, et al. Altered calcium homeostasis and mitochondrial dysfunction in cortical synaptic compartments of presenilin-1 mutant mice. J Neurochem. 1999;72:1030–1039. doi: 10.1046/j.1471-4159.1999.0721030.x. [DOI] [PubMed] [Google Scholar]
- 40.Chan SL, et al. Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem. 2000;275:18195–18200. doi: 10.1074/jbc.M000040200. [DOI] [PubMed] [Google Scholar]
- 41.Pack-Chung E, et al. Presenilin 2 interacts with sorcin, a modulator of the ryanodine receptor. J Biol Chem. 2000;275:14440–14445. doi: 10.1074/jbc.m909882199. [DOI] [PubMed] [Google Scholar]
- 42.Stabler SM, Ostrowski LL, Janicki SM, Monteiro MJ. A myristoylated calcium-binding protein that preferentially interacts with the Alzheimer’s disease presenilin 2 protein. J Cell Biol. 1999;145:1277–1292. doi: 10.1083/jcb.145.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buxbaum JD, et al. Calsenilin: a calcium-binding protein that interacts with the presenilins and regulates the levels of a presenilin fragment. Nat Med. 1998;4:1177–1181. doi: 10.1038/2673. [DOI] [PubMed] [Google Scholar]
- 44.Green KN, Demuro A, Akbari Y, Hitt BD, Smith IF, Parker I, LaFerla FM. SERCA pump activity is physiogically regulated by presenilin and regulates amyloid β production. J Cell Biol. 2008 doi: 10.1083/jcb.200706171. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cai C, et al. The presenilin-2 loop peptide perturbs intracellular Ca2+ homeostasis and accelerates apoptosis. J Biol Chem. 2006;281:16649–16655. doi: 10.1074/jbc.M512026200. [DOI] [PubMed] [Google Scholar]
- 46.Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM-Y, Foskett JK. Mechanism of Ca2+ disruption in Alzheimer’s Disease by presenilin regulation of InsP3 receptor channel gating. Neuron. 2008;53 doi: 10.1016/j.neuron.2008.04.015. in press June 26, 2008 scheduled. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stutzmann GE, et al. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci. 2006;26:5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tu H, et al. Presenilins form ER Ca2+ leak channels, a function disrupted by familial Alzheimer’s disease-linked mutations. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson O, et al. Familial Alzheimer disease-linked mutations specifically disrupt Ca2+ leak function of presenilin 1. J Clin Invest. 2007;117:1230–1239. doi: 10.1172/JCI30447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cedazo-Minguez A, et al. The presenilin 1 deltaE9 mutation gives enhanced basal phospholipase C activity and a resultant increase in intracellular calcium concentrations. J Biol Chem. 2002;277:36646–36655. doi: 10.1074/jbc.M112117200. [DOI] [PubMed] [Google Scholar]
- 51.Dumanchin C, et al. Biological effects of four PSEN1 gene mutations causing Alzheimer disease with spastic paraparesis and cotton wool plaques. Hum Mutat. 2006 Oct;27:1063. doi: 10.1002/humu.9458. [DOI] [PubMed] [Google Scholar]
- 52.McKee AC, et al. Hippocampal neurons predisposed to neurofibrillary tangle formation are enriched in type II calcium/calmodulin-dependent protein kinase. J Neuropathol Exp Neurol. 1990;49:49–63. doi: 10.1097/00005072-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 53.Nixon RA. The calpains in aging and aging-related diseases. Ageing Res Rev. 2003;2:407–418. doi: 10.1016/s1568-1637(03)00029-1. [DOI] [PubMed] [Google Scholar]
- 54.Mattson MP. Antigenic changes similar to those seen in neurofibrillary tangles are elicited by glutamate and Ca2+ influx in cultured hippocampal neurons. Neuron. 1990;4:105–117. doi: 10.1016/0896-6273(90)90447-n. [DOI] [PubMed] [Google Scholar]
- 55.Furukawa K, et al. Alteration in calcium channel properties is responsible for the neurotoxic action of a familial frontotemporal dementia tau mutation. J Neurochem. 2003;87:427–436. doi: 10.1046/j.1471-4159.2003.02020.x. [DOI] [PubMed] [Google Scholar]
