Abstract
myr 5 is an unconventional myosin (class IX) from rat that contains a Rho-family GTPase-activating protein (GAP) domain. Herein we addressed the specificity of the myr 5 GAP activity, the molecular mechanism by which GAPs activate GTP hydrolysis, the consequences of myr 5 overexpression in living cells, and its subcellular localization. The myr 5 GAP activity exhibits a high specificity for Rho. To achieve similar rates of GTPase activation for RhoA, Cdc42Hs, and Rac1, a 100-fold or 1000-fold higher concentration of recombinant myr 5 GAP domain was needed for Cdc42Hs or Rac1, respectively, as compared with RhoA. Cell lysates from Sf9 insect cells infected with recombinant baculovirus encoding myr 5 exhibited increased GAP activity for RhoA but not for Cdc42Hs or Rac1. Analysis of Rho-family GAP domain sequences for conserved arginine residues that might contribute to accelerate GTP hydrolysis revealed a single conserved arginine residue. Mutation of the corresponding arginine residue in the myr 5 GAP domain to a methionine (M1695) virtually abolished Rho-GAP activity. Expression of myr 5 in Sf9 insect cells induced the formation of numerous long thin processes containing occasional varicosities. Such morphological changes were dependent on the myr 5 Rho-GAP activity, because they were induced by expressing the myr 5 tail or just the myr 5 Rho-GAP domain but not by expressing the myr 5 myosin domain. Expression of myr 5 in mammalian normal rat kidney (NRK) or HtTA-1 HeLa cells induced a loss of actin stress fibers and focal contacts with concomitant morphological changes and rounding up of the cells. Similar morphological changes were observed in HtTA-1 HeLa cells expressing just the myr 5 Rho-GAP domain but not in cells expressing myr 5 M1695. These morphological changes induced by myr 5 were inhibited by coexpression of RhoV14, which is defective in GTP hydrolysis, but not by RhoI117. myr 5 was localized in dynamic regions of the cell periphery, in the perinuclear region in the Golgi area, along stress fibers, and in the cytosol. These results demonstrate that myr 5 has in vitro and in vivo Rho-GAP activity. No evidence for a Rho effector function of the myr 5 myosin domain was obtained.
INTRODUCTION
Myosins are molecular motors capable of converting chemical energy stored in ATP into directed mechanical force along actin filaments. In recent years, a multitude of novel myosins have been discovered (for review, see Mooseker and Cheney, 1995). All these myosins share a more or less conserved motor domain. This motor domain consists of a head domain that contains actin- and ATP-binding sites and a neck domain that contains one or more binding sites for associated light chains. The myosin light chains are members of the calmodulin/EF-hand protein family. On the basis of sequence comparisons of their homologous head domains, myosins have been grouped into different classes (Cheney et al., 1993; Goodson and Spudich, 1993). In addition to a motor domain, each class of myosin also exhibits a characteristic tail domain that differs between individual classes. Although the head domains have class-specific features, it is thought that the tail domains specify function by binding to cellular components. In support of this hypothesis, several myosin tail domains contain binding motifs found in other proteins, such as α-helical coiled-coil forming motifs, SH3 motifs, AF-6/canoe homology motifs, zinc-binding motifs, and motifs with homology to Rho-GTPase activating proteins (Bähler, 1996). However, currently little is known about myosin binding partners and the functional significance of fusing a myosin motor domain to the mentioned binding motifs.
The recently discovered rat myosin myr 5 and its human homologue myosin IXb contain several intriguing sequence features (Reinhard et al., 1995; Wirth et al., 1996). In their head domains, they have an extension at the N terminus and a 15-kDa insertion at a putative actin binding site. Nevertheless, myr 5 can bind actin filaments in an ATP-regulated manner as is characteristic for other myosin molecules (Reinhard et al., 1995). The neck domains encode four light chain binding sites. The tail domains contain two sequence motifs previously identified in other proteins, namely, a zinc-binding motif and a GAP motif for members of the Rho-subfamily of small G-proteins. The zinc-binding motif consists of a Cys6His2 motif that binds two zinc ions. A similar motif is also found in protein kinase C isoforms, the raf kinase, the rho nucleotide exchange factor lfc, the rac-GAP chimaerin, and other proteins (Reinhard et al., 1995; Whitehead et al., 1995). C-terminal to the Cys6His2 motif, as in the proteins chimaerin (Hall et al., 1990; Ahmed et al., 1994) and rotund (Agnel et al., 1992), myr 5 and myosin IXb contain a region with homology to GAPs for the Rho subfamily of small GTPases. Small GTPases act as molecular switches cycling between GTP-bound active and GDP-bound inactive conformations (Bourne et al., 1991). The cycling between the two conformations is regulated by accessory proteins. GAPs act as negative regulators facilitating inactivation of small GTPases by stimulating GTP hydrolysis. It has been proposed that Ras-GAPs might accelerate the Ras-GTPase reaction by the supply of residues into the active site of Ras where they participate in catalysis, possibly by stabilizing the transition state (Mittal et al., 1996; Scheffzek et al., 1996). Such residues could be arginines that in other proteins have been shown to have such a function (Coleman et al., 1994; Sondek et al., 1994). Several GAPs specific for the Rho subfamily have been identified (Lamarche and Hall, 1994). These GAPs exhibit in vitro a preference for one or more members of the Rho subfamily, but currently it is not possible to predict their substrate specificity based on amino acid sequence comparison. The Rho subfamily of small GTPases includes Rho, Rac, and Cdc42. They have been demonstrated to play important roles in growth-factor–regulated actin organization and cell adhesion. In fibroblasts active Rho induces stress fiber and focal contact formation (Ridley and Hall, 1992); active Rac induces lamellipodia, membrane ruffle, and focal complex formation (Ridley et al., 1992; Nobes and Hall, 1995); and active Cdc42 induces filopodia and focal complex formation (Kozma et al., 1995; Nobes and Hall, 1995). The molecular pathways leading from the active GTPase to the changes in actin organization are only beginning to be elucidated. In this context it is of interest that in myr 5 a GAP region is connected to an actin-binding myosin domain. However, it is not known whether and how the two domains are coupled functionally. To begin to elucidate myr 5 function(s), we determined the specificity of myr 5 GAP activity both in vitro and in vivo and analyzed whether myr 5 serves an effector function in GTPase-dependent actin reorganization and cell adhesion. We found that both the isolated GAP region (expressed in Escherichia coli) and intact myr 5 (expressed in Sf9 insect cells) in vitro efficiently stimulate the GTPase activity of Rho A but not Cdc42 and Rac1. Overexpression of myr 5 in Sf9 insect cells leads to the formation of cell protrusions that correlate with the expression of Rho-GAP activity. Overexpression of myr 5 in NRK and HeLa cells causes a loss of actin stress fibers and focal contacts, leading to changes in cell morphology. These effects of myr 5 overexpression are due to inactivation of Rho. No evidence for an effector function of myr 5 in Rho signaling was observed. Mutation of a single arginine residue in myr 5 that is conserved in all GAPs for the Rho subfamily to a methionine virtually abolished its in vitro and in vivo Rho-GAP activity, demonstrating the importance of this charged residue.
MATERIALS AND METHODS
Plasmids for the Baