Abstract
The present study evaluated the extension of a new rat model of depression, repeated open-space swimming, which overcomes drawbacks of existing models, to mice. Mice were swum for 15 min daily in a large tank of tepid water for 4 days and thereafter at 4 day intervals for a period of 3 weeks. Some of the animals were provided with an active coping (escape) response. Variables measured included time floating, distance swum, immobility on a subsequent tail-suspension test, sucrose preference and brain cell proliferation (Ki67 immunohistochemistry) as well as responses to 2 antidepressant drugs, desmethylimipramine and fluoxetine, and 2 non-antidepressant drugs, haloperidol and diazepam. The repeated swims were found to increase time floating and tail-suspension immobility and to decrease distance swum, sucrose preference and brain cell proliferation. Both chronic antidepressant drugs as well as the active coping response attenuated the increased time floating while neither of the non-antidepressant drugs had this effect. The distance swum measure was found to be more variable. Chronic fluoxetine also reversed the increased tail-suspension immobility, reduced sucrose preference and reduced brain cell proliferation caused by the model. It is concluded that repeated open-space swim represents a useful new model of depression in the mouse.
Keywords: depression, forced swim, open space, antidepressant, tail-suspension test, sucrose preference, brain cell proliferation, mouse
INTRODUCTION
Numerous animal models of depression are currently available to investigators (Borsini and Meli, 1988; Cryan et al., 2005b). However, virtually all have significant drawbacks. The popular forced swim and tail suspension tests, while predictive of antidepressant activity, are unlike human depression in that they respond to acute administration of antidepressants (Cryan et al., 2005a). The commonly used learned helplessness and chronic mild stress models, also predictive of antidepressant activity, utilize severe stress or require a lengthy treatment time (4 weeks). Furthermore, the chronic mild stress model can be difficult to administer in a reliable fashion (Dalvi and Lucki, 1999).
It would be useful to have a mild and relatively rapid procedure that is simple to administer for reliably inducing prolonged depressive behavior. A variant of the forced swim model that has been developed by Sun and Alkon (2003; 2004) may have these characteristics. The latter authors utilized a modification of the Porsolt forced swim procedure in which rats are swum initially 3 times for 15 min/day on 3 consecutive days in a large tank of water and thereafter are given 15 min tests in the form of maintenance swims at weekly intervals for up to one month. This procedure produces a progressive reduction in distance swum and a corresponding increase in time floating that persists unchanged for at least one month. The open-space procedure has a number of advantages over the original forced swim method in that a) it permits a more objective measure of immobility from reduced distance swum; b) it permits tracking the development of inactivity over a series of swim sessions which allows time course studies of acquisition; c) the long-lasting reduction of active swimming is more amenable to analysis of the chronic effects of antidepressants, and, is sensitive to chronic but not acute administration of antidepressants (Sun and Alkon, 2003; 2004), and d) the method can readily detect the antidepressant effects of serotonin-selective reuptake inhibitors (Sun and Alkon, 2003) which had been problematic for the original Porsolt test (Lucki et al., 2001; Bourin et al., 1996; Borsini and Meli, 1988). The modified model may also have advantages over the chronic mild stress model in that it is more rapidly acting (1–2 weeks versus 4 weeks), is simpler to administer and is milder and shorter than most of the stressors involved in the chronic mild stress procedure.
Since mice are increasingly used as subjects in depression research and can be swum in smaller open-space tanks such as rat tub cages we attempted to replicate the open space model in this species. Because of their smaller bodies and greater susceptibility to hypothermia than rats, we utilized tepid water (31–33°C) for all swims which also reduced the physiological stressfulness of the procedure. We also chose to use an outbred strain, Swiss Webster, that is likely to yield results that are generalizable to a broad range of other mouse strains.
To produce chronic immobility, we adapted Sun and Alkon’s procedure of swimming the animals on 4 consecutive days the first week and thereafter at approximate 4 day intervals for the following 2 weeks. The repeated swims served both as behavioral tests and to maintain the chronic depressive state. In a previous study using this procedure we had found that mice, like rats, show a progressive decline in swimming activity over the first 3 swims and respond to antidepressant therapy given prior to the swims (Stone et al., 2007). In the present study we further evaluated the model by determining if it increased immobility in the tail suspension test, produced anhedonia (reduction in sucrose preference) and reduced brain cell proliferation rate, three additional signs of depression in rodents. We also measured its sensitivity to reversal after the initial swims by two effective antidepressants representing a norepinephrine-selective (desmethylimipramine) and serotonin-selective reuptake inhibitor (SSRI) (fluoxetine) and by the availability of a coping response (escape from the tank) as well as its specificity for antidepressants by testing whether two non-antidepressants (haloperidol and diazepam) were without effect. Since drugs have relatively short half lives in mice, to more closely mimic the human condition in which blood levels are maintained for prolonged periods, all agents were administered by osmotic minipump. This also avoided repeated injections of the animals which are stressful for mice. We now report that the repeated open-space swim method produces a number of positive signs of depression in the mouse and is likely to yield an improved model in this species.
