Abstract
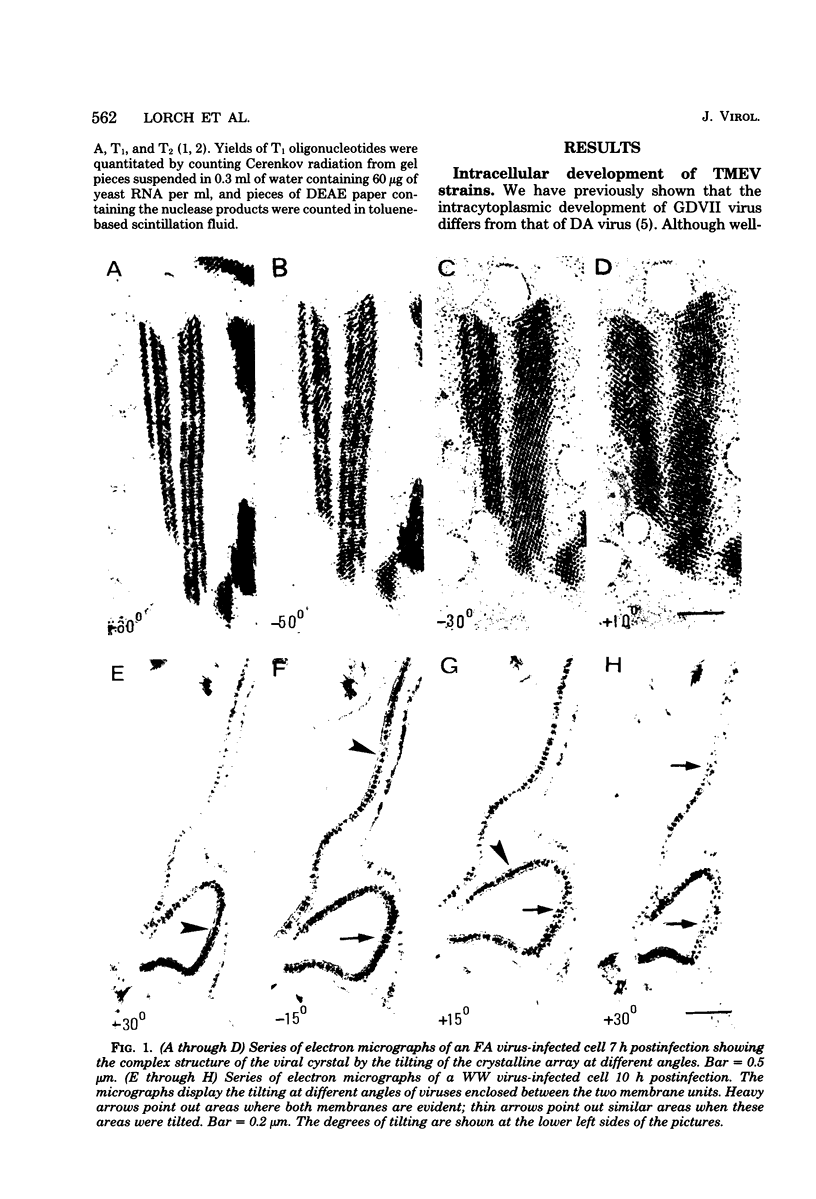
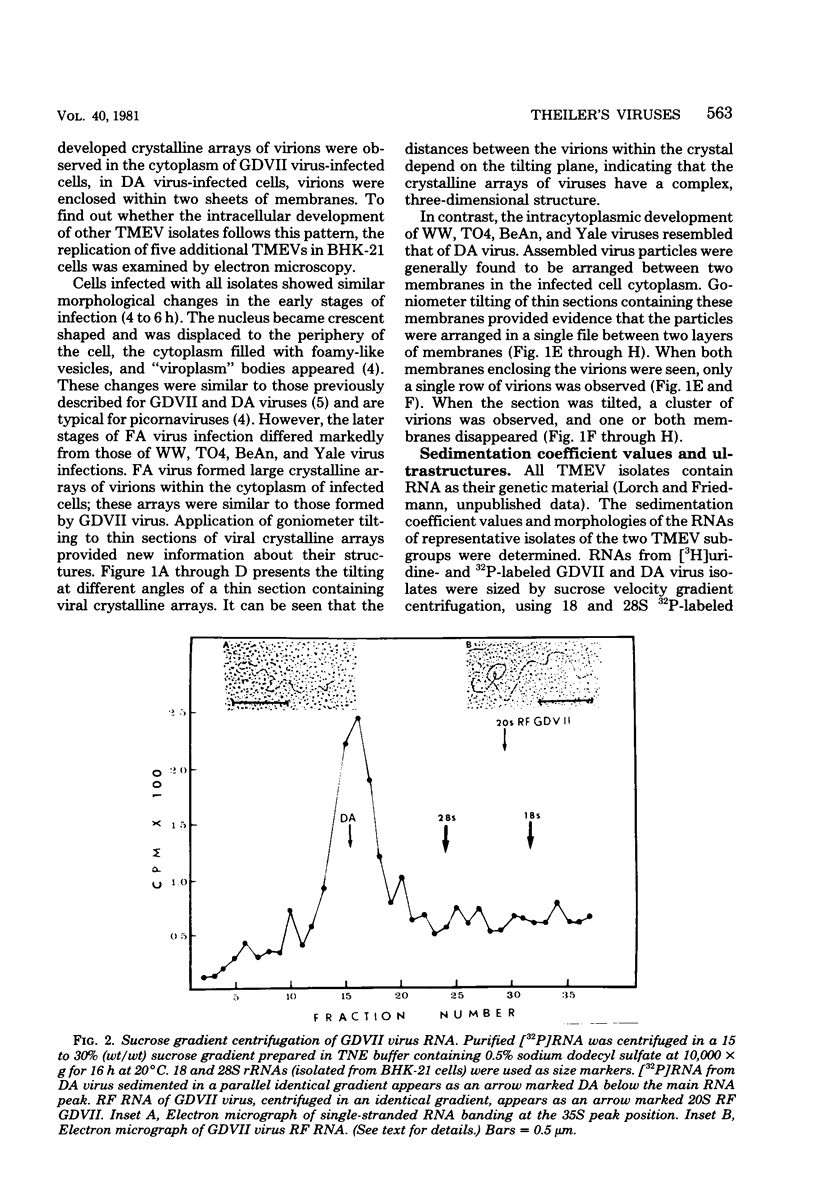
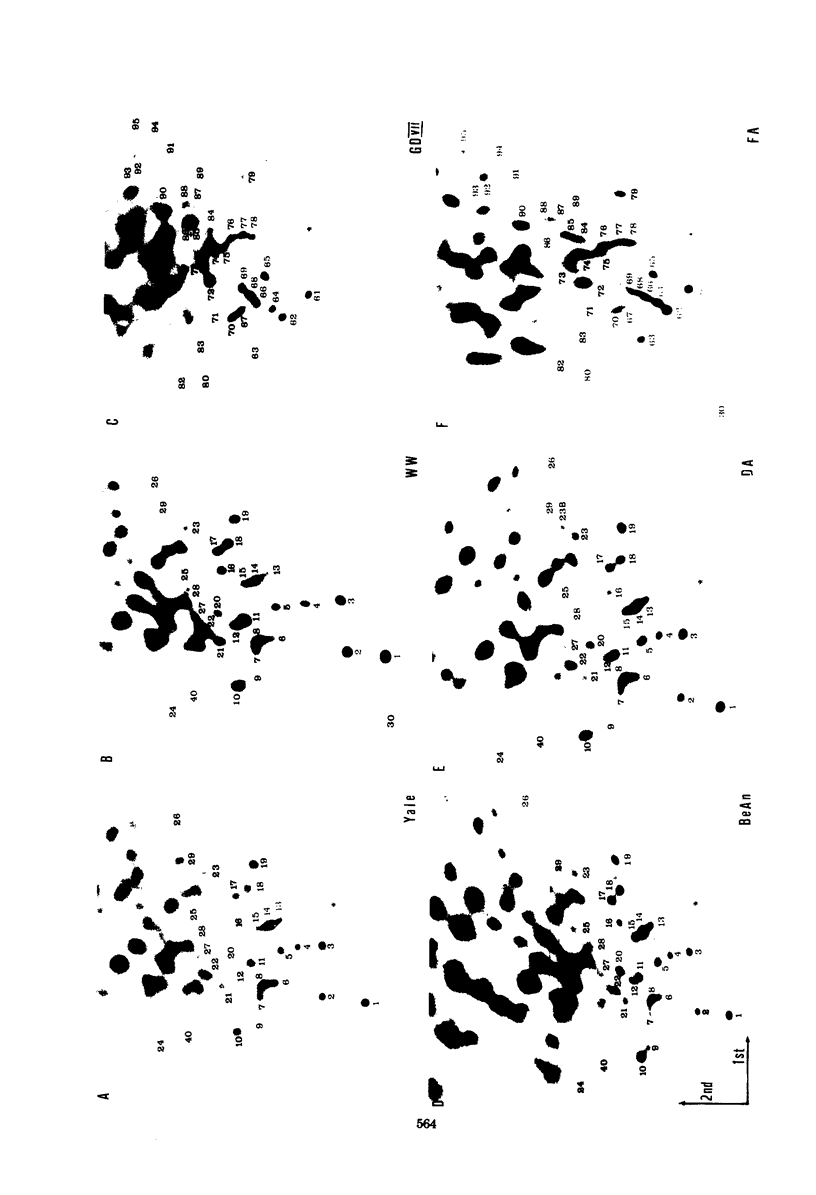
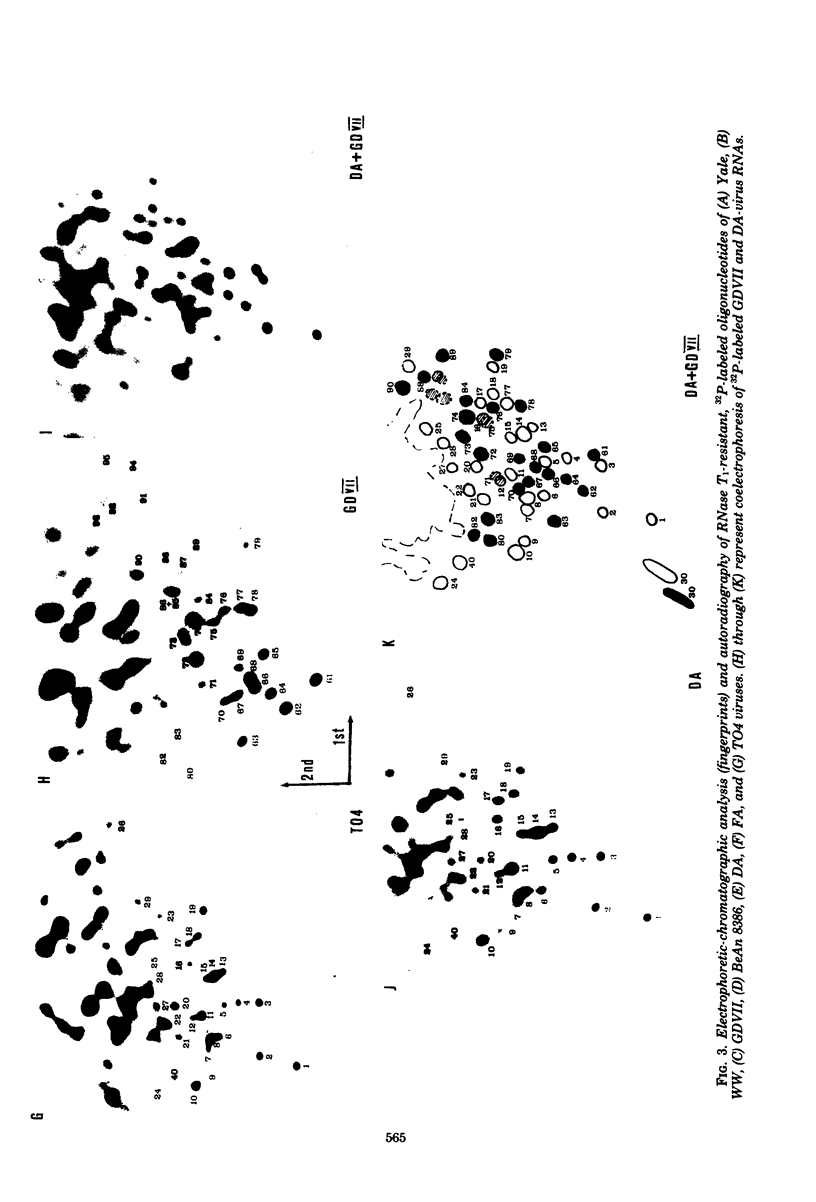
The intracellular development and RNA composition of Theiler's murine encephalomyelitis virus (TMEV) isolates were determined by electron microscopy, sucrose gradient centrifugation, and RNase T1 fingerprinting. Replication of FA virus, a virulent strain of TMEV, was characterized by the appearance of viral crystalline arrays in the cytoplasm of infected cells. In contrast, cells infected with the less virulent isolates (WW, TO4, BeAn 8386, and Yale) showed no crystalline arrays; instead, virions were found to be arranged between two layers of membranes in the cytoplasm of infected cells. Analysis of the RNAs of TMEV isolates showed that the RNAs were single-stranded molecules having sedimentation coefficients of 35S. RNase T1 fingerprinting of TMEV RNA revealed that striking differences between the virulent and less virulent TMEV isolates existed. Moreover, base composition analysis of RNase