Abstract
CD46 is a complement regulatory molecule expressed on every cell type, except for erythrocytes. While initially described as a regulator of complement activity, it later became a ‘magnet for pathogens’, binding to several viruses and bacteria. More recently, an alternative role for such complement molecules has emerged: they do regulate T-cell immunity, affecting T-cell proliferation and differentiation. In particular, CD46 stimulation induces Tr1 cells, regulatory T cells characterized by massive production of interleukin-10 (IL-10), a potent anti-inflammatory cytokine. Hence, CD46 is likely to control inflammation. Indeed, data from CD46 transgenic mice highlight a role for CD46 in inflammation, with antagonist roles depending on the cytoplasmic tail being expressed. Furthermore, recent data have shown that CD46 is defective in multiple sclerosis, IL-10 production being severely impaired in these patients. This lack of IL-10 production probably participates in the inflammation observed in patients with multiple sclerosis. This review will summarize the data on CD46 and T cells, and how CD46 is likely involved in multiple sclerosis.
Keywords: CD46, inflammation, interleukin-10, multiple sclerosis, regulatory T cell type 1, T-cell regulation
Introduction
The control mechanisms for accurate T-cell activation and T-cell differentiation are essential to maintain good immune homeostasis. Complete understanding of the factors controlling immunity is therefore likely to help to understand human pathologies. Among autoimmune diseases, multiple sclerosis (MS) is a chronic inflammatory disease, with inflammation in the brain.1,2 It is a complex disease that involves multiple aspects (immunological, genetic and environmental), and although several mechanisms have been uncovered, the understanding of MS pathogenesis is far from complete. CD46 recently appeared as a new player in the regulation of the acquired immune response. Indeed, on top of its role in innate immunity, CD46 controls T-cell fate.3–5 Its activation leads to T-cell proliferation, differentiation of regulatory type 1 T cells (Tr1) and interleukin-10 (IL-10) production. Moreover, in a transgenic mouse model, a definite role of CD46 in the control of inflammation was demonstrated.6 Similarly, a role in inflammation in humans was recently suggested by a study analysing patients with MS. In most patients, IL-10 production was severely impaired upon CD46 stimulation, suggesting that Tr1 differentiation was defective in patients with MS.7,8 Hence, CD46 is a regulator of T-cell function, and it is likely that it has a significant role in human pathologies.
CD46 structure
CD46 is a transmembrane protein ubiquitously expressed, except for red blood cells. It is composed of four short consensus repeat structures [also called Sushi domains or complement control protein (CCP) modules] in the extracellular part.9 The binding of different ligands involves different CCP modules. For example, CCP2, CCP3 and CCP4 are necessary for C3b/C4b binding, whereas CCP1 and CCP2 are necessary for measles virus binding.10,11 These are followed by three exons enriched in serine-threoine-proline residues (STP A, B and C). Then, a short transmembrane domain is present, followed by a common exon and several exons coding for a cytoplasmic tail. Multiple isoforms are produced as the result of alternative splicing.12–17 Of note, two major intracytoplasmic isoforms are produced by Cyt1 splicing (called C1 and C2). As there is a stop codon in the Cyt1 exon, C1 and C2 sequences are distinct (Fig. 1). The main isoforms produced result from a combination of splices of the STP exons and of the cytoplasmic ones, resulting in isoforms C1, C2, BC1 and BC2.
Figure 1.
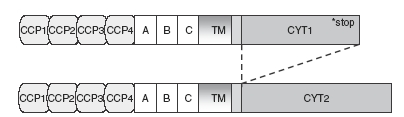
Schematic structure of CD46 cytoplasmic isoforms. The two cytoplasmic isoforms, C1 and C2, derived from the alternative splicing of the Cyt1 exon. They exhibit different sequences, because of the stop codon present in the Cyt1 sequence. CCP, complement control protein modules.