METHODS
Subjects
Swiss Webster male mice, 8–10 weeks old, were subjects. Because the animals were to be implanted with minipumps, all mice were housed singly for the duration of the experiment (3 weeks). Single housing in male Swiss Webster mice is known not to induce behavioral depression in the forced swim test (Hilakivi et al., 1989; Brain, 1975). Furthermore pilot studies in our laboratory have failed to show an effect of intermittent social stimulation by female mice (3 h/week) on the response of isolated males to repeated swims. Standard mouse cages with nesting material were maintained at a room temperature of 22 ± 1° C under a 12 hr light/dark cycle (lights on 0500 hr). Food and water were available ad libitum. All experiments were conducted in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals (1996) and were approved by the New York University School of Medicine IUCAC.
Swim procedure
Swimming was carried out in rat tub cages (24 × 43 × 23 cm, w × h × l) filled with 13 cm high tepid tap water (31–33°C). Mice were handled on day 1 (Monday), swum individually for 15–20 min/day on 4 consecutive days (Tuesday-Friday, Days 2–5) and then at approximate 4 day intervals until 3 weeks had passed (Days 8, 11, 15, 19, 22). When drugs were administered the schedule was slightly altered so that the animals were implanted on Day 8 and then swum on Days 10, 15, 18, 22 (Post drug days 2, 7, 10, 14). All swims were videotaped from above. No special procedures were used to dry or warm the animals as they rapidly dried themselves with no observable episodes of shivering. Because of the large volumes of water used (13.4 L), the water was not changed until 4 mice had swum except to maintain the tepid temperature.
Drug studies
Osmotic minipumps delivering 6 μl/24 h for 14 d were used (Alzet, #1002). Desmethylimipramine (DMI) was dissolved in saline at a concentration designed to give a dose of 10–11.5 mg/kg/day in a 35–40 g mouse (66.6 mg/ml). Fluoxetine was dissolved at the same concentration in a vehicle of 50% dimethylsulfoxide (DMSO)/H2O to give the same dosage while halodoperidol was dissolved in 25% DMSO/H2O to yield a dose of 0.3–0.34 mg/kg/day (2 mg/ml) and diazepam in 100% DMSO to yield a dose of 1–1.1 mg/kg/day (6.66 mg/ml). Pilot experiments indicated that the drugs used did not produce more than a 5% change in body weight over the two week treatment period. The doses were chosen on the basis of previous experiments showing acute antidepressant effects of DMI and fluoxetine (Cryan et al., 2004), an acute anti-conditioned avoidance response effect of haloperidol (Arenas et al., 1999) and an acute anxiolytic effect of diazepam (Cole and Rodgers,1995) at the above doses.
Vehicle treated animals received pumps containing either saline, 25, 50% or 100% DMSO. Pumps were implanted subcutaneously and stitched closed between the scapulae in Nembutal anesthetized mice (60 mg/kg, i.p.) after the animals had been matched on time floating scores (fourth swim) into vehicle and drug groups.
Two separate drug experiments were run, one for the antidepressants and the other for the non-antidepressant drugs. The antidepressant experiment comprised three groups of 6 animals each and consisted of a DMI, fluoxetine and vehicle group (half with each of the vehicles for DMI and fluoxetine). The non-antidepressant experiment comprised three groups of 6 animals each for a haloperidol, diazepam and vehicle group (combined vehicles for haloperidol, diazepam).
Behavioral measures and procedures
Swimming behaviors
Videotapes were rated for Time floating (drifting with no observable movements of the limbs or tail) and Distance swum (number of tank quadrants entered) by an observer unaware of the animals’ treatments.
Tail suspension test
Animals were randomly assigned to four groups comprising a 1) non-swum-vehicle, 2) swum-vehicle, 3) non swum-fluoxetine, and 4) swum-fluoxetine. Animals from the swum groups were swum on days 2, 3, 4 and at approximate 4 day intervals up to the 18th day of the experiment. Pumps containing vehicle or fluoxetine at the above concentrations were implanted on Day 7. On Day 18 (24 h after the last swim) each animal was individually taped by the tail 72 cm above a platform for 6 min during which time it was videotaped. Tapes were subsequently rated for Time immobile during the last 4 min of the test by an observer unaware of the animals’ treatments.
Sucrose preference test
All animals were first familiarized with 1% sucrose solution in tap water placed on the cages for 72 h together with water bottles. The animals were then randomly assigned to one of four groups: non-swum vehicle, non-swum fluoxetine, swum vehicle, and swum-fluoxetine. The swum animals were subjected to forced swimming on days 2–4 and thereafter at approximate 4 day intervals. After the 3rd swim the animals were implanted with minipumps containing either vehicle or fluoxetine at the above concentrations. Nightly sucrose and water consumption was then measured on Days 4, 8, 11 and 15 of the procedure. Sucrose preference scores were calculated from the sucrose intakes divided by the combined sucrose plus water intakes.
