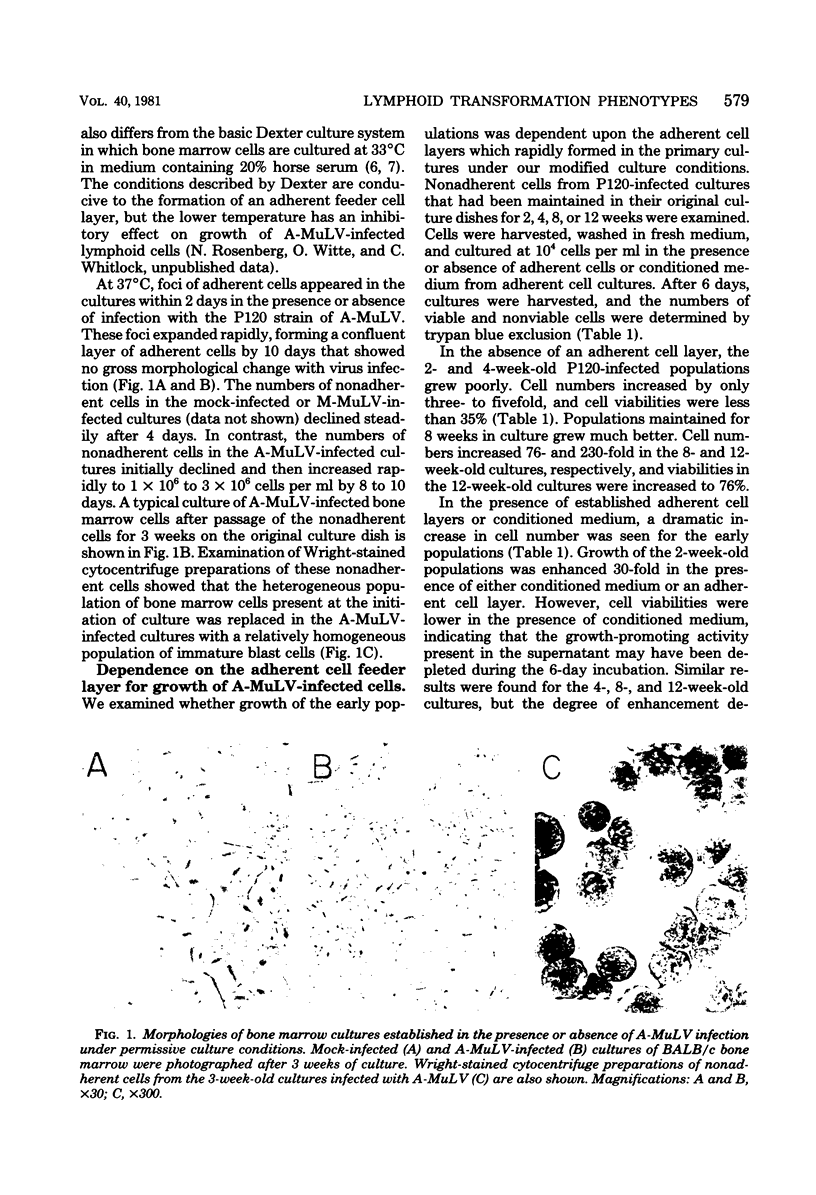
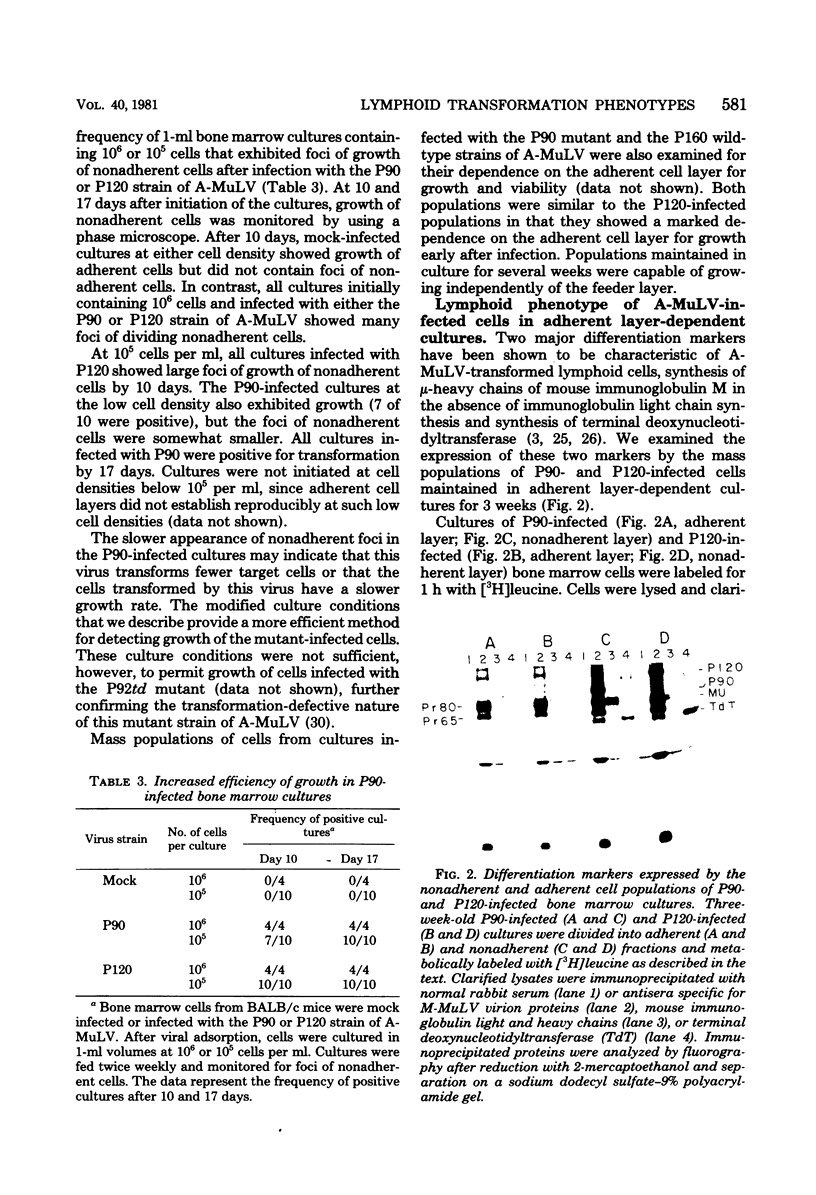
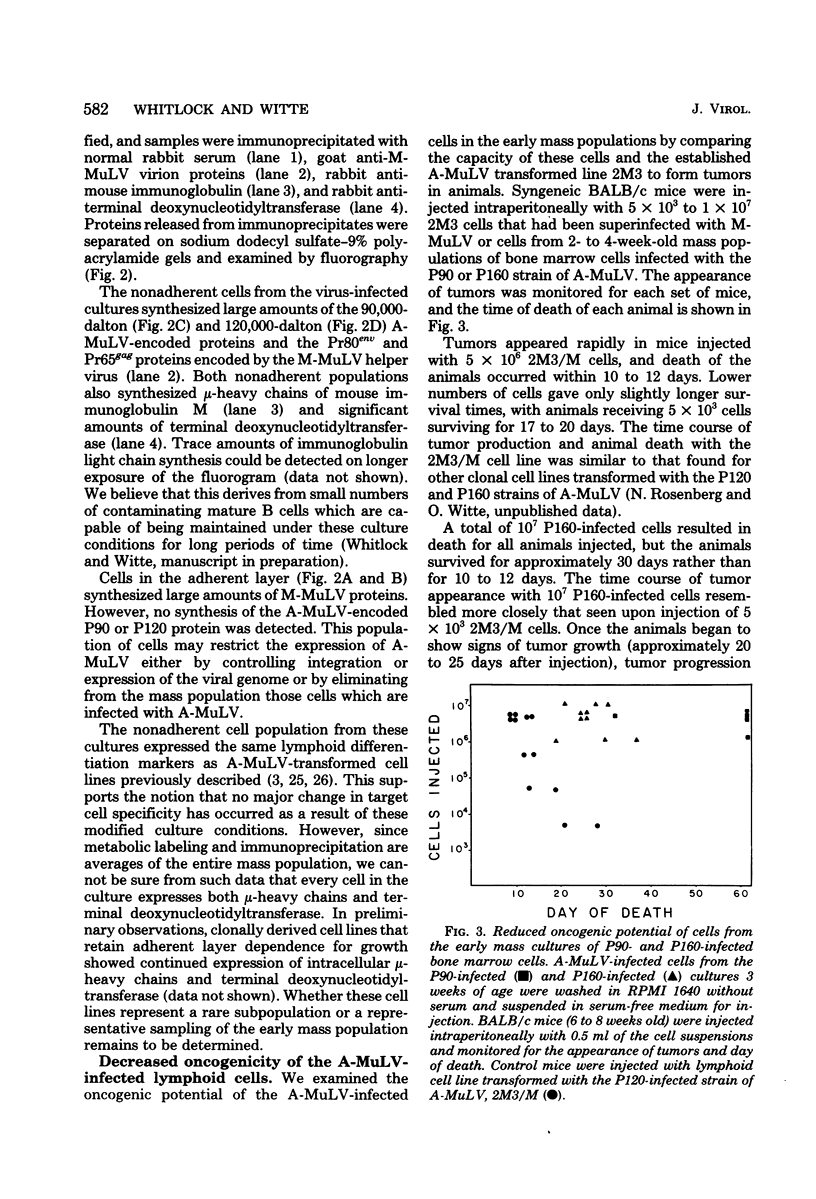
Abstract
We have designed a method for growing bone marrow cells infected with Abelson murine leukemia virus which permits examination of target cell growth early after infection. This culture system increases the efficiency of target cell growth by favoring rapid growth of a mixed population of adherent cells in the primary culture. The nonadherent Abelson virus-infected cell populations expressed pre-B-cell differentiation markers characteristic of Abelson virus-transformed cells (mu-heavy chains of immunoglobulin M and terminal deoxynucleotidyltransferase). Early after infection, these cell populations exhibited restricted in vitro and in vivo growth properties which differed from those of an established Abelson virus-transformed cell line, 2M3. These included a marked dependency upon the adherent cell layer for growth and viability, a lower efficiency of agar colony formation, and a lower capacity for tumor production in syngeneic animals. Growth of the early populations could be maintained in the absence of the adherent cell layer by using conditioned medium from long-term adherent cell cultures established in the absence of viral infection. After passage of the populations for several weeks, the in vitro growth properties gradually shifted toward that of the 2M3 cell line. Twelve-week-old populations grew independently of the adherent cell layer and showed an increased efficiency of agar colony formation. These data indicate that many lymphoid target cells exhibit an intermediate transformed phenotype when infected with Abelson virus. Growth of these cells in culture is mediated via a synergistic interaction between intracellular