Abstract
Epididymitis represents a serious threat to male fertility and usually develops following secondary bacterial infection of the epididymis such as urinary tract infections or sexually transmitted diseases. Surprisingly, very little is known about the innate host response triggered by bacterial infection in the male reproductive tract. In this study we investigated the regulation and function of Nod2 in epididymal epithelial cells following lipopolysaccharide (LPS) stimulation. The immortalized epididymal epithelial cell line PC1 (proximal caput 1) constitutively expressed Toll-like receptor 4, MD-2, CD-14 but not Nod2 messenger RNA. Lipopolysaccharide (LPS; 0·5 μg/ml) rapidly induced IκB phosphorylation and degradation, RelA nuclear translocation and phosphorylation, which correlated with enhanced transcriptional activity (four-fold) in PC1 cells. The LPS and lipid A rapidly (1 hr) induced Nod2 messenger RNA accumulation in a dose-dependent manner. RelA and RNApolII recruitment to the Nod2 gene promoter was enhanced in LPS-stimulated cells. Molecular blockade of nuclear factor-κB signalling with adenovirus 5 (Ad5) IκBAA or adenovirus 5 double-negative (Ad5dn) IKKβ prevented LPS-induced Nod2 gene expression. Functionally, Nod2 upregulation enhanced muramyl dipeptide (MDP) -induced tumour necrosis factor messenger RNA accumulation in PC1 cells. We conclude that epididymal epithelial cells mount an innate response following LPS exposure which leads to upregulation of Nod2 and enhanced responsiveness to the microbial product MDP. The rapid Nod2 upregulation in epididymal epithelial cells is probably part of a complex innate host response aimed at protecting the male reproductive tract from the deleterious impact of bacteria.
Keywords: epididymal epithelial cells, innate immunity, Nod2, NF-κB, transcription
Introduction
The evolution of a species is dictated by those members that are able to successfully reproduce and pass on their genetic material. In this regard a healthy germline is of key importance to the survival of any sexually reproducing organism. Thus, it is vital that germ cells have an environment that is conductive to their proper growth and development. Spermatozoa that leave the testis are immature and lack forward mobility and fertilizing ability.1 From the efferent ductules spermatozoa slowly transit through the epididymis, which will provide the environment critical to proper maturation, storage and transport. During their transit in the epididymis a wide variety of proteins secreted by the epithelial cells are added to the sperm membrane by a process called ‘sperm maturation’, which results in acquisition of forward motility and the ability to undergo capacitation, two major factors in male fertility.2 The epithelia of this organ are responsible for maintaining this environment and protecting the spermatozoa thereby producing viable gametes.3 The epithelia accomplish this through various secretory and resorptive activities, ensuring that the spermatozoa receive nutrients, extratesticular fluid is absorbed, old spermatozoa are phagocytosed, and mucus and defensins are secreted providing protection against invading pathogens.1
Epididymitis represents a serious clinical condition and is characterized by inflammation and obstruction of sperm movement, resulting from the retrograde extension of microorganisms from the vas deferens.4,5 If left untreated, the infection could lead to the formation of epididymal and testicular abscesses.3 The incidence of epididymitis is approximately 600 000 cases per year in the United States, with the highest prevalence observed in young men aged 19–35 years.6,7 The infection can be the result of sexually transmitted pathogens such as Chlamydia, Neisseria gonorrohoea or of common pathogenic bacteria such as Escherichia coli.4,6
A number of pattern recognition receptors, including Toll-like receptors (TLR) and nucleotide-binding oligomerization domain (NOD), play a pivotal role in mediating the innate host response to various infectious microorganisms. For example, cell surface TLR4 recognizes lipopolysaccharide (LPS), whereas intracellular Nod2 (CARD15) recognizes peptidoglycan-derived muramyl dipeptide (MDP), a component of Gram-positive and Gram-negative bacterial cell walls.8–10 Thus Nod2 functions as a general sensor of most, if not all, bacteria whereas TLR4 is selective toward Gram-negative bacteria.11 Interestingly, although initiation of proximal TLR4 and Nod2 signalling is quite distinct, both cascades ultimately activate the nuclear factor-κB (NF-κB) transcriptional system. Indeed, TLR4 and Nod2 signalling converge at receptor-interacting protein-2 (RIP-2), which then activates the IκB kinase (IKK) complex.12 This enzyme complex phosphorylates the NF-κB inhibitor IκB on serine residues 32 and 36, causing its ubiquitination and degradation. Elimination of IκB liberates NF-κB from its inhibitory effect and permits the nuclear translocation of the transcription factor, binding to κB-promoter elements and induction of gene transcription.13 Genes that are targeted by NF-κB include those encoding cytokines, chemokines, adhesion molecules, acute-phase proteins and antimicrobial peptides. The epithelial host response to colonization and/or infection of microorganisms has been extensively studied in organ systems such as the digestive, respiratory and female reproductive tracts.14–16 However, very little information is available about the innate host response and the mechanisms of signal transduction in the male reproductive tract.17–19 Furthermore, the presence of NOD proteins and their role in the male reproductive tract are unknown. It is possible that this system may have a unique immune signalling mechanism that differs from other organ systems. In this study, we showed that epididymal epithelial cells rapidly respond to LPS by inducing Nod2 gene expression through a NF-κB-dependent mechanism. Nod2-expressing cells become responsive to MDP and upregulate the chemokine gene tumour necrosis factor (TNF). The rapid Nod2 upregulation in epididymal epithelial cells is probably part of a complex innate host response aimed at protecting the male reproductive tract from the deleterious impact of bacteria.
Materials and methods
Cell culture and treatment of PC1 cells
The immortalized murine epididymal epithelial cell line, PC1 (a gift from Dr Susan Hall, University of North Carolina at Chapel Hill, Chapel Hill, NC) was used between passages 35 and 55.7 Cells were grown to near confluency in six-well plates (Costar, Corning Incorporated, NY) at 32° in Iscove's modified Dulbecco's medium (Life Technologies, Grand Island, NY) supplemented with non-essential amino acids (0·1 mm, Life Technologies), sodium pyruvate (1 mm, Life Technologies), l-glutamine (4 mm, Life Technologies), 5α-dihydrotestosterone (1 nm, Sigma, St. Louis, MO), 10% fetal calf serum (Sigma), and antibiotics (penicillin G 50 U/ml and streptomycin 50 mg/ml; Life Technologies). Approximately 90% confluent monolayers of PC1 cells were stimulated for 1 hr or prestimulated with LPS (1 μg/ml; from E. coli 0111:B4; Sigma) followed by stimulation with MDP (10 μg/ml; Sigma). All experiments were conducted in media containing 1% serum.
Chromatin immunoprecipitation (ChIP) analysis
Proximal caput cells were stimulated with LPS (1 μg/ml; from E. coli 0111:B4; Sigma) for 0, 30, 60 and 120 min and the ChIP assay was performed using a ChIP assay kit (Upstate-Cell Signaling Solutions, Temecula, CA) according to the manufacturer's specification as described previously.20 Immunoprecipitation was carried out overnight with 2 μg p65 (c-20, Santa Cruz Biotechnology, Santa Cruz, CA) or RNA Polymerase II (c-21, Santa Cruz Biotechnology) antibodies. Polymerase chain reaction (PCR) was performed with total DNA (5 μl) and immunoprecipitated DNA (5 μl) using Nod2 promoter-specific primers: murine Nod 2 (mNod2) proximal promoter forward: 5′-ACGTTGCATGGGACTGACA-3′, mNod2 proximal promoter reverse: 5′-CCCCTCCTGATTTCCCTC-3′, mNod2 distal promoter forward: 5′-ATGGATGGAATACTCACCCTCTA-3′ and mNod2 distal promoter reverse 5′-GCGGATGAAATAACCCAATAC-3′. The PCR was carried out in a volume of 50 μl containing final concentrations of 1×Taq buffer (Applied Biosystems, Foster City, CA), 50 nm primers, 0·5 mm dNTPs, and 1 U Thermo aquaticus polymerase (Applied Biosystems) using a 9700 Gene-Amp PCR system cycler (Applied Biosystems). The PCR temperatures used were 95° for 30 seconds (denaturating), 56° for 60 seconds (annealing), and 72° for 1 min (polymerization) followed by an extension of 5 min at 72°. The PCR products were subjected to electrophoresis on 2% agarose gels containing gel Star fluorescent dye (Cambrex BioScience Rockland, Rockland, ME). Fluorescence staining was captured using an Alpha Imager 2000 (AlphaInnotech, San Leandro, CA).
RNA extraction and reverse transcription-PCR analysis
RNA was isolated using Trizol (Invitrogen, Carlsbad, CA), reverse transcribed (1 μg RNA), and amplified using specific primers for mouse Nod2, TLR4, CD14, MD-2, TNF-α and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Murine Nod2 forward 5′-GCCCTACAGCTGGATTACAAC-3′, mNod2 reverse: 5′-CGCCTGTGATGTGATTGTTC-3′; mTNF-α forward: 5′-ATGAGCACAGAAAGCATGATC3′, mTNF-α reverse: 5′-TACAGGCTTGTCACTCGAATT-3′; GAPDH forward: 5′-GGTGAAGGTCGGTGTGAACGGA-3′, GAPDH reverse: 5′-GAGGGATCTCGCTCCTGGAAGA-3′; mCD14 forward: 5′-CGCGGATTCCTAGTCGG-3′; mCD14 reverse: 5′-CGCAGGAAAAGTTGAGTGAGT-3′; mMD2 forward: 5′-CTCCGATGCAATTATTTCCTAC-3′; mMD2 reverse: 5′-TGGCACAGAACTTCCTTACG-3′ mTLR4 forward 5′ATTCAGAGCCGTTGGTGTATC-3′; mTLR4 reverse: 5′-TTCGAGGCTTTTCCATCCAATAGG-3′. The PCR products (8 μl) were subjected to electrophoresis on 2% agarose gels containing GelStar fluorescent dye (Cambrex BioScience Rockland). Fluorescence staining was captured using an Alpha Imager 2000 (Alpha Innotech, San Leandro, CA). The Nod2, TLR4, CD14, MD-2, TNF-α and GAPDH amplicons were 277 base pairs (bp), 242 bp, 267 bp, 207 bp, 276 bp and 240 bp respectively. The amplicons were sequenced using the Taq dye-terminator chemistry method and the identity of each product was confirmed by sequence alignment using ncbi blast. To precisely quantify the expression of TNF-α and GAPDH real-time PCR was conducted with an Applied Biosystems 7900HT Fast Real-Time PCR System. For PCR 2 μl cDNA preparation, 150 nm final concentration of forward and reverse primers and 6 μl QuantiTect SYBR Green PCR Master Mix (Qiagen, Valencia, CA) in a total of 12 μl were applied. The following PCR programme was performed: 15 min at 95° (initial denaturation); 20°/second temperature transition rate up to 95° for 30 seconds, 45 seconds at 56°, 45 seconds at 72°, with acquisition mode single, repeated 40 times (amplification). The PCR was evaluated by melting curve analysis following the manufacturer's instructions and checking the PCR products on 1·8% agarose gels.
Western immunoblots
Proximal caput cells were pretreated with the IκB-phosphorylation inhibitor BAY 11-7082 (25 μm) (Calbiochem, SanDiego, CA) for 1 hr where indicated followed by stimulation with LPS (0·5 μg/ml; from E. coli 0111:B4, Sigma) at various-time points or were prestimulated with LPS for 16 hr followed by MDP (Sigma) stimulation (10 μg/ml) for various time-points or left untreated. Cells were harvested and lysed in 1× Laemmli buffer, and the protein concentration was measured using a Bio-Rad quantification assay (Bio-Rad Laboratories, Hercules, CA). Twenty micrograms of the protein extracts was subjected to electrophoresis on 10% sodium dodecyl sulphate (SDS)–polyacrylamide gels and transferred to Hybond-C Extra nitrocellulose membranes (Amersham Biosciences, Piscataway, NJ). Anti-phosphoserine IκBα (S32, Cell Signaling, Beverly, MA), anti-IκB (C-21, Santa Cruz Biotechnology) and anti-β-actin (ICN, Costa Mesa, CA) antibodies were used to detect immunoreactive phospho-IκBα, IκBα and β-actin respectively. All antibodies were used at a 1 : 1000 dilution in a solution containing 5% milk in tris-buffered saline Tween-20 (TBS-T). Immunoreactive proteins were detected using the enhanced chemiluminescence light (ECL) detecting kit (Amersham Biosciences).