Brain Cell proliferation
At 24 hr after the tail suspension tests, the four groups of animals used in the above experiment were terminally anesthetized with halothane plus urethane (2 g/kg, i.p.) and perfused intracardially with 25 ml normal saline followed by 45 ml of 4% paraformaldehyde. Brains were postfixed for 24 h and then submerged in 30% sucrose for 48 h prior to sectioning. Frozen brains were sectioned at 35 μ on a cryostat. Sections were then blocked with 4% normal goat serum and incubated with primary antibody (rabbit anti-Ki67, Vector Laboratories, 1:1000) overnight followed by application of a secondary biotinylated antibody (goat-anti-rabbit) and avidin-biotin-peroxidase complex. Color was developed with diaminobenzidine intensified by nickel.
Ki67 positive cells were counted in the wall of the lateral cerebral ventricle with a profile method using ImageJ (NIH) at two planes corresponding approximately to −1.82 and −2.06 mm Bregma. To minimize sampling bias the entire length of the wall of the lateral ventricle was outlined in these sections and counted for every positive cell. The two sections were chosen at random from a larger set of adequately stained sections at these planes.
Effect of an active coping response
Mice were randomly assigned to two groups, a passive swum and active-coping swum group. Both groups were swum 5, 10, 15, 15 and 15 min on Days 1,2,3,4 and 5 of the procedure with the difference being that the passive swum group was not given an active escape response but was simply picked up from the pool by the tail as in the standard procedure whereas the active escape group was presented with a platform (inverted flower pot) at the end of the swim onto which they readily learned to climb. On the last day all animals were videotaped and rated for distance swum and time floating over the 15 min swim as above.
Drugs used
Desmethylimipramine HCl, fluoxetine, haloperidol and diazepam were obtained from RBI.
Statistics
Distance swum and Time floating measures in the antidepressant and non-antidepressant studies were analyzed by two-way (Drug × Day) ANOVAs for repeated measures on the Day variable using Bonferroni-corrections for two mean comparisons. Sucrose preference data was analyzed by a 3-way (Swim × Drug × Day) ANOVA with repeated measures on the Day variable. Tail suspension and cell proliferation data were analyzed by two-way (Swim ×Drug) factorial ANOVAs.
RESULTS
Effects of Antidepressants
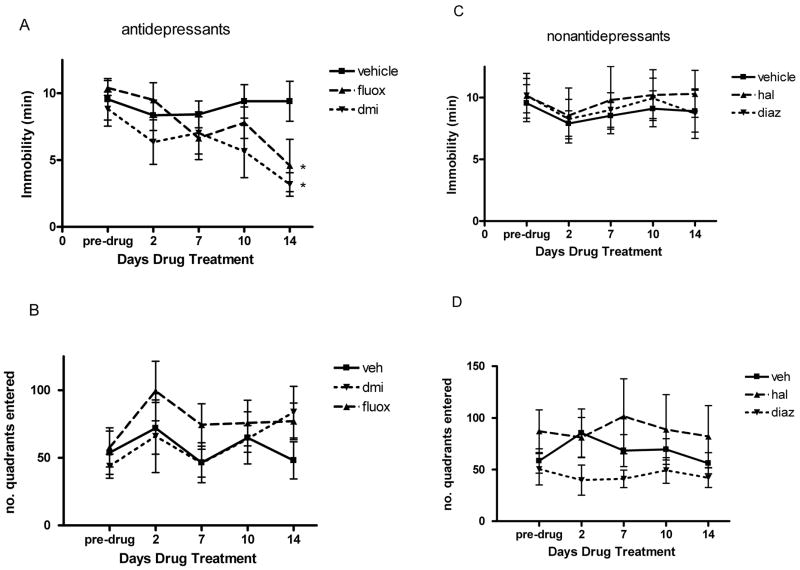
We replicated our earlier findings showing a progressive increase in Time floating and a corresponding decrease in Distance swum over the first 3–4 swims (data not shown). The effects of antidepressant drug treatment begun after week 1 are shown in Fig. 1A and B. Pre-drug values in this figure represent the scores on the fourth swim when the animals had become inactive. As there was no significant difference between the two types of vehicle used in this experiment, these groups have been combined into a single vehicle group. As can be seen from the figure both chronic antidepressants gradually reduced Time floating but had variable effects on Distance swum. For the Time floating data, there was no significant main effect of Drug (F2,15 = 2.50, p > 0.1) but there was a highly significant Drug x Day interaction (F6,45 = 4.41, p < 0.002). Bonferroni-corrected planned comparisons of the groups on the 2nd and 14th days of treatment showed that neither drug was effective on day 2 (DMI F1,15 = 0.08, NS; fluoxetine, F1,15 = 2.71, NS) but both significantly reduced floating on Day 14 (DMI, F1,15 = 16.55, p < 0.01; fluoxetine, F1,15 = 12.39, p < 0.02). The final reductions amounted to drops of 64.0% for DMI and 55.7% for fluoxetine. For Distance swum, there was no significant effect of Drug or significant Drug × Day interaction.