- 56.Querfurth HW, Selkoe DJ. Calcium ionophore increases amyloid beta peptide production by cultured cells. Biochemistry. 1994;33:4550–4561. doi: 10.1021/bi00181a016. [DOI] [PubMed] [Google Scholar]
- 57.Zhang X, et al. Hypoxia-inducible factor 1alpha (HIF-1alpha)-mediated hypoxia increases BACE1 expression and beta-amyloid generation. J Biol Chem. 2007;282:10873–10880. doi: 10.1074/jbc.M608856200. [DOI] [PubMed] [Google Scholar]
- 58.Buxbaum JD, et al. Calcium regulates processing of the Alzheimer amyloid protein precursor in a protein kinase C-independent manner. Proc Natl Acad Sci U S A. 1994;91:4489–4493. doi: 10.1073/pnas.91.10.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Petryniak MA, Wurtman RJ, Slack BE. Elevated intracellular calcium concentration increases secretory processing of the amyloid precursor protein by a tyrosine phosphorylation-dependent mechanism. Biochem J. 1996;320:957–963. doi: 10.1042/bj3200957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lazarov O, et al. Evidence that synaptically released beta-amyloid accumulates as extracellular deposits in the hippocampus of transgenic mice. J Neurosci. 2002;22:9785–9793. doi: 10.1523/JNEUROSCI.22-22-09785.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Spires-Jones, et al. Impaired spine stability underlies plaque-related spine loss in an Alzheimer’s disease mouse model. Am J Pathol. 2007;171:1304–1311. doi: 10.2353/ajpath.2007.070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Celsi F, et al. Beta-amyloid causes downregulation of calcineurin in neurons through induction of oxidative stress. Neurobiol Dis. 2007;26:342–352. doi: 10.1016/j.nbd.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 63.Lacor PN, et al. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci. 2007;27:796–807. doi: 10.1523/JNEUROSCI.3501-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Janus C, et al. A beta peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408:979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 65.Palop JJ. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron. 2007;55:697–711. doi: 10.1016/j.neuron.2007.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Priller C, et al. Mutant presenilin 1 alters synaptic transmission in cultured hippocampal neurons. J Biol Chem. 2007;282:1119–1127. doi: 10.1074/jbc.M605066200. [DOI] [PubMed] [Google Scholar]
- 67.Shemer I, et al. Non-fibrillar beta-amyloid abates spike-timing-dependent synaptic potentiation at excitatory synapses in layer 2/3 of the neocortex by targeting postsynaptic AMPA receptors. Eur J Neurosci. 2006;23:2035–2047. doi: 10.1111/j.1460-9568.2006.04733.x. [DOI] [PubMed] [Google Scholar]
- 68.Haughey NJ, et al. Disruption of neurogenesis by amyloid beta-peptide, and perturbed neural progenitor cell homeostasis, in models of Alzheimer’s disease. J Neurochem. 2002;83:1509–1524. doi: 10.1046/j.1471-4159.2002.01267.x. [DOI] [PubMed] [Google Scholar]
- 69.Huang Y. Apolipoprotein E and Alzheimer disease. Neurology. 2006;66:S79–85. doi: 10.1212/01.wnl.0000192102.41141.9e. [DOI] [PubMed] [Google Scholar]
- 70.Hartmann H, Eckert A, Muller WE. Apolipoprotein E and cholesterol affect neuronal calcium signalling: the possible relationship to beta-amyloid neurotoxicity. Biochem Biophys Res Commun. 1994;200:1185–1192. doi: 10.1006/bbrc.1994.1576. [DOI] [PubMed] [Google Scholar]
- 71.Chen Y, Beffert U, Ertunc M, Tang TS, Kavalali ET, Bezprozvanny I, Herz J. Reelin modulates NMDA receptor activity in cortical neurons. J Neurosci. 2005;25:8209–16. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Botella-Lopez A, et al. Reelin expression and glycosylation patterns are altered in Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:5573–5578. doi: 10.1073/pnas.0601279103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chin J, et al. Reelin depletion in the entorhinal cortex of human amyloid precursor protein transgenic mice and humans with Alzheimer’s disease. J Neurosci. 2007;27:2727–2733. doi: 10.1523/JNEUROSCI.3758-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Iritani S, Niizato K, Emson PC. Relationship of calbindin D28K-immunoreactive cells and neuropathological changes in the hippocampal formation of Alzheimer’s disease. Neuropathology. 2001;21:162–167. doi: 10.1046/j.1440-1789.2001.00393.x. [DOI] [PubMed] [Google Scholar]