Adenoviruses and cell infection
Proximal caput cells were infected with the various adenoviral vectors (∼ 2 × 108 plaque-forming units/μl) in serum-reduced media (1%) at optimal multiplicity of infection (MOI) for 12 hr. The adenovirus was washed off, fresh serum-reduced medium was added and cells were stimulated where indicated. The various adenoviral vectors containing dominant negative molecules of the NF-κB pathway (Ad5IκBAA and Ad5dnIKΚβ, MOI: 50),21 an NF-κB luciferase reporter gene (Ad5NF-κB-LUC, MOI: 10)22 or green-fluorescent protein (Ad5GFP; negative control)23 were amplified and maintained as described previously.
Immunofluorescence
Where indicated, PC1 cells were infected for 12 hr with Ad5dnIKKβ, stimulated with LPS (500 ng/ml) for 1 hr, and fixed with 100% ice-cold methanol for 10 min at 4° immediately after stimulation. Cells were blocked with 10% non-immune goat serum (NGS; Sigma) for 30 min, then probed with rabbit anti-RelA antibody (Santa Cruz Biotechnology) diluted 1 : 200 in 10% NGS in phosphate-buffered saline (PBS) for 30 min, followed by tetramethyl rhodamine isothiocyanate (TRITC)-conjugated goat anti-rabbit immunoglobulin G antibody (Jackson ImmunoResearch, West Grove, PA) diluted 1 : 100 in 10% NGS in PBS for 30 min. Slides were mounted in 50% glycerol in deionized water before microscopic analysis. RelA nuclear staining was imaged using an Olympus IMT2 Microscope (Olympus, Melville, NY) fitted with a rhodamine-specific filter. Images were captured using a digital Optronics DEI 750 camera (Optronics, Goleta, CA) and processed with scion image software (Scion Corporation, Frederick, MD). Identical exposure times were used for each data-point within an individual experiment. Original magnification set at 400×.
NF-κB-luciferase reporter assay
Proximal caput cells were infected for 16 hr with an adenoviral vector encoding an NF-κB-luciferase reporter gene (Ad5NF-κB-LUC) as previously described.23 Cell extracts were prepared using luciferase cell lysis buffer (PharMingen, San Diego, CA). Luciferase assays were performed using an Lmax luminometer microplate reader (Molecular Devices, Sunnyvale, CA), and results were normalized for protein extract concentrations measured with the Bio-Rad protein assay kit.
Preparation of bacterial lysate
Briefly, E. coli (UNC mouse strain) or N. gonorrhoeae (serovar B, FA 1090) were cultured in liquid Luria–Bertani and Thayer–Martin media respectively for 18–24 hr until the medium was cloudy. Cultures were pelleted by centrifugation. After centrifugation, the pellet was washed twice with sterile 1× PBS. The pellet was then resuspended in 1–2 ml 1× sterile PBS and 250 μl MD solution (0·1 m MgCl2 and 100 μg/ml DNAse in sterile water). After addition of MD solution, 1 ml of this bacterial suspension was added to 1 ml of 0·1-mm glass beads and cells were disrupted at 2200 g in a Mini-Bead Beater (BioSpec Products, Bartlesville, OK) for 3 min and then iced. The glass beads and unlysed cells were removed by centrifuging at 5000 g for 5 min. The lysates were filter sterilized by a 0·2 μg syringe filter. The protein concentration was measured with the Bio-Rad protein assay kit.
Results
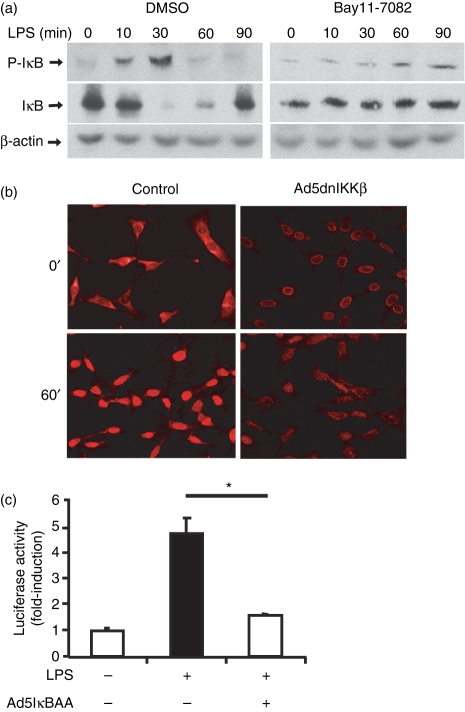
The simian virus 40 (SV40) immortalized epididymal epithelial cell line PC1, derived from the proximal caput region of the epididymis was utilized to investigate the expression pattern of the innate receptors CD14, MD-2, TLR4 and Nod2. As seen in Fig. 1, PC1 cells constitutively expressed CD-14, MD-2 and TLR4 messenger RNA (mRNA), whereas Nod2 was minimally expressed at steady-state conditions. Because the NF-κB transcriptional system is a well-characterized downstream effect target of TLR4 signalling, we next investigated the functional impact of TLR4/MD2 expression in PC1 cells by studying LPS-induced NF-κB signalling.24 Epididymal cells were treated with LPS (0·5 μg/ml) for various times and IκB phosphorylation/degradation was analysed by Western blot using specific antibodies. As seen in Fig. 2(a), LPS induced IκBα phosphorylation (at 10 and 30 min) with concomitant protein degradation (left panel) in PC1 cells. The IκB-phosphorylation inhibitor Bay 11-7082 (25 μm) strongly reduced LPS-induced IκB-phosphorylation and degradation in PC1 cells (Fig. 2a right panel). The LPS-induced IκB degradation was followed by RelA nuclear translocation as assayed by immunofluorescence staining (Fig. 2b). Importantly, adenoviral delivery of a dominant negative IκBkinaseβ (Ad5dnIKKβ) abrogates LPS-induced RelA nuclear translocation in PC1 cells. To further demonstrate the functional impact of LPS-induced NF-κB signalling, PC1 cells were infected with an adenoviral vector encoding an NF-κB-luciferase reporter gene (Ad5NF-κB-LUC) alone or with Ad5IκBαAA to selectively block NF-κB activation. As shown in Fig. 2c, LPS increased NF-κB transcriptional activity by more than four-fold, which was significantly inhibited in Ad5IκBαAA-infected cells. These findings indicate that epididymal cells respond to LPS stimulation by inducing the NF-κB signalling pathway.