Fig. 1.
Effect of antidepressants (A,B) and non-antidepressants (C,D) on Time floating (immobility, A,C) and Distance swum (number of tank quadrants entered, B,D) in repeatedly swum mice. DMI (desmethylimipramine, 10 mg/kg); fluox (fluoxetine, 10 mg/kg); hal (haloperidol, 0.3 mg/kg); diaz (diazepam, 1 mg/kg). Mice had been swum 3 times prior to pre-drug test, implanted with drug-containing osmotic minipumps and then swum on post-drug implantation days as indicated. Means and SEMs are shown for N = 6 mice/group. * p < 0.02 versus vehicle group by ANOVA.
Effect of Non-antidepressants
The effects of haloperidol and diazepam on Time floating and Distance swum are shown in Fig. 1C,D. The same analyses applied to the non-antidepressants failed to show either a significant main effect of Drug (Time floating, F2, 15 = 0.26, NS; Distance swum, F2,15 = 1.48, NS), interaction of Drug × Day (Time floating, F6,45 = 0.64, NS; Distance swum, F6,45 = 0.79, NS) or significant difference between either of the drug groups and vehicle for Day 14 floating (Hal, F1,15 = 0.97, p >0.1: Diaz, F1,15 = 0.1, p >0.1) or distance scores (Hal, F1,15 = 0.90, p >0.1: Diaz, F1,15 = 0.26, p >0.1)..
Effect on tail-suspension test
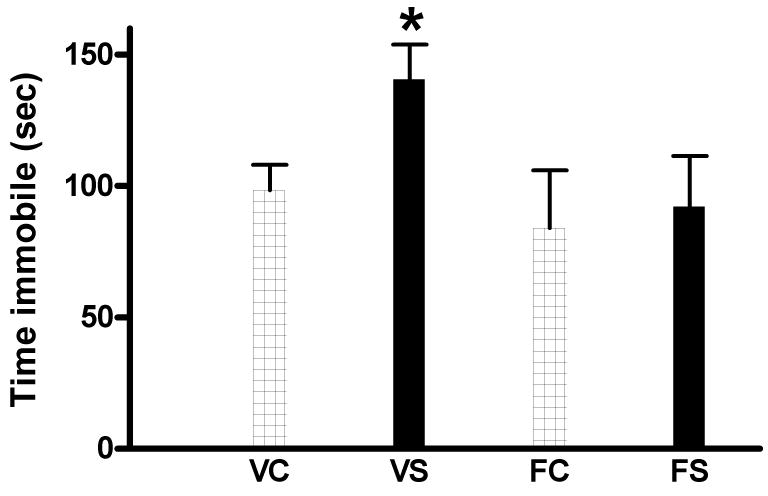
The effects of the swim procedure with and without fluoxetine treatment on immobility in the tail-suspension test are shown in Fig. 2. ANOVA revealed a significant effect of Drug (F1,24 = 5.57, p < 0.05) but no significant overall effect of the swim procedure or significant Swim × Drug interaction. However, individual planned comparisons revealed a significant increase in immobility in the swum versus non-swum animals in the vehicle condition (F1,24 = 3.53, p = 0.05) but no difference between these groups in the fluoxetine condition (F1,24 = 0.07, NS).
Fig. 2.
Effect of repeated swim procedure with or without chronic fluoxetine treatment on immobility in the tail suspension test. N= 5–6/group. VC, vehicle-control; VS, vehicle-swum; FC, fluoxetine-control; FS, fluoxetine-swum. * p < 0.05 versus VC.