expression of the viral transforming gene and an exogenous growth-promoting activity which can be provided by cultures of adherent bone marrow cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Rabstein L. S. Lymphosarcoma: virus-induced thymic-independent disease in mice. Cancer Res. 1970 Aug;30(8):2213–2222. [PubMed] [Google Scholar]
- Baltimore D., Shields A., Otto G., Goff S., Besmer P., Witte O., Rosenberg N. Structure and expression of the Abelson murine leukemia virus genome and its relationship to a normal cell gene. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):849–854. doi: 10.1101/sqb.1980.044.01.090. [DOI] [PubMed] [Google Scholar]
- Boss M., Greaves M., Teich N. Abelson virus-transformed haematopoietic cell lines with pre-B-cell characteristics. Nature. 1979 Apr 5;278(5704):551–553. doi: 10.1038/278551a0. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Cline M. J., Golde D. W. Cellular interactions in haematopoiesis. Nature. 1979 Jan 18;277(5693):177–181. doi: 10.1038/277177a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Lajtha L. G. Proliferation of haemopoietic stem cells in vitro. Br J Haematol. 1974 Dec;28(4):525–530. doi: 10.1111/j.1365-2141.1974.tb06671.x. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Paraskeva C. A study to determine the reasons for differences in the tumorigenicity of rat cell lines transformed by adenovirus 2 and adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):703–713. doi: 10.1101/sqb.1980.044.01.075. [DOI] [PubMed] [Google Scholar]