Figure 1.
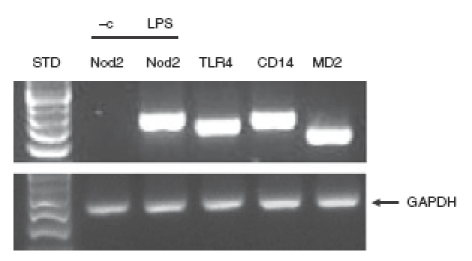
PC1 cells constitutively express Toll-like receptor 4 (TLR4), CD14 and MD-2. PC1 cells were lysed in trizol, and total RNA was extracted, reverse-transcribed (1 μg), and amplified by polymerase chain reaction using specific murine TLR4, CD14, MD-2 and Nod2 primers for constitutive expression. For induction of Nod2 mRNA expression PC1 cells were stimulated for 3 hr with 0·5 μg/ml lipopolysaccharide (LPS) or phosphate-buffered saline, vehicle control (– c). The housekeeping gene GAPDH was used to ascertain similar loading. Results are representative of three independent experiments.
Figure 2.
Lipopolysaccharide (LPS)-induced nuclear factor-κB (NF-κB) signalling in PC1 cells. (a) LPS-induced IκB degradation in PC1 cells. Cells were pretreated for 1 hr with dimethylsulphoxide (DMSO; left panel, vehicle control) or 25 μm Bay 11-7082 (right panel) and then stimulated with LPS (500 ng/ml) for the indicated times. Total protein was extracted and 20 μg was subjected to sodium dodecyl sulphate–polyacrylamide gel electrophoresis followed by immunoblotting with phospho-IκB-α (Ser 32), IκB-α, and β-actin specific antibodies. (b) LPS-induced RelA translocation in PC1 cells. Cells were infected with Ad5dnIKKβ [multiplicity of infection (MOI): 50] to selectively block NF-κB signalling or with Ad5 enhanced green fluorescent protein (EGFP) control virus (MOI: 50), for 12 hr. Cells were then stimulated with LPS (500 ng/ml) for 1 hr and RelA localization was visualized using anti-RelA primary antibody followed by TRITC-labelled detecting antibody. (c) LPS-induced NF-κB-transcriptional activity in PC1 cells. Cells were infected for 12 hr with the reporter Ad5NF-κB-LUC adenoviral vector (MOI: 10) and, where indicated, were coinfected with Ad5IκBAA (MOI: 50) for an additional 12 hr to block the κB-luciferase activity. Cells were then stimulated with LPS (500 ng/ml) and luciferase activity was measured after 16 hr using an Lmax microplate reader. Results were normalized for extract protein concentrations (*P < 0·05 unpaired Student's t-test).
Previous reports showed that Nod2 is induced in intestinal epithelial cells following TNF/interferon-γ stimulation.25 We next tested whether LPS induced Nod2 expression in PC1 cells, a process that could enhance the innate host response to microbial products. PC1 cells were stimulated with various amounts of LPS and Nod2 expression was determined by RT-PCR. Interestingly, Nod2 mRNA accumulation is induced with LPS concentration as low as 0·1 and peaked at 1 ng/ml (Fig. 3a). In addition, similar amounts of lipid A triggered Nod2 mRNA accumulation, showing that the LPS effect was not a result of cross-contamination of endotoxin in the preparation (Fig. 3a). LPS-induced Nod2 mRNA expression was time-dependent with a peak of expression between 2 and 3 hr (Fig. 3b). We next tested whether bacteria known to cause epididymitis (N. gonorrhoeae and E. coli) are able to trigger Nod2 expression. Interestingly bacterial lysates from both N. gonorrhoeae and E. coli induced Nod2 mRNA expression similar to that of LPS in epithelial cells (Fig. 4). These findings showed that epithelial cells induce Nod2 expression in response to microbial products.
Figure 3.
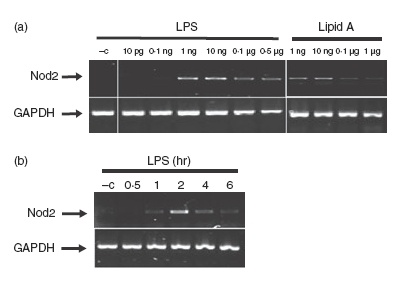
Lipopolysacchairde (LPS) time- and dose-dependently induced Nod2 mRNA expression in PC1 cells. (a) PC1 cells were stimulated with different concentrations of LPS and lipid A for 3 hr, or with a less than 0·5% working concentration in culture media of a chloroform : methanol : water (74 : 23 : 3) mixture, lipid A vehicle (–c). PC1 cells were also separately stimulated with LPS (500 ng/ml) for the indicated times, or with phosphate-buffered saline, LPS vehicle (–c). (b) Cells were lysed in trizol and total RNA was extracted, reverse-transcribed (1 μg), and amplified by polymerase chain reaction using specific murine Nod2 primers. The housekeeping gene GAPDH was utilized to ascertain similar loading. Results are representative of three independent experiments.
Figure 4.
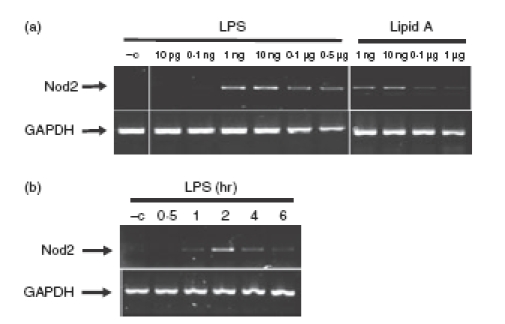
Lysates from pathogenic bacteria Neisseria gonorrhoeae and Escherichia coli induce Nod2 mRNA expression in PC1 cells. PC1 cells were stimulated for 3 hr with different concentrations of lipopolysaccharide (LPS) or bacterial lysates from Gram-negative bacteria, N. gonorrhoeae (serovar B, FA 1090) or E. coli (UNC mouse strain) or phosphate-buffered saline, LPS vehicle control (–c). Cells were lysed in Trizol, total RNA was extracted, reverse-transcribed (1 μg), and amplified by polymerase chain reaction using specific murine Nod2 primers. The housekeeping gene GAPDH was utilized to ascertain similar loading. Results are representative of three independent experiments.