Effect on sucrose preference
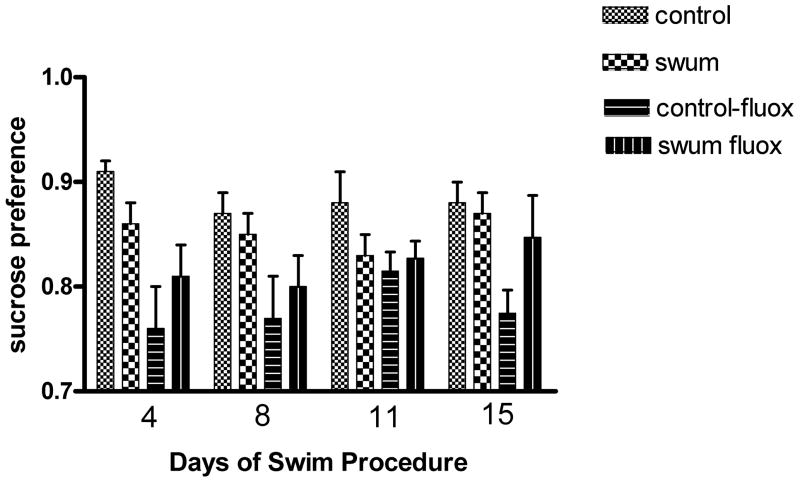
The effect of the swim procedure with and without fluoxetine treatment on sucrose preference between days 6–18 is shown in Fig. 3. A three-way (Swim x Fluoxetine x Day) ANOVA for repeated measures on the Day variable revealed a marked and significant reducing effect of fluoxetine (F1,16 = 23.02, p < 0.001) and a significant Swim × Fluoxetine interaction (F1,16 = 15.36, p < 0.002). There were no significant interactions of these variables with Day of experiment. A planned comparison revealed that the swim procedure in the vehicle-treated animals produced a significant reduction of overall sucrose preference (averaged across all days) (F118 = 4.66, p < 0.05) although the effect was small and the two groups were not significantly different on any individual day. In the swum groups, fluoxetine treatment reversed this overall reduction and produced a significant increase compared to vehicle (F1,18 = 5.37, p < 0.05)
Fig 3.
Effect of repeated swim and fluoxetine treatment on sucrose preference tests on days indicated. N=5–6/group. For statistics see text.
Effect on brain cell proliferation
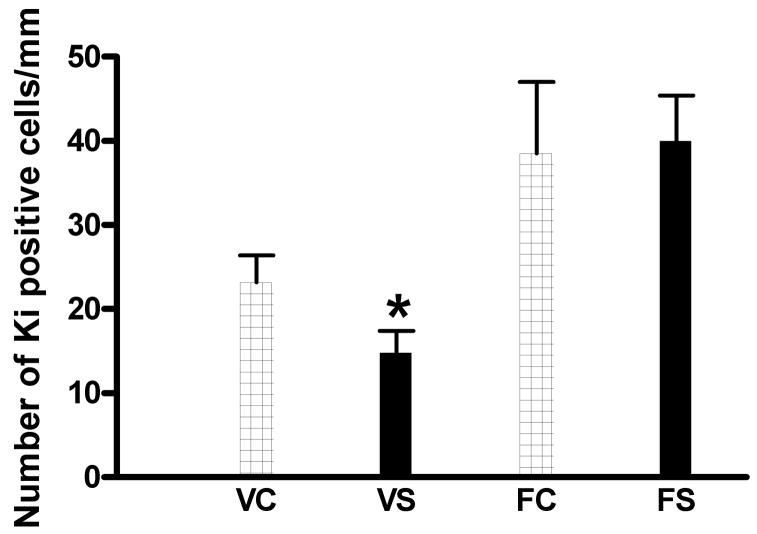
The effect of the swim procedure in the presence and absence of fluoxetine treatment on the number of Ki67 positive cells in the wall of the lateral cerebral ventricle is shown in Fig. 4. ANOVA revealed a significant main effect for fluoxetine (F1,20 = 20.36, p < 0.001) with no significant main effect of the swim procedure or significant swim × drug interaction. However, planned comparisons revealed a significant reduction in the swum versus non-swum animals of the vehicle (F1, 20 = 5.40, p < 0.05) but not fluoxetine condition.
Fig. 4.