CD46 function as a complement binding protein
CD46 was formerly called membrane cofactor protein. Its primary function is to protect autologous cells from complement attack. Indeed, it binds to C3b and C4b and acts as a cofactor for the protease factor I, allowing the proteolytic cleavage of C3b and C4b.18 Therefore, it functions as a complement inhibitor, along with CD55 (DAF) and CD59.3,19 More recently, it was shown that CD46 was shed from the cell surface of apoptotic and necrotic cells, allowing their efficient removal by phagocytes.20 Finally, the importance of CD46 in regulating complement activation is further demonstrated by genetic analyses. In humans, mutations in CD46 are associated with haemolytic–uremic syndrome, and even a heterozygous deficiency of CD46 predisposes to this disease.21–23 In the mouse, deficiency of the homologous protein Crry, leads to embryonic lethality.24
CD46 described as a ‘magnet for pathogens’
In the early 1990s, it was reported that CD46 was able to act as a receptor for measles virus.25,26 This is true for the vaccine strains such as Edmonston, but some clinical isolates do not bind to CD46 but to SLAM.27 A series of manuscripts then reported the role of CD46 on the control of immunity, aiming at understanding the immunosuppression induced by measles virus infection.28–30 This was followed by description of the binding to CD46 of other viruses such as human herpes virus 6 (HHV6) (subgroups A and B)31–33 as well as adenoviruses.34,35 Several strains of bacteria have also been shown to bind to CD46, including Streptococcus pyogenes36,37 and the pathogenic Neisseria strain Neisseria gonorrhoeae,38 which leads to meningitis in a mouse model.39 Altogether, seven human pathogens, so far, have been found binding to CD46. Hence, it was properly baptized a ‘pathogens’ magnet’.40 Of note, HHV7, which does not bind to CD46, enhances CD46 expression on infected human T cells, which is likely to allow the virus to evade the immune system.41
CD46 affects T-cell fate
CD46 as a costimulatory molecule for human T cells
While CD46 was described as a receptor for several pathogens, several groups tried to identify the intracellular mediators involved after CD46 activation. Indeed, CD46 ligation, by crosslinking of antibodies or binding of natural ligands at the surface of several cell types, induces intracellular signalling, such as calcium flux, NO production, and phosphorylation of intracellular substrates.42–47 Notably, the phosphorylation of two substrates previously identified in T-cell signalling was observed: LAT and p120CBL were tyr-phosphorylated in human T cells upon CD46 activation.44 Hence, a role of CD46 in T-cell activation was investigated; it turned out that costimulation of CD46 and CD3 led to potent proliferation of human T cells, with intensity comparable to that of CD28.44 Moreover, a similar role in T-cell costimulation has also been observed for Crry, the murine homologue of CD46 that is not expressed in mouse except on testis.48–50 This highlights the new role of these complement regulatory molecules in the control of T-cell activation. The expression of CD46 in testis might be explained by its role in reproduction.51
CD46 alters T-cell shape and polarity
CD46 activation also leads to morphological changes in human T cells that are suggestive of a role in migration.52 CD46 plays a crucial role in the regulation of T-cell polarity53,54 by interacting with members of the PDZ-containing Scribble complex.54 While CD46 acts as a costimulatory molecule when coengaged with CD3, its ligation alone causes capping at the distal site in antigen-presenting cell : T-cell interaction, which prevents the immunological synapse formation, subsequent T-cell activation and natural killer cell cytotoxicity. In human primary T cells, CD46 localizes at the uropod, a protrusion formed during migration, and colocalizes with DGL4, which binds specifically to Cyt1 but not Cyt2.55 The polarization of the CD46–DGL4 complex is also dependent on the functional expression of Scribble. Therefore, the triggering of CD46 at the surface of human T cells induces a redistribution of the PDZ polarity network, and regulates in fine T-cell shape and migration.54 Therefore, CD46 influences T-cell fate by modulating T-cell proliferation and affecting T-cell shape.
CD46, an important source of IL-10
Regulatory T cells
There has been a resurgence of interest in regulatory T cells (Treg). Such cells play a role in tolerance by switching off the activation of effector T cells.56–59 Several Treg subsets have been described. Briefly, the main ‘natural Tregs’ express high levels of CD25 and Foxp3 and require cell-to-cell contact to exert their suppressive activity.60 Several classes of ‘induced’ Treg have also been described, which act mainly through the release of soluble mediators. They do not need cell-to-cell contact to exert suppression. Among these, Tr1 cells are characterized by the large amount of IL-10 secreted61 whereas Th3 cells produce mainly transforming growth factor-β.62 The possibility of inducing such cells in vitro has generated a lot of interest because of their possible use in therapies, aimed at turning off unwanted immune responses, such as those observed in autoimmune diseases. Indeed, data on asthma patients suggest that a combination of drugs can restore in vivo Tr1 function, measured by IL-10 production.63 Hence, the manipulation of Tregs in vivo, either by inducing their differentiation or by modulating their activity, is an attractive tool for future immunotherapies.