The human Nod2 promoter region has been previously characterized and shown to possess three NF-κB binding sites.25 As opposed to the human promoter, little is known about the cis-acting elements present in the murine Nod2 gene promoter. Bioinformatic analysis using tess (transcription element search software) revealed two potential NF-κB binding sites in the murine Nod2 promoter region at positions –191 and –1307 relative to the first ATG before the exon start. To study the functional relevance of these sites, we performed ChIP assays on LPS-stimulated PC1 cells using RelA and RNA polymerase II antibodies. Interestingly, RelA was recruited to both binding sites with more pronounced recruitment to the proximal promoter region (Fig. 5). Additionally, RNApolII was recruited to the proximal but not distal site following LPS stimulation. These findings demonstrate an association between LPS-induced NF-κB recruitment to the Nod2 gene promoter and enhanced mRNA accumulation.
Figure 5.
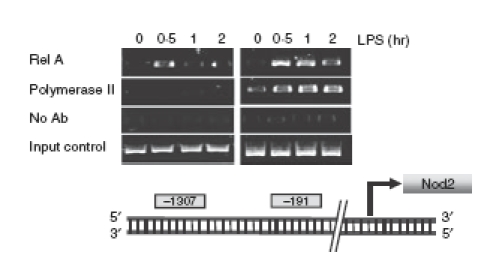
Lipopolysacchairde (LPS)-induced RelA and RNA Polymerase II recruitment to nuclear factor-κB (NF-κB) binding sites present in the Nod2 gene promoter. PC1 cells were stimulated with LPS (1 μg/ml) for the indicated times and chromatin immunoprecipitation assays were performed using anti-RelA or anti-RNA polymerase II antibodies as described in the Materials and methods. PCR was performed using primers specific for the NF-κB consensus sites within the Nod2 gene promoter (locations of the newly described NF-κB binding sites are shown). The input control shows the result of target sequences amplified from the total pool of whole cell DNA before immunoprecipitation. Data are representative of three independent experiments.
To investigate the functional role of NF-κB in LPS-induced Nod2 expression, we utilized the molecular inhibitor Ad5IκBAA (super-repressor). The LPS-induced Nod2 mRNA expression was blocked in Ad5IκBAA-infected epididymal epithelial cells (Fig. 6). We next tested the functional consequence of LPS-induced Nod2 expression in PC1 cells. Cells were stimulated with LPS for 16 hr and then infected with Ad5NF-κB-LUC for 8 hr followed by MDP stimulation. Interestingly, while MDP alone failed to induce NF-κB-dependent luciferase activity, pretreatment with LPS significantly enhanced MDP-induced transcriptional activity as a result of upregulation of Nod2 (Fig. 7). To validate this observation, we investigated the expression pattern of the NF-κB-dependent proinflammatory cytokine TNF. As seen in Fig. 8a, MDP-induced TNF mRNA accumulation is enhanced in cells pretreated with LPS. Altogether, these findings suggest that epididymal epithelial cells might utilize TLR4 and Nod2 signalling pathway to mount an innate response following bacterial infection.
Figure 6.
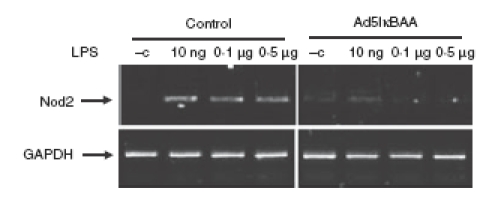
Lipopolysaccharide (LPS) -induced Nod2 mRNA accumulation is dependent on nuclear factor-κB (NF-κB) pathway. PC1 cells were infected with Ad5IκBAA (multiplicity of infection 50) where indicated for 12 hr to selectively block NF-κB signalling and cells were then stimulated with different concentrations of LPS for 3 hr. Cells were lysed in trizol, total RNA was extracted, reverse-transcribed (1 μg), and amplified by polymerase chain reaction using specific murine Nod2 primers. The housekeeping gene GAPDH was utilized to ascertain similar loading. Results are representative of three independent experiments.
Figure 7.
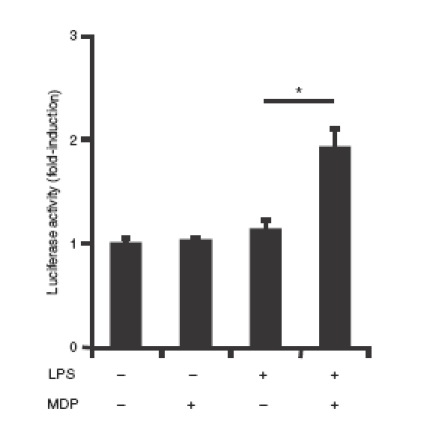
Enhanced nuclear factor-κB (NF-κB) signalling in lipopolysaccharide (LPS) pretreated PC1 cells. PC1 cells were infected for 12 hr with Ad5NF-κB-LUC adenoviral vector encoding a NF-κB-luciferase reporter (multiplicity of infection 10) for 12 hr. Cells were prestimulated with LPS (1 μg/ml) for 16 hr followed by stimulation with MDP (10 μg/ml) for another 16 hr. Cells were lysed and luciferase activity was measured using an Lmax microplate reader and results were normalized for extract protein concentrations (*P < 0·05 unpaired Student's t-test).
Figure 8.
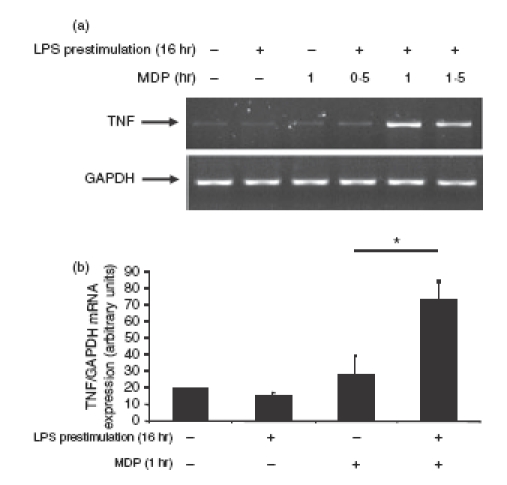
Enhanced Nod2 expression increased muramyl dipeptide (MDP)-induced tumour necrosis factor (TNF) mRNA accumulation. PC1 cells were prestimulated with LPS (1 μg/ml) for 16 hr after which cells were washed and fresh media containing MDP (10 μg/ml) was added for the indicated times. Cells were lysed in trizol, total RNA was extracted, reverse-transcribed (1 μg), and amplified by polymerase chain reaction (PCR) (a) or real-time PCR (b) using specific murine TNF primers. The housekeeping gene GAPDH was utilized to ascertain similar loading (a) or fold induction of TNF mRNA expression relative to fold induction of GAPDH mRNA (b). Results are representative of three independent experiments (*P < 0·05 unpaired Student's t-test).