Effect of repeated swim and fluoxetine treatment on number of Ki67 positive cells in the wall of the lateral ventricle at the level of the hippocampus. For abbreviations see legend to Fig. 2. N=5 mice/group. *p < 0.05 versus VC
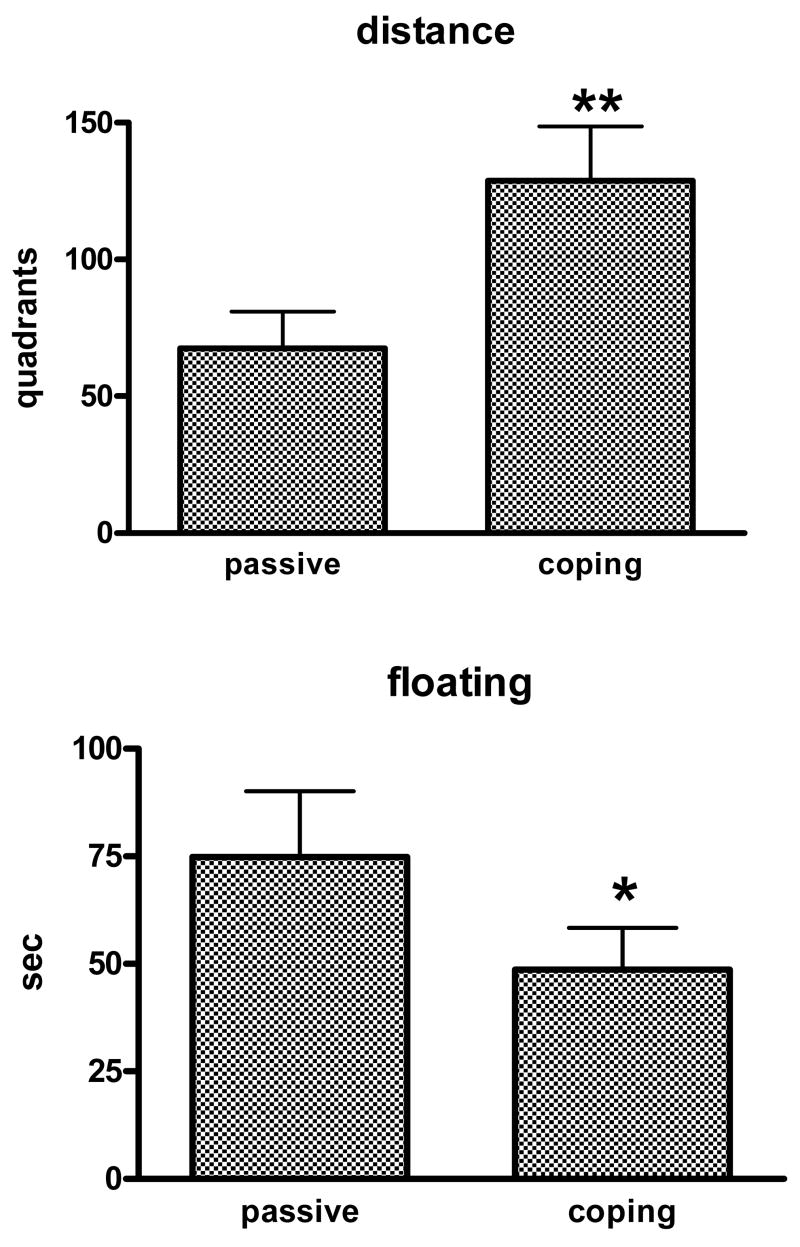
Effect of active coping response
The effect of an active escape coping response on the distance swum and time floating is shown in Fig. 5. The active-coping response significantly increased distance swum (t14 = 2.55, p < 0.05) and produced a borderline reduction in time floating (t14 = 1.82, p = 0.09) compared to the passive condition.
Fig 5.
Effect of an active escape coping response on the development of depressed behavior in repeatedly swum animals. N=8 mice/group. ** p < 0.05, * = 0.09 versus passive group.
DISCUSSION
The present results together with our previous findings extend Sun and Alkon’s findings in rats to mice. Like rats, mice show a progressive, marked diminution of active swimming and a corresponding increase in floating behavior with repeated open-space swims that persists for several weeks with occasionally repeated swims. Moreover, like rats, the increased inactivity was reversed selectively by antidepressant drugs in that DMI and fluoxetine, a tricyclic and an SSRI, inhibited the increased floating behavior whereas haloperidol and diazepam, an antipsychotic and an anxiolytic, failed to have this effect. Although only one dose was used for each of the non-antidepressants, numerous previous studies have shown that the doses employed have effective anti-anxiety (diazepam) and anti-conditioned avoidance response (haloperidol) effects in this species (cited above). Moreover the effects of the antidepressants were found when the drugs were administered after the onset of depressive behavior and with chronic and not acute administration. Sun and Alkon (2003; 2004) have shown in rats that the antidepressants imipramine (nonspecific tricylcic), mianserin (atypical), alaprocate (SSRI), and iproniazid (monoamine oxidase inhibitor) were effective with chronic administration in the open-space swim model whereas the anxiolytic, buspirone, was not.
Unlike rats, the measure that proved most effective in revealing the difference between antidepressants and non-antidepressants was floating behavior. Distance swum, although it was affected in the same antidepressant direction as floating, tended to be much more variable in this species. This is in agreement with the common usage of immobility rather than active swimming as the target behavior in the classical Porsolt forced swim test for both mice and rats (Porsolt et al., 1977).
Further evidence for a depressing effect of repeated open-space swimming came from the tail suspension and sucrose preference tests and the measure of brain cell proliferation. Mice subjected to 1–2 weeks of this procedure were found to show a higher level of immobility on the tail suspension test, a lower preference for sucrose solution, and a reduced expression of Ki67, a marker of brain cell proliferation. These signs are known to be characteristic of rodent models of depression. Moreover, each of these effects was reversed by chronic treatment with fluoxetine. Although it may be argued that the reduced preference for sucrose and reduced cell proliferation are the results of nonspecific stress (Rosenbrock et al., 2005), the present study and numerous previous studies have shown that they are reversed by treatment with antidepressant agents (Cryan et al., 2005a; Chen et al., 2006; Duman and Monteggia,2006; Bekris et al., 2005; Casorotto and Andreatini,2007; Grippo et al., 2007) and therefore are likely to be related to the depressive effects of the stressors. It should be noted that fluoxetine administration, while it reversed the swim-induced reduction, produced a marked and significant reduction in sucrose preference by itself. This effect has been noted by others and may be related to the initial anorexic effects of the drug (Sammut et al., 2002).