CD46 and Tr1
In 2003, Kemper and colleagues showed that activation of naïve human CD4+ T cells by CD46 and CD3 in the presence of IL-2 led to differentiation towards a Tr1 phenotype.4,64 These CD46-induced cells secrete large amounts of IL-10 and are able to inhibit the proliferation of bystander CD4 effector cells. Hence CD46 is a potential tool for therapy to dampen an unwanted immune response; one can imagine that its triggering could induce Tr1 and IL-10 production in vivo. Importantly, the authors reported that a natural ligand of CD46, S. pyogenes, also leads to a Tr1 phenotype, demonstrating the physiological relevance of CD46-induced Tr1 cells.65 Further studies have then shown that these Tr1-induced cells secrete granzyme B as well as IL-10, and have the potential to kill autologous targets.66,67
CD46 controls inflammation
As mentioned earlier, multiple isoforms of CD46 exist, including two main cytoplasmic tails, C1 and C2. These two isoforms are coexpressed in human cells, rendering their individual analysis difficult. Transgenic mice were produced that expressed either the C1 or the C2 isoform. This model, although in the mouse, allowed the analysis of each cytoplasmic tail individually.6 When T-cell-dependent inflammation was induced in a contact hypersensitivity model, striking opposite effects were observed for each cytoplasmic tail (summarized in Fig. 2). While the C1 isoform inhibited the overall inflammation, the C2 isoform increased it. This was the result of differential effects on CD4+ proliferation and CD8+ cytotoxicity and of different profiles of cytokine released. In particular, IL-2 was decreased by C1 expression whereas IL-10 secretion was decreased by C2 expression. Hence, it appears that CD46 function is quite complex since, depending on which cytolasmic tail is expressed or activated, a regulatory or proinflammatory phenotype can be observed. This implies that CD46 function in humans must be thoroughly examined and understood before CD46 is applied in therapies, because opposite and undesired results could be obtained.
Figure 2.
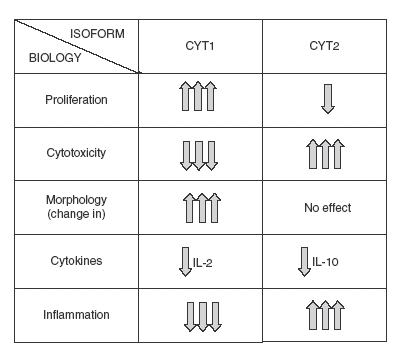
Opposite role of CD46 cytoplasmic isoforms in inflammation. C1 and C2 isoforms were expressed in transgenic mice, and the hypersensitivity contact reaction to dinitrofluorobenzene was assessed in each strain, after stimulation by heat-inactivated measles virus (which binds to CD46). Purified T cells were then costimulated in vitro by CD3/CD46, and proliferation, cytokine production and cytotoxicity were determined.6
CD46 is altered in patients with MS
Defective immune regulation by natural CD4+ CD25+ Tregs has been widely reported in the community in the context of autoimmunity and in particular in MS.68–70 When Tr1 induction was analysed upon CD46 activation, a defect in Tr1 differentiation was observed in a large proportion of patients with MS who were in the relapsing–remitting phase.8 This was characterized by a defect in IL-10 secretion, specifically upon CD46 activation. IL-10 secretion upon CD28 was normal in these patients. This suggests that Tr1 differentiation is abnormal in patients with MS. The expression of both cytoplasmic isoforms was analysed in patients with MS and in healthy donors. While no difference was observed in freshly isolated T cells between healthy volunteers and patients, an increased C2 expression was detected in some patients upon activation. These data, although preliminary, correlate with the findings obtained in the murine model, in which C2-expressing mice have increased inflammation and decreased IL-10 secretion. Moreover, CD46 is also altered in dendritic cells from patients with MS. In these patients, triggering of CD46 on DCs leads to increased IL-23 production and exacerbated chemokine secretion compared to healthy donors.71 There is a profound decrease in CCL2 expression, whereas both CCL3 and CCL5 secretions are increased. Hence, CD46 might be a key element in MS pathogenesis with a likely role in the inflammatory process.