- 75.Geula C, et al. Loss of calbindin-D28k from aging human cholinergic basal forebrain: relation to neuronal loss. J Comp Neurol. 2003;455:9–59. doi: 10.1002/cne.10475. [DOI] [PubMed] [Google Scholar]
- 76.Mikkonen M, et al. Subfield- and layer-specific changes in parvalbumin, calretinin and calbindin-D28K immunoreactivity in the entorhinal cortex in Alzheimer’s disease. Neuroscience. 1999;92:515–532. doi: 10.1016/s0306-4522(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 77.Mishizen-Eberz, et al. Biochemical and molecular studies of NMDA receptor subunits NR1/2A/2B in hippocampal subregions throughout progression of Alzheimer’s disease pathology. Neurobiol Dis. 2004;15:80–92. doi: 10.1016/j.nbd.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 78.Lazarov O, et al. Environmental enrichment reduces Abeta levels and amyloid deposition in transgenic mice. Cell. 2005;120:701–713. doi: 10.1016/j.cell.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 79.Wolf SA, et al. Cognitive and physical activity differently modulate disease progression in the amyloid precursor protein (APP)-23 model of Alzheimer’s disease. Biol Psychiatry. 2006;60:1314–1323. doi: 10.1016/j.biopsych.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 80.Halagappa VK, et al. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer’s disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 81.Mattson MP, Maudsley S, Martin B. BDNF and 5-HT: a dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004;27:589–594. doi: 10.1016/j.tins.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 82.Schmidt B, Baumann S, Braun HA, Larbig G. Inhibitors and modulators of beta-and gamma-secretase. Curr Top Med Chem. 2006;6:377–392. doi: 10.2174/156802606776287027. [DOI] [PubMed] [Google Scholar]
- 83.Lipton SA. Paradigm shift in neuroprotection by NMDA receptor blockade: memantine and beyond. Nat Rev Drug Discov. 2006;5:160–170. doi: 10.1038/nrd1958. [DOI] [PubMed] [Google Scholar]
- 84.Bachurin S, et al. Antihistamine agent Dimebon as a novel neuroprotector and a cognition enhancer. Ann N Y Acad Sci. 2001;939:425–435. doi: 10.1111/j.1749-6632.2001.tb03654.x. [DOI] [PubMed] [Google Scholar]
- 85.Grigorev VV, Dranyi OA, Bachurin SO. Comparative study of action mechanisms of dimebon and memantine on AMPA- and NMDA-subtypes glutamate receptors in rat cerebral neurons. Bull Exp Biol Med. 2003;136:474–477. doi: 10.1023/b:bebm.0000017097.75818.14. [DOI] [PubMed] [Google Scholar]
- 86.Lindholm D, Wootz H, Korhonen L. ER stress and neurodegenerative diseases. Cell Death Differ. 2006;13:385–392. doi: 10.1038/sj.cdd.4401778. [DOI] [PubMed] [Google Scholar]
- 87.Chan SL, et al. Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. J Biol Chem. 2004;279:28733–28743. doi: 10.1074/jbc.M404272200. [DOI] [PubMed] [Google Scholar]
- 88.Thiffault C, Bennett JP., Jr Cyclical mitochondrial deltapsiM fluctuations linked to electron transport, F0F1 ATP-synthase and mitochondrial Na+/Ca+2 exchange are reduced in Alzheimer’s disease cybrids. Mitochondrion. 2005;5:109–119. doi: 10.1016/j.mito.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 89.Chan SL, et al. Mitochondrial uncoupling protein-4 regulates calcium homeostasis and sensitivity to store depletion-induced apoptosis in neural cells. J Biol Chem. 2006;281:37391–37403. doi: 10.1074/jbc.M605552200. [DOI] [PubMed] [Google Scholar]