- Gartner S., Kaplan H. S. Long-term culture of human bone marrow cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4756–4759. doi: 10.1073/pnas.77.8.4756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff S. P., Gilboa E., Witte O. N., Baltimore D. Structure of the Abelson murine leukemia virus genome and the homologous cellular gene: studies with cloned viral DNA. Cell. 1980 Dec;22(3):777–785. doi: 10.1016/0092-8674(80)90554-1. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Neel B. G., Hayward W. S., Robinson H. L., Fang J., Astrin S. M. Avian leukosis virus-induced tumors have common proviral integration sites and synthesize discrete new RNAs: oncogenesis by promoter insertion. Cell. 1981 Feb;23(2):323–334. doi: 10.1016/0092-8674(81)90128-8. [DOI] [PubMed] [Google Scholar]
- Payne G. S., Courtneidge S. A., Crittenden L. B., Fadly A. M., Bishop J. M., Varmus H. E. Analysis of avian leukosis virus DNA and RNA in bursal tumours: viral gene expression is not required for maintenance of the tumor state. Cell. 1981 Feb;23(2):311–322. doi: 10.1016/0092-8674(81)90127-6. [DOI] [PubMed] [Google Scholar]
- Pollack R., Lo A., Steinberg B., Smith K., Shure H., Blanck G., Verderame M. SV40 and cellular gene expression in the maintenance of the tumorigenic syndrome. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):681–688. doi: 10.1101/sqb.1980.044.01.072. [DOI] [PubMed] [Google Scholar]
- Reynolds F. H., Jr, Sacks T. L., Deobagkar D. N., Stephenson J. R. Cells nonproductively transformed by Abelson murine leukemia virus express a high molecular weight polyprotein containing structural and nonstructural components. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3974–3978. doi: 10.1073/pnas.75.8.3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Potter M., Rowe W. P. Abelson virus-induced lymphomagenesis in mice. J Exp Med. 1978 Sep 1;148(3):714–726. doi: 10.1084/jem.148.3.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. E., Clark D. R., Witte O. N. Abelson murine leukemia virus mutants deficient in kinase activity and lymphoid cell transformation. J Virol. 