What's next?
Overall, within the past years, CD46 has become a new regulator of T-cell function. Besides being just another costimulatory molecule for T cells, it is an inductor of Tr1 cells, and hence a potent provider of IL-10. Importantly, this very function is altered in patients with MS. It will be important to discover whether CD46 functions in other diseases. Is CD46 defective in other autoimmune diseases? Also, one could imagine that in allergic reactions, the Tr1 function of CD46 is altered – and no IL-10 is secreted by T cells from atopic patients. Similarly, in patients with asthma who are resistant to steroids, dexamethasone treatment does not induce IL-10-producing Tr1. One obvious question is: are CD46-induced Tr1 also defective in these refractory patients? Finally, by analysing patients with tumours, we could determine if CD46 is overreacting in such patients. Is CD46 involved and secreting high amounts of IL-10, suppressing a very much wanted immune response? Another fascinating study could be the analysis of an alternative ligand for CD46. Although a multitude of natural ligands have already been described, it is possible that an endogenous ligand, expressed on dendritic cells or other antigen-presenting cells, exists. This is suggested by Gasque and collaborators who recorded specific binding of a CD46–IgG4 fusion protein to THP1, HL60 and polymorphonuclear cells.20 Finally, it should be noted that MS relapses are often associated with HHV6 infection.72,73 As HHV6 binds to CD46, this suggests that HHV6 could be the trigger for CD46 stimulation in the brains of these patients.
Conclusion
Much more work is needed to further investigate the role of CD46 in T-cell regulation and inflammation. The combined efforts of several investigators will likely improve our common knowledge on CD46, thus improving our understanding of how to modulate the immune response, with potential therapeutic benefits.
Acknowledgments
Part of the work presented here was made possible through support from CNRS (France), from the NMSS and from Professor Chantal Rabourdin-Combe (INSERM U851, Lyon, France) and Professor David Hafler (Harvard Medical School, Boston).
References
- 1.Hafler DA, Slavik JM, Anderson DE, O’Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis. Immunol Rev. 2005;204:208–31. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 2.McQualter JL, Bernard CC. Multiple sclerosis: a battle between destruction and repair. J Neurochem. 2007;100:295–306. doi: 10.1111/j.1471-4159.2006.04232.x. [DOI] [PubMed] [Google Scholar]
- 3.Riley-Vargas RC, Gill DB, Kemper C, Liszewski MK, Atkinson JP. CD46: expanding beyond complement regulation. Trends Immunol. 2004;25:496–503. doi: 10.1016/j.it.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Kemper C, Atkinson JP. T-cell regulation: with complements from innate immunity. Nat Rev Immunol. 2007;7:9–18. doi: 10.1038/nri1994. [DOI] [PubMed] [Google Scholar]
- 5.Russell S. CD46: a complement regulator and pathogen receptor that mediates links between innate and acquired immune function. Tissue Antigens. 2004;64:111–8. doi: 10.1111/j.1399-0039.2004.00277.x. [DOI] [PubMed] [Google Scholar]
- 6.Marie JC, Astier AL, Rivailler P, Rabourdin-Combe C, Wild TF, Horvat B. Linking innate and acquired immunity: divergent role of CD46 cytoplasmic domains in T cell induced inflammation. Nat Immunol. 2002;3:659–66. doi: 10.1038/ni810. [DOI] [PubMed] [Google Scholar]
- 7.Astier AL, Hafler DA. Abnormal Tr1 differentiation in multiple sclerosis. J Neuroimmunol. 2007;191:70–8. doi: 10.1016/j.jneuroim.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Astier AL, Meiffren G, Freeman S, Hafler DA. Alterations in CD46-mediated Tr1 regulatory T cells in patients with multiple sclerosis. J Clin Invest. 2006;116:3252–7. doi: 10.1172/JCI29251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liszewski MK, Post TW, Atkinson JP. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu Rev Immunol. 1991;9:431–55. doi: 10.1146/annurev.iy.09.040191.002243. [DOI] [PubMed] [Google Scholar]