Discussion
While various aspects of the innate immune response to bacterial invasion of the epithelial cells lining the lungs and digestive tract are fairly well characterized, the molecular response of epididymal epithelial cells to bacterial infections remains poorly characterized.26–28 In this study, we showed that epididymal epithelial cells possess a functional TLR4/NF-κB signalling machinery and rapidly detect bacterial products, which leads to the production of the proinflammatory cytokine TNF and the induction of the intracellular pattern recognition receptor Nod2. In addition, we showed that newly synthesized Nod2 renders epididymal epithelial cells responsive to MDP with subsequent induction of TNF mRNA. Therefore, experiments with the epididymal cell line suggest that LPS detection by TLR4 and upregulation of Nod2 may be part of an effective innate host response aimed at preserving the male reproductive tract against bacterial infection/colonization. The presence of innate immune machinery as demonstrated earlier lends support to the fact that the immune mechanisms observed in this study may also be operating in the organ system.26–28 However, further in vivo studies are needed to confirm the physiological impact of our findings.
Bacterial load differs significantly in different organ systems of the body, which probably shapes the nature of the immune response. For example, intestinal epithelial cells are in physical proximity to luminal microorganisms and must refrain from being constantly stimulated by the microbiota.17,29,30 A sophisticated regulatory system exists in these cells to prevent uncontrolled responses to ubiquitous luminal bacteria, including low TLR and Nod2 expression, differential apical and basolateral receptor distribution, and elevated expression of negative regulators such as Toll-interacting protein (TOLLIP) and single immunoglobulin and Toll-interleukin 1 receptor domain (SIGIRR).31–33 However, the bacterial load in the male reproductive tract is negligible and sudden bacterial exposure must be rapidly cleared because prolonged bacterial infection could have a deleterious impact on the host. Interestingly, numerous TLR proteins are detected in the male reproductive tract of mice, including TLR2 and TLR4.17,29,30 Similarly, the expression of LPS-binding protein, an acute-phase protein that is known to play a central role in defence against Gram-negative bacteria, was demonstrated in the human epididymis.34 Furthermore, CD14, a 54 000 molecular weight glycolipid-anchored membrane glycoprotein expressed on myeloid cells, which functions as a member of the LPS receptor complex, has been found in the seminal plasma and also on the sperm membranes, suggesting the presence of a potentially functional innate response machinery in the epididymis.35 Similarly, we showed that epididymal epithelial cells constitutively expressed TLR4, CD14 and MD-2 and further extended these observations by showing the functional impact of these proteins. Indeed, we demonstrated that LPS induced IκB-phosphorylation/degradation, RelA nuclear translocation, NF-κB transcriptional activity and TNF mRNA accumulation in epididymal epithelial cells. These findings clearly established the functionality of the TLR4/NF-κB signalling cascade in these cells.
The male reproductive tract is a relatively sterile environment and bacterial infection could impair fertility by interfering with the development of spermatozoa.1,36 Bacterial detection by epididymal epithelial cells through TLR/NF-κB signalling probably helps to maintain and preserve a functional reproductive tract. Another important finding reported here is the rapid and robust upregulation of the intracellular receptor Nod2 gene following LPS exposure. Nod2 is an important intracellular receptor sensing microbial components derived from bacterial peptidoglycan.11,12 Because lipid A, the structure responsible for the initiation of LPS-mediated cell activation, increased Nod2 expression this argues against the presence of an LPS contaminant.36–38 Interestingly, bacterial lysates derived from E. coli and N. gonorrhoeae, two bacteria commonly associated with epididymitis, also increased Nod2 mRNA expression in epididymal epithelial cells. Importantly, the synthetic Nod2 ligand MDP significantly increased NF-κB transcriptional activity leading to TNF mRNA accumulation in epididymal epithelial cells pretreated with LPS. This shows that Nod2 upregulation is biologically functional and renders epithelial cells responsive to MDP challenge. Therefore, the combined presence of TLR4 signalling machinery, which initially detects bacteria, and the upregulation of Nod2 might enhance the ability of epididymal epithelial cells to combat and eliminate invading bacteria. Nod2 expression has been shown to have a negative impact on intracellular bacterial survival in epithelial and monocytic cells.39–41 Although we observed a reduced survival rate of the invasive E. coli in Nod2-expressing PC1 cells over control cells, these differences did not reach statistical significance (data not shown). Nevertheless, because multiple bacteria species could potentially invade epididymal epithelial cells, a general bacterial sensor such as Nod2 might provide an effective surveillance mechanism.
Previous reports have demonstrated the key role of NF-κB in controlling Nod2 gene expression.8,12,25,42 However, a paucity of information exists on the molecular mechanism controlling murine Nod2 gene expression. We showed that NF-κB plays a critical role in controlling LPS-induced Nod2 gene expression. The ChIP assay showed that RelA binds to two NF-κB consensus sites (–1307 and –191) identified by bioinformatics. Additionally, RNApolII located in the proximal –191 site of Nod2 suggests a transcriptionally active gene. Moreover, molecular blockage using Ad5IκBAA totally prevented LPS-induced Nod2 gene expression. Altogether, these findings demonstrate that expression of the murine Nod2 gene is regulated by the transcription factor NF-κB in epididymal epithelial cells. Interestingly, three NF-κB-binding sites were identified in the human Nod2 gene and colonic epithelial cells were shown to have increased Nod2 expression following stimulation with TNF and IFN.25 However, Nod2 upregulation by cytokine stimulation requires a long stimulation period (> 12 hr) in IEC, whereas LPS rapidly triggers Nod2 mRNA accumulation (2 hr) in epididymal epithelial cells.25,43 The differential kinetics of Nod2 induction may reflect the particular microenvironment in which these cells evolved. IECs are constantly exposed to high concentration of luminal microorganisms whereas epididymal epithelial cells evolved in a virtually sterile environment.29,30 A swift and robust innate response of epididymal epithelial cells to bacterial colonization is necessary to preserve the biological integrity of the male reproductive tract. Thus it seems logical that natural selection would drive the evolution of such a potent protective immune response.