T1-resistant oligonucleotides of two TMEV isolates which represented the two subgroups indicated that there were no substantial oligonucleotides common to both subgroups. Based on these findings and the known difference in virulence, we suggest that the TMEV group contains two genetically district subgroups of viruses.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Billeter M. A., Parsons J. T., Coffin J. M. The nucleotide sequence complexity of avian tumor virus RNA. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3560–3564. doi: 10.1073/pnas.71.9.3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M., Billeter M. A. A physical map of the Rous sarcoma virus genome. J Mol Biol. 1976 Jan 25;100(3):293–318. doi: 10.1016/s0022-2836(76)80065-4. [DOI] [PubMed] [Google Scholar]
- DALES S., EGGERS H. J., TAMM I., PALADE G. E. ELECTRON MICROSCOPIC STUDY OF THE FORMATION OF POLIOVIRUS. Virology. 1965 Jul;26:379–389. doi: 10.1016/0042-6822(65)90001-2. [DOI] [PubMed] [Google Scholar]
- Dal Canto M. C., Lipton H. L. Recurrent demyelination in chronic central nervous system infection produced by Theiler's murine encephalomyelitis virus. J Neurol Sci. 1979 Aug;42(3):391–405. doi: 10.1016/0022-510x(79)90172-2. [DOI] [PubMed] [Google Scholar]
- Friedmann A., Lipton H. L. Replication of Theiler's murine encephalomyelitis viruses in BHK21 cells: an electron microscopic study. Virology. 1980 Mar;101(2):389–398. doi: 10.1016/0042-6822(80)90452-3. [DOI] [PubMed] [Google Scholar]
- Jacobson A. B., Spahr P. F. Studies on the secondary structure of single-stranded RNA from the bacteriophage MS2. II Analysis of the RNase IV cleavage products. J Mol Biol. 1977 Sep 25;115(3):279–294. doi: 10.1016/0022-2836(77)90155-3. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Characterization of the TO strains of Theiler's mouse encephalomyelitis viruses. Infect Immun. 1978 Jun;20(3):869–872. doi: 10.1128/iai.20.3.869-872.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L., Friedmann A. Purification of Theiler's murine encephalomyelitis virus and analysis of the structural virion polypeptides: correlation of the polypeptide profile with virulence. J Virol. 1980 Mar;33(3):1165–1172. doi: 10.1128/jvi.33.3.1165-1172.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton H. L. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect Immun. 1975 May;11(5):1147–1155. doi: 10.1128/iai.11.5.1147-1155.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Jacobson A., Lee Y. F., Dunn J., Wimmer E. Defective interfering particles of poliovirus: mapping of the deletion and evidence that the deletions in the genomes of DI(1), (2) and (3) are located in the same region. J Mol Biol. 1979 Feb 25;128(2):179–196. doi: 10.1016/0022-2836(79)90125-6. [DOI] [PubMed] [Google Scholar]
- Powell H. C., Lehrich J. R., Arnason B. G. Electron-microscopic appearance of the DA virus, a demyelinating murine virus. J Neurol Sci. 1977 Oct;34(1):15–23. doi: 10.1016/0022-510X(77)90087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziola B. R., Scraba D. G. Structure of the Mengo virion. I. Polypeptide and ribonucleate components of the virus particle. Virology. 1974 Feb;57(2):531–542. doi: 10.1016/0042-6822(74)90192-5. [DOI] [PubMed] [Google Scholar]