- 10.Manchester M, Gairin JE, Patterson JB, Alvarez J, Liszewski MK, Eto DS, Atkinson JP, Oldstone MB. Measles virus recognizes its receptor, CD46, via two distinct binding domains within SCR1-2. Virology. 1997;233:174–84. doi: 10.1006/viro.1997.8581. [DOI] [PubMed] [Google Scholar]
- 11.Buchholz CJ, Koller D, Devaux P, Mumenthaler C, Schneider-Schaulies J, Braun W, Gerlier D, Cattaneo R. Mapping of the primary binding site of measles virus to its receptor CD46. J Biol Chem. 1997;272:22072–9. doi: 10.1074/jbc.272.35.22072. [DOI] [PubMed] [Google Scholar]
- 12.Liszewski MK, Tedja I, Atkinson JP. Membrane cofactor protein (CD46) of complement. Processing differences related to alternatively spliced cytoplasmic domains. J Biol Chem. 1994;269:10776–9. [PubMed] [Google Scholar]
- 13.Manchester M, Liszewski MK, Atkinson JP, Oldstone MB. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc Natl Acad Sci USA. 1994;91:2161–5. doi: 10.1073/pnas.91.6.2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnstone RW, Russell SM, Loveland BE, McKenzie IF. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol Immunol. 1993;30:1231–41. doi: 10.1016/0161-5890(93)90038-d. [DOI] [PubMed] [Google Scholar]
- 15.Johnstone RW, Russell S, Loveland BE, McKenzie IF. Tissue-specific expression of CD46 protein isoforms due to production of RNA splice variants. Transplant Proc. 1994;26:1248. [PubMed] [Google Scholar]
- 16.Purcell DF, Russell SM, Deacon NJ, Brown MA, Hooker DJ, McKenzie IF. Alternatively spliced RNAs encode several isoforms of CD46 (MCP), a regulator of complement activation. Immunogenetics. 1991;33:335–44. doi: 10.1007/BF00216692. [DOI] [PubMed] [Google Scholar]
- 17.Xing PX, Russell S, Prenzoska J, McKenzie I. Discrimination between alternatively spliced STP-A and -B isoforms of CD46. Immunology. 1994;83:122–7. [PMC free article] [PubMed] [Google Scholar]
- 18.Seya T, Atkinson JP. Functional properties of membrane cofactor protein of complement. Biochem J. 1989;264:581–8. doi: 10.1042/bj2640581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Longhi MP, Harris CL, Morgan BP, Gallimore A. Holding T cells in check – a new role for complement regulators? Trends Immunol. 2006;27:102–8. doi: 10.1016/j.it.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Elward K, Griffiths M, Mizuno M, Harris CL, Neal JW, Morgan BP, Gasque P. CD46 plays a key role in tailoring innate immune recognition of apoptotic and necrotic cells. J Biol Chem. 2005;280:36342–54. doi: 10.1074/jbc.M506579200. [DOI] [PubMed] [Google Scholar]
- 21.Kavanagh D, Richards A, Atkinson JP. Complement regulatory genes and hemolytic uremic syndromes. Annu Rev Med. 2008;59:293–309. doi: 10.1146/annurev.med.59.060106.185110. [DOI] [PubMed] [Google Scholar]
- 22.Richards A, Kemp EJ, Liszewski MK, et al. Mutations in human complement regulator, membrane cofactor protein (CD46), predispose to development of familial hemolytic uremic syndrome. Proc Natl Acad Sci U S A. 2003;100:12966–71. doi: 10.1073/pnas.2135497100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Noris M, Brioschi S, Caprioli J, Todeschini M, Bresin E, Porrati F, Gamba S, Remuzzi G. Familial haemolytic uraemic syndrome and an MCP mutation. Lancet. 2003;362:1542–7. doi: 10.1016/S0140-6736(03)14742-3. [DOI] [PubMed] [Google Scholar]
- 24.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]
- 25.Dorig RE, Marcil A, Chopra A, Richardson CD. The human CD46 molecule is a receptor for measles virus (Edmonston strain) Cell. 1993;75:295–305. doi: 10.1016/0092-8674(93)80071-l. [DOI] [PubMed] [Google Scholar]