In addition to these similarities, the repeated open-space swim model also appears to possess construct validity in that it has been found to produce changes in central neural activity that are similar to those occurring in human depression and that have been postulated as underlying depressive behavior. Thus we have shown that the increased fos expression in a number of brain regions involved in approach and active coping behavior (secondary motor cortex, piriform cortex, posterior cingulate gyrus, nucleus accumbens) in response to a challenge swim is significantly attenuated in repeatedly swum animals. However the same response in a brain region involved in stress responses (paraventricular nucleus of hypothalamus) is relatively unchanged (Stone et al., 2007). This same neural pattern, which was shown to be prevented by prior antidepressant treatment, has been found in 4 other mouse models of depression involving either chronic subordination stress, intraventricular galanin injection, lipopolysaccharide or reserpine administration. A similar shift of neural activity away from brain regions involved in positively motivated, executive behavior toward regions associated with emotional stress has been observed in numerous neuroimaging studies of human depression and has been postulated to be the final common neural pathway for this disorder (Mayberg,2007; Drevets,2001; Stone et al., 2008).
An argument that has been proferred against the use of repeated swims as a model of depression asserts that the progressive inactivity of rodents in this test is simply a learned adaptive passive coping response which may not represent true depression in which coping responses are minimal or absent. However, it should be noted that passive responding, whether learned or unconditioned, is, in many situations, stressful to both humans and animals and is accompanied by activation of the paraventricular nucleus of the hypothalamus (Matsuda et al., 1996; Stone et al., 2007; Guzman et al., 1989) and an elevated rate of depression (Mercado et al., 2005). In support of this notion, the present study showed that if mice were permitted to learn an active coping (escape) response in the form of climbing on a presented platform at the end of each swim they developed much less depressive behavior in terms of both distance swum and time floating. Similar results have been found in the Morris water maze with rats during extinction of a previously learned spatial response (Schulz et al., 2007). As active coping responses are known to ameliorate depression (Wagner et al., 1977), it is reasonable to propose that the forced passivity as a result of repeated open-space swimming with no possibility of active escape may be sufficient to induce a state of depression in mice and rats.
Acknowledgments
Supported in part by NS048594.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arenas MC, Vinader-Caerols C, Monleon S, Parra A, Simon VM. Dose dependency of sex differences in the effects of repeated haloperidol administration in avoidance conditioning in mice. Pharmacol Biochem Behav. 1999;62:703–9. doi: 10.1016/s0091-3057(98)00207-x. [DOI] [PubMed] [Google Scholar]
- Bekris S, Antoniou K, Daskas S, Papadopoulou-Daifoti Z. Behavioural and neurochemical effects induced by chronic mild stress applied to two different rat strains. Behav Brain Res. 2005;161:45–59. doi: 10.1016/j.bbr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology. 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- Bourin M, Hascoet M, Colombel MC, Redrobe JP, Baker GB. Differential effects of clonidine, lithium and quinine in the forced swimming test in mice for antidepressants: possible roles of serotoninergic systems. Eur Neuropsychopharmacol. 1996;6:231–6. doi: 10.1016/0924-977x(96)00025-9. [DOI] [PubMed] [Google Scholar]
- Brain P. What does individual housing mean to a mouse? Life Sci. 1975;16:187–200. doi: 10.1016/0024-3205(75)90017-x. [DOI] [PubMed] [Google Scholar]
- Casorotto PC, Andreatini R. Repeated paroxetine treatment reverses anhedonia induced in rats by chronic mild stress or dexamethasone. Eur Neuropsychopharmacol. 2007 doi: 10.1016/j.euroneuro.2007.03.001. In press. [DOI] [PubMed] [Google Scholar]
- Chen H, Pandey GN, Dwivedi Y. Hippocampal cell proliferation regulation by repeated stress and antidepressants. NeuroReport. 2006;17:863–7. doi: 10.1097/01.wnr.0000221827.03222.70. [DOI] [PubMed] [Google Scholar]