In summary we demonstrated the presence of a functional TLR4/NF-κB-signalling machinery in epididymal epithelial cells. Furthermore Nod2 upregulation following LPS stimulation renders epithelial cells responsive to MDP challenge and causes a significant increase of TNF expression. Epididymal epithelial cells likely utilize TLR4 and Nod2 signalling to prevent/limit damage to the male reproductive tract caused by bacterial infection.
Acknowledgments
We thank Brigitte Allard for excellent technical assistance, Christopher E. Thomas PhD (UNC Dept. of Med./Div. of Infect.) for providing us with N. gonorrhoeae cultures and the Microscopy Services Laboratory (UNC Dept. of Path. and Lab. Med.). This work was supported by NIH ROI DK 47700 to C. Jobin and by the German Research Foundation (DFG) MU2301/2-1 to Marcus Mühlbauer.
Abbreviations
- Ad
adenoviral
- ChIP
chromatin immunoprecipitation
- dn
dominant negative
- ECL
enhanced chemiluminescence
- EGFP
enhanced green fluorescent protein
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- IEC
intestinal epithelial cell
- IFN-γ
interferon-γ
- IKK
IκB kinase
- LPS
lipopolysaccharide
- MDP
muramyl dipeptide
- MOI
multiplicity of infection
- NF-κB
nuclear transcription factor-κB
- NGS
non-immune goat serum
- NOD
nucleotide-binding oligomerization domain
- PBS
phosphate-buffered saline
- PC1
proximal caput
- PCR
polymerase chain reaction
- RNApolII
RNA polymerase II
- TBS-T
tris-buffered saline Tween-20
- TESS
transcription element search software
- TLR
Toll-like receptor
- TNF
tumour necrosis factor
- TRITC
tetramethyl rhodamine isothiocyanate
References
- 1.Hall SH, Hamil KG, French FS. Host defense proteins of the male reproductive tract. J Androl. 2002;23:585–97. [PubMed] [Google Scholar]
- 2.Araki Y, Suzuki K, Matusik RJ, et al. Immortalized epididymal cell lines from transgenic mice overexpressing temperature-sensitive simian virus 40 large T-antigen gene. J Androl. 2002;23:854–69. [PubMed] [Google Scholar]
- 3.Chan PT, Schlegel PN. Inflammatory conditions of the male excurrent ductal system. Part II. J Androl. 2002;23:461–9. [PubMed] [Google Scholar]
- 4.Hagley M. Epididymo-orchitis and epididymitis: a review of causes and management of unusual forms. Int J STD AIDS. 2003;14:372–7. doi: 10.1258/095646203765371240. [DOI] [PubMed] [Google Scholar]
- 5.Horner PJ. European guideline for the management of epididymo-orchitis and syndromic management of acute scrotal swelling. Int J STD AIDS. 2001;12(Suppl. 3):88–93. doi: 10.1258/0956462011924010. [DOI] [PubMed] [Google Scholar]
- 6.Nickel JC, Teichman JM, Gregoire M, et al. Prevalence, diagnosis, characterization, and treatment of prostatitis, interstitial cystitis, and epididymitis in outpatient urological practice: the Canadian PIE Study. Urology. 2005;66:935–40. doi: 10.1016/j.urology.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Luzzi GA, O'Brien TS. Acute epididymitis. BJU Int. 2001;87:747–55. doi: 10.1046/j.1464-410x.2001.02216.x. [DOI] [PubMed] [Google Scholar]
- 8.Strober W, Murray PJ, Kitani A, et al. Signalling pathways and molecular interactions of NOD1 and NOD2. Nat Rev Immunol. 2006;6:9–20. doi: 10.1038/nri1747. [DOI] [PubMed] [Google Scholar]
- 9.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 11.Kufer TA, Banks DJ, Philpott DJ. Innate immune sensing of microbes by Nod proteins. Ann N Y Acad Sci. 2006;1072:19–27. doi: 10.1196/annals.1326.020. [DOI] [PubMed] [Google Scholar]
- 12.Windheim M, Lang C, Peggie M, et al. Molecular mechanisms involved in the regulation of cytokine production by muramyl dipeptide. Biochem J. 2007;404:179–90. doi: 10.1042/BJ20061704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25:6680–4. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 14.Abreu MT, Fukata M, Arditi M. TLR signaling in the gut in health and disease. J Immunol. 2005;174:4453–60. doi: 10.4049/jimmunol.174.8.4453. [DOI] [PubMed] [Google Scholar]
- 15.Whitsett JA. Intrinsic and innate defenses in the lung: intersection of pathways regulating lung morphogenesis, host defense, and repair. J Clin Invest. 2002;109:565–9. doi: 10.1172/JCI15209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quayle AJ. The innate and early immune response to pathogen challenge in the female genital tract and the pivotal role of epithelial cells. J Reprod Immunol. 2002;57:61–79. doi: 10.1016/s0165-0378(02)00019-0. [DOI] [PubMed] [Google Scholar]
- 17.Albiger B, Dahlberg S, Henriques-Normark B, et al. Role of the innate immune system in host defence against bacterial infections: focus on the Toll-like receptors. J Intern Med. 2007;261:511–28. doi: 10.1111/j.1365-2796.2007.01821.x. [DOI] [PubMed] [Google Scholar]
- 18.Chaudhuri N, Whyte MK, Sabroe I. Reducing the toll of inflammatory lung disease. Chest. 2007;131:1550–6. doi: 10.1378/chest.06-2869. [DOI] [PubMed] [Google Scholar]
- 19.Wira CR, Fahey JV, Sentman CL, et al. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–35. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 20.Karrasch T, Steinbrecher KA, Allard B, et al. Wound-induced p38MAPK-dependent histone H3 phosphorylation correlates with increased COX-2 expression in enterocytes. J Cell Physiol. 2006;207:809–15. doi: 10.1002/jcp.20626. [DOI] [PubMed] [Google Scholar]