- 26.Naniche D, Varior-Krishnan G, Cervoni F, Wild TF, Rossi B, Rabourdin-Combe C, Gerlier D. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J Virol. 1993;67:6025–32. doi: 10.1128/jvi.67.10.6025-6032.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tatsuo H, Ono N, Tanaka K, Yanagi Y. SLAM (CDw150) is a cellular receptor for measles virus. Nature. 2000;406:893–7. doi: 10.1038/35022579. [DOI] [PubMed] [Google Scholar]
- 28.Karp CL, Wysocka M, Wahl LM, Ahearn JM, Cuomo PJ, Sherry B, Trinchieri G, Griffin DE. Mechanism of suppression of cell-mediated immunity by measles virus. Science. 1996;273:228–31. doi: 10.1126/science.273.5272.228. Published erratum appears in Science 1997; 275:1053. [DOI] [PubMed] [Google Scholar]
- 29.Kurita M, Yanagi Y, Hara T, Nagasawa S, Matsumoto M, Seya T. Human lymphocytes are more susceptible to measles virus than granulocytes, which is attributable to the phenotypic differences of their membrane cofactor protein (CD46) Immunol Lett. 1995;48:91–5. doi: 10.1016/0165-2478(95)02447-6. [DOI] [PubMed] [Google Scholar]
- 30.Schnorr JJ, Xanthakos S, Keikavoussi P, Kampgen E, ter Meulen V, Schneider-Schaulies S. Induction of maturation of human blood dendritic cell precursors by measles virus is associated with immunosuppression. Proc Natl Acad Sci U S A. 1997;94:5326–31. doi: 10.1073/pnas.94.10.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santoro F, Kennedy PE, Locatelli G, Malnati MS, Berger EA, Lusso P. CD46 is a cellular receptor for human herpesvirus 6. Cell. 1999;99:817–27. doi: 10.1016/s0092-8674(00)81678-5. [DOI] [PubMed] [Google Scholar]
- 32.Greenstone HL, Santoro F, Lusso P, Berger EA. Human herpesvirus 6 and Measles virus employ distinct CD46 domains for receptor function. J Biol Chem. 2002;277:39112–8. doi: 10.1074/jbc.M206488200. [DOI] [PubMed] [Google Scholar]
- 33.Smith A, Santoro F, Di Lullo G, Dagna L, Verani A, Lusso P. Selective suppression of IL-12 production by human herpesvirus 6. Blood. 2003;102:2877–84. doi: 10.1182/blood-2002-10-3152. [DOI] [PubMed] [Google Scholar]
- 34.Segerman A, Atkinson JP, Marttila M, Dennerquist V, Wadell G, Arnberg N. Adenovirus type 11 uses CD46 as a cellular receptor. J Virol. 2003;77:9183–91. doi: 10.1128/JVI.77.17.9183-9191.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaggar A, Shayakhmetov DM, Lieber A. CD46 is a cellular receptor for group B adenoviruses. Nat Med. 2003;9:1408–12. doi: 10.1038/nm952. [DOI] [PubMed] [Google Scholar]
- 36.Giannakis E, Jokiranta TS, Ormsby RJ, et al. Identification of the streptococcal M protein binding site on membrane cofactor protein (CD46) J Immunol. 2002;168:4585–92. doi: 10.4049/jimmunol.168.9.4585. [DOI] [PubMed] [Google Scholar]
- 37.Okada N, Liszewski MK, Atkinson JP, Caparon M. Membrane cofactor protein (CD46) is a keratinocyte receptor for the M protein of the group A streptococcus. Proc Natl Acad Sci U S A. 1995;92:2489–93. doi: 10.1073/pnas.92.7.2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kallstrom H, Liszewski MK, Atkinson JP, Jonsson AB. Membrane cofactor protein (MCP or CD46) is a cellular pilus receptor for pathogenic Neisseria. Mol Microbiol. 1997;25:639–47. doi: 10.1046/j.1365-2958.1997.4841857.x. [DOI] [PubMed] [Google Scholar]
- 39.Johansson L, Rytkonen A, Bergman P, et al. CD46 in meningococcal disease. Science. 2003;301:373–5. doi: 10.1126/science.1086476. [DOI] [PubMed] [Google Scholar]
- 40.Cattaneo R. Four viruses, two bacteria, and one receptor: membrane cofactor protein (CD46) as pathogens’ magnet. J Virol. 2004;78:4385–8. doi: 10.1128/JVI.78.9.4385-4388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Takemoto M, Yamanishi K, Mori Y. Human herpesvirus 7 infection increases the expression levels of CD46 and CD59 in target cells. J Gen Virol. 2007;88:1415–22. doi: 10.1099/vir.0.82394-0. [DOI] [PubMed] [Google Scholar]