1980 Dec;36(3):766–774. doi: 10.1128/jvi.36.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. The effect of helper virus on Abelson virus-induced transformation of lymphoid cells. J Exp Med. 1978 Apr 1;147(4):1126–1141. doi: 10.1084/jem.147.4.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Witte O. N. Abelson murine leukemia virus mutants with alterations in the virus-specific P120 molecule. J Virol. 1980 Jan;33(1):340–348. doi: 10.1128/jvi.33.1.340-348.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Siden E. J., Baltimore D., Clark D., Rosenberg N. E. Immunoglobulin synthesis by lymphoid cells transformed in vitro by Abelson murine leukemia virus. Cell. 1979 Feb;16(2):389–396. doi: 10.1016/0092-8674(79)90014-x. [DOI] [PubMed] [Google Scholar]
- Silverstone A., Sun L., Witte O. N., Baltimore D. Biosynthesis of murine terminal deoxynucleotidyltransferase. J Biol Chem. 1980 Jan 25;255(2):791–796. [PubMed] [Google Scholar]
- Witte O. N., Goff S., Rosenberg N., Baltimore D. A transformation-defective mutant of Abelson murine leukemia virus lacks protein kinase activity. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4993–4997. doi: 10.1073/pnas.77.8.4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte O. N., Rosenberg N., Paskind M., Shields A., Baltimore D. Identification of an Abelson murine leukemia virus-encoded protein present in transformed fibroblast and lymphoid cells. Proc Natl Acad Sci U S A. 1978 May;75(5):2488–2492. doi: 10.1073/pnas.75.5.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zaane D., Bloemers H. P. The genome of the mammalian sarcoma viruses. Biochim Biophys Acta. 1978 Nov 17;516(3):249–268. doi: 10.1016/0304-419x(78)90010-0. [DOI] [PubMed] [Google Scholar]