- 21.Russo MP, Bennett BL, Manning AM, et al. Differential requirement for NF-kappaB-inducing kinase in the induction of NF-kappaB by IL-1beta, TNF-alpha, and Fas. Am J Physiol Cell Physiol. 2002;283:347–57. doi: 10.1152/ajpcell.00166.2001. [DOI] [PubMed] [Google Scholar]
- 22.Jobin C, Morteau O, Han DS, et al. Specific NF-kappaB blockade selectively inhibits tumour necrosis factor-alpha-induced COX-2 but not constitutive COX-1 gene expression in HT-29 cells. Immunology. 1998;95:537–43. doi: 10.1046/j.1365-2567.1998.00646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haller D, Russo MP, Sartor RB, et al. IKK beta and phosphatidylinositol 3-kinase/Akt participate in non-pathogenic Gram-negative enteric bacteria-induced RelA phosphorylation and NF-kappa B activation in both primary and intestinal epithelial cell lines. J Biol Chem. 2002;277:38168–78. doi: 10.1074/jbc.M205737200. [DOI] [PubMed] [Google Scholar]
- 24.Carmody RJ, Chen YH. Nuclear factor-kappaB: activation and regulation during toll-like receptor signaling. Cell Mol Immunol. 2007;4:31–41. [PubMed] [Google Scholar]
- 25.Rosenstiel P, Fantini M, Brautigam K, et al. TNF-alpha and IFN-gamma regulate the expression of the NOD2 (CARD15) gene in human intestinal epithelial cells. Gastroenterology. 2003;124:1001–9. doi: 10.1053/gast.2003.50157. [DOI] [PubMed] [Google Scholar]
- 26.Palladino MA, Johnson TA, Gupta R, et al. Members of the Toll-like receptor family of innate immunity pattern-recognition receptors are abundant in the male rat reproductive tract. Biol Reprod. 2007;76:958–64. doi: 10.1095/biolreprod.106.059410. [DOI] [PubMed] [Google Scholar]
- 27.Nishimura M, Naito S. Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet. 2005;20:452–77. doi: 10.2133/dmpk.20.452. [DOI] [PubMed] [Google Scholar]
- 28.Zhang D, Zhang G, Hayden MS, et al. A toll-like receptor that prevents infection by uropathogenic bacteria. Science. 2004;303:1522–6. doi: 10.1126/science.1094351. [DOI] [PubMed] [Google Scholar]
- 29.Dann SM, Eckmann L. Innate immune defenses in the intestinal tract. Curr Opin Gastroenterol. 2007;23:115–20. doi: 10.1097/MOG.0b013e32803cadf4. [DOI] [PubMed] [Google Scholar]
- 30.Heidemann J, Domschke W, Kucharzik T, et al. Intestinal microvascular endothelium and innate immunity in inflammatory bowel disease: a second line of defense? Infect Immun. 2006;74:5425–32. doi: 10.1128/IAI.00248-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Otte JM, Cario E, Podolsky DK. Mechanisms of cross hyporesponsiveness to Toll-like receptor bacterial ligands in intestinal epithelial cells. Gastroenterology. 2004;126:1054–70. doi: 10.1053/j.gastro.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 32.Melmed G, Thomas LS, Lee N, et al. Human intestinal epithelial cells are broadly unresponsive to Toll-like receptor 2-dependent bacterial ligands: implications for host–microbial interactions in the gut. J Immunol. 2003;170:1406–15. doi: 10.4049/jimmunol.170.3.1406. [DOI] [PubMed] [Google Scholar]
- 33.Wald D, Qin J, Zhao Z, et al. SIGIRR, a negative regulator of Toll-like receptor-interleukin 1 receptor signaling. Nat Immunol. 2003;4:920–7. doi: 10.1038/ni968. [DOI] [PubMed] [Google Scholar]
- 34.Malm J, Nordahl EA, Bjartell A, et al. Lipopolysaccharide-binding protein is produced in the epididymis and associated with spermatozoa and prostasomes. J Reprod Immunol. 2005;66:33–43. doi: 10.1016/j.jri.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 35.Harris CL, Vigar MA, Rey Nores JE, et al. The lipopolysaccharide co-receptor CD14 is present and functional in seminal plasma and expressed on spermatozoa. Immunology. 2001;104:317–23. doi: 10.1046/j.1365-2567.2001.01312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schletter J, Heine H, Ulmer AJ, et al. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995;164:383–9. doi: 10.1007/BF02529735. [DOI] [PubMed] [Google Scholar]
- 37.Lien E, Chow JC, Hawkins LD, et al. A novel synthetic acyclic lipid A-like agonist activates cells via the lipopolysaccharide/toll-like receptor 4 signaling pathway. J Biol Chem. 2001;276:1873–80. doi: 10.1074/jbc.M009040200. [DOI] [PubMed] [Google Scholar]
- 38.Poltorak A, Ricciardi-Castagnoli P, Citterio S, et al. Physical contact between lipopolysaccharide and toll-like receptor 4 revealed by genetic complementation. Proc Natl Acad Sci USA. 2000;97:2163–7. doi: 10.1073/pnas.040565397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–4. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 40.Hisamatsu T, Suzuki M, Reinecker HC, et al. CARD15/NOD2 functions as an antibacterial factor in human intestinal epithelial cells. Gastroenterology. 2003;124:993–1000. doi: 10.1053/gast.2003.50153. [DOI] [PubMed] [Google Scholar]
- 41.Voss E, Wehkamp J, Wehkamp K, et al. NOD2/CARD15 mediates induction of the antimicrobial peptide human beta-defensin-2. J Biol Chem. 2006;281:2005–11. doi: 10.1074/jbc.M511044200. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi Y, Isuzugawa K, Murase Y, et al. Up-regulation of NOD1 and NOD2 through TLR4 and TNF-alpha in LPS-treated murine macrophages. J Vet Med Sci. 2006;68:471–8. doi: 10.1292/jvms.68.471. [DOI] [PubMed] [Google Scholar]
- 43.Begue B, Dumant C, Bambou JC, et al. Microbial induction of CARD15 expression in intestinal epithelial cells via toll-like receptor 5 triggers an antibacterial response loop. J Cell Physiol. 2006;209:241–52. doi: 10.1002/jcp.20739. [DOI] [PubMed] [Google Scholar]