- 42.Kallstrom H, Islam MS, Berggren PO, Jonsson AB. Cell signaling by the type IV pili of pathogenic Neisseria. J Biol Chem. 1998;273:21777–82. doi: 10.1074/jbc.273.34.21777. [DOI] [PubMed] [Google Scholar]
- 43.Yant S, Hirano A, Wong TC. Identification of a cytoplasmic Tyr-X-X-Leu motif essential for down regulation of the human cell receptor CD46 in persistent measles virus infection. J Virol. 1997;71:766–70. doi: 10.1128/jvi.71.1.766-770.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Astier A, Trescol-Biemont MC, Azocar O, Lamouille B, Rabourdin-Combe C. Cutting edge: CD46, a new costimulatory molecule for T cells, that induces p120CBL and LAT phosphorylation. J Immunol. 2000;164:6091–5. doi: 10.4049/jimmunol.164.12.6091. [DOI] [PubMed] [Google Scholar]
- 45.Hirano A, Kurita-Taniguchi M, Katayama Y, Matsumoto M, Wong TC, Seya T. Ligation of human CD46 with purified complement C3b or F(ab′)2 of monoclonal antibodies enhances isoform-specific interferon gamma-dependent nitric oxide production in macrophages. J Biochem. 2002;132:83–91. doi: 10.1093/oxfordjournals.jbchem.a003203. [DOI] [PubMed] [Google Scholar]
- 46.Lee SW, Bonnah RA, Higashi DL, Atkinson JP, Milgram SL, So M. CD46 is phosphorylated at tyrosine 354 upon infection of epithelial cells by Neisseria gonorrhoeae. J Cell Biol. 2002;156:951–7. doi: 10.1083/jcb.200109005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kurita-Taniguchi M, Fukui A, Hazeki K, et al. Functional modulation of human macrophages through CD46 (measles virus receptor): production of IL-12 p40 and nitric oxide in association with recruitment of protein-tyrosine phosphatase SHP-1 to CD46. J Immunol. 2000;165:5143–52. doi: 10.4049/jimmunol.165.9.5143. [DOI] [PubMed] [Google Scholar]
- 48.Fernandez-Centeno E, de Ojeda G, Rojo JM, Portoles P. Crry/p65, a membrane complement regulatory protein, has costimulatory properties on mouse T cells. J Immunol. 2000;164:4533–42. doi: 10.4049/jimmunol.164.9.4533. [DOI] [PubMed] [Google Scholar]
- 49.Gaglia JL, Mattoo A, Greenfield EA, Freeman GJ, Kuchroo VK. Characterization of endogenous Chinese hamster ovary cell surface molecules that mediate T cell costimulation. Cell Immunol. 2001;213:83–93. doi: 10.1006/cimm.2001.1867. [DOI] [PubMed] [Google Scholar]
- 50.Jimenez-Perianez A, Ojeda G, Criado G, Sanchez A, Pini E, Madrenas J, Rojo JM, Portoles P. Complement regulatory protein Crry/p65-mediated signaling in T lymphocytes: role of its cytoplasmic domain and partitioning into lipid rafts. J Leukoc Biol. 2005;78:1386–96. doi: 10.1189/jlb.1104642. [DOI] [PubMed] [Google Scholar]
- 51.Rooney IA, Heuser JE, Atkinson JP. GPI-anchored complement regulatory proteins in seminal plasma. An analysis of their physical condition and the mechanisms of their binding to exogenous cells. J Clin Invest. 1996;97:1675–86. doi: 10.1172/JCI118594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zaffran Y, Destaing O, Roux A, Ory S, Nheu T, Jurdic P, Rabourdin-Combe C, Astier AL. CD46/CD3 costimulation induces morphological changes of human T cells and activation of Vav, Rac, and extracellular signal-regulated kinase mitogen-activated protein kinase. J Immunol. 2001;167:6780–5. doi: 10.4049/jimmunol.167.12.6780. [DOI] [PubMed] [Google Scholar]