- Cole JC, Rodgers RJ. Ethological comparison of the effects of diazepam and acute/chronic imipramine on the behaviour of mice in the elevated plus-maze. Pharmacol Biochem Behav. 1995;52:473–8. doi: 10.1016/0091-3057(95)00163-q. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Mombereau C, Vassout A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci Biobehav Revs. 2005a;29:571–625. doi: 10.1016/j.neubiorev.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Cryan JF, O’Leary OF, Jin SH, Friedland JC, Ouyang M, Hirsch BR, et al. Norepinephrine-deficient mice lack responses to antidepressant drugs, including selective serotonin reuptake inhibitors. Proc Natl Acad Sci U S A. 2004;101:8186–91. doi: 10.1073/pnas.0401080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Valentino RJ, Lucki I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci Biobehav Revs. 2005b;29:547–69. doi: 10.1016/j.neubiorev.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Dalvi A, Lucki I. Murine models of depression. Psychopharmacology. 1999;147:14–6. doi: 10.1007/s002130051131. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging and neuropathological studies of depression: implications for the cognitive-emotional features of mood disorders. Curr Opin Neurobiol. 2001;11:240–9. doi: 10.1016/s0959-4388(00)00203-8. [DOI] [PubMed] [Google Scholar]
- Duman RS, Monteggia LM. A neurotrophic model for stress-related mood disorders. Biol Psychiatry. 2006;59:1116–27. doi: 10.1016/j.biopsych.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Cushing BS, Carter CS. Depression-like behavior and stressor-induced neuroendocrine activation in female prairie voles exposed to chronic social isolation. Psychosom Med. 2007;69:149–57. doi: 10.1097/PSY.0b013e31802f054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman NA, Hernandez L, Hoebel BG. Capillary electrophoresis: a new era in microseparations. BioPharm. 1989 Jan;:22–37. [Google Scholar]
- Hilakivi LA, Ota M, Lister RG. Effect of isolation on brain monoamines and the bahavior of mice in tests of exploration, locomotion, anxiety and behavioural ‘despair’. Pharmacol Biochem Behav. 1989;33:371–4. doi: 10.1016/0091-3057(89)90516-9. [DOI] [PubMed] [Google Scholar]
- Lucki I, Dalvi A, Mayorga AJ. Sensitivity to the effects of pharmacologically selective antidepressants in different strains of mice. Psychopharmacology. 2001;155:315–22. doi: 10.1007/s002130100694. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Peng H, Yoshimura H, Wen TC, Fukuda T, Sakanaka M. Persistent c-fos expression in the brains of mice with chronic social stress. Neurosci Res. 1996;26:157–70. [PubMed] [Google Scholar]
- Mayberg HS. Defining the neural circuitry of depression: toward a new nosology with therapeutic implications. Biol Psychiatry. 2007;61:729–30. doi: 10.1016/j.biopsych.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Mercado AC, Carroll LJ, Cassidy JD, Côté P. Passive coping is a risk factor for disabling neck or low back pain. Pain. 2005;117:51–7. doi: 10.1016/j.pain.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Bertin A, Jalfre M. Behavioural despair in mice: a primary screening test for antidepressants. Arch int Pharmacodyn Ther. 1977;229:327–36. [PubMed] [Google Scholar]
- Rosenbrock H, Koros E, Bloching A, Podhorna J, Borsini F. Effect of chronic intermittent restraint stress on hippocampal expression of marker proteins for synaptic plasticity and progenitor cell proliferation in rats. Brain Res. 2005;1040:55–63. doi: 10.1016/j.brainres.2005.01.065. [DOI] [PubMed] [Google Scholar]
- Sammut S, Bethus I, Goodall G, Muscat R. Antidepressant reversal of interferon-α-induced anhedonia. Physiol Behav. 2002;75:765–72. doi: 10.1016/s0031-9384(02)00677-7. [DOI] [PubMed] [Google Scholar]
- Schulz D, Buddenberg T, Huston JP. Extinction-induced “despair” in the water maze, exploratory behavior and fear: effects of chronic antidepressant treatment. Neurobiol Learn Mem. 2007;87:624–34. doi: 10.1016/j.nlm.2006.12.001. [DOI] [PubMed] [Google Scholar]
- Stone E, Lehmann M, Lin Y, Quartermain D. Reduced evoked fos expression in activity-related brain regions in animal models of depression. Prog Neuropsychopharmacol Biol Psychiat. 2007;31:1196–207. doi: 10.1016/j.pnpbp.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Stone E, Lin Y, Quartermain D. A final common pathway for depression? Progress toward a general conceptual framework. Neurosci Biobehav Revs. 2008;32:508–24. doi: 10.1016/j.neubiorev.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun MK, Alkon DL. Open space swimming test to index antidepressant activity. J Neurosci Methods. 2003;126:35–40. doi: 10.1016/s0165-0270(03)00068-2. [DOI] [PubMed] [Google Scholar]
- Sun MK, Alkon DL. Induced depressive behavior impairs learning and memory in rats. Neuroscience. 2004;129:129–39. doi: 10.1016/j.neuroscience.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Wagner HR, Hall TL, Cote IL. The applicability of inescapable shock as a source of animal depression. J Gen Psychol. 1977;96(2d Half):313–8. doi: 10.1080/00221309.1977.9920828. [DOI] [PubMed] [Google Scholar]