- 53.Oliaro J, Pasam A, Waterhouse NJ, Browne KA, Ludford-Menting MJ, Trapani JA, Russell SM. Ligation of the cell surface receptor, CD46, alters T cell polarity and response to antigen presentation. Proc Natl Acad Sci U S A. 2006;103:18685–90. doi: 10.1073/pnas.0602458103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ludford-Menting MJ, Oliaro J, Sacirbegovic F, et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–48. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 55.Ludford-Menting MJ, Thomas SJ, Crimeen B, Harris LJ, Loveland BE, Bills M, Ellis S, Russell SM. A functional interaction between CD46 and DLG4: a role for DLG4 in epithelial polarization. J Biol Chem. 2002;277:4477–84. doi: 10.1074/jbc.M108479200. [DOI] [PubMed] [Google Scholar]
- 56.Thompson C, Powrie F. Regulatory T cells. Curr Opin Pharmacol. 2004;4:408–14. doi: 10.1016/j.coph.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 57.Paust S, Cantor H. Regulatory T cells and autoimmune disease. Immunol Rev. 2005;204:195–207. doi: 10.1111/j.0105-2896.2005.00247.x. [DOI] [PubMed] [Google Scholar]
- 58.Bluestone JA, Tang Q. How do CD4+CD25+ regulatory T cells control autoimmunity? Curr Opin Immunol. 2005;17:638–42. doi: 10.1016/j.coi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 59.Sakaguchi S, Powrie F. Emerging challenges in regulatory T cell function and biology. Science. 2007;317:627–9. doi: 10.1126/science.1142331. [DOI] [PubMed] [Google Scholar]
- 60.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor foxp3. Science. 2003;299:1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 61.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 62.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xystrakis E, Kusumakar S, Boswell S, et al. Reversing the defective induction of IL-10-secreting regulatory T cells in glucocorticoid-resistant asthma patients. J Clin Invest. 2006;116:146–55. doi: 10.1172/JCI21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kemper C, Chan AC, Green JM, Brett KA, Murphy KM, Atkinson JP. Activation of human CD4(+) cells with CD3 and CD46 induces a T-regulatory cell 1 phenotype. Nature. 2003;421:388–92. doi: 10.1038/nature01315. [DOI] [PubMed] [Google Scholar]
- 65.Price JD, Schaumburg J, Sandin C, Atkinson JP, Lindahl G, Kemper C. Induction of a regulatory phenotype in human CD4+ T cells by streptococcal M protein. J Immunol. 2005;175:677–84. doi: 10.4049/jimmunol.175.2.677. [DOI] [PubMed] [Google Scholar]
- 66.Grossman WJ, Verbsky JW, Tollefsen BL, Kemper C, Atkinson JP, Ley TJ. Differential expression of granzymes A and B in human cytotoxic lymphocyte subsets and T regulatory cells. Blood. 2004;104:2840–8. doi: 10.1182/blood-2004-03-0859. [DOI] [PubMed] [Google Scholar]
- 67.Grossman WJ, Verbsky JW, Barchet W, Colonna M, Atkinson JP, Ley TJ. Human T regulatory cells can use the perforin pathway to cause autologous target cell death. Immunity. 2004;21:589–601. doi: 10.1016/j.immuni.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Haas J, Hug A, Viehover A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–52. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 69.Huan J, Culbertson N, Spencer L, et al. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 70.Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–9. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vaknin-Dembinsky A, Murugaiyan G, Hafler DA, Astier AL, Weiner HL. Increased IL-23 secretion and altered chemokine production by dendritic cells upon CD46 activation in patients with multiple sclerosis. J Neuroimmunol. 2008 doi: 10.1016/j.jneuroim.2008.01.002. doi: 10.1016/j.jneuroim.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Soldan SS, Berti R, Salem N, et al. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat Med. 1997;3:1394–7. doi: 10.1038/nm1297-1394. [DOI] [PubMed] [Google Scholar]
- 73.Alvarez-Lafuente R, Garcia-Montojo M, De las Heras V, Bartolome M, Arroyo R. Clinical parameters and HHV-6 active replication in relapsing–remitting multiple sclerosis patients. J Clin Virol. 2006;37(Suppl. 1):S24–6. doi: 10.1016/S1386-6532(06)70007-5. [DOI] [PubMed] [Google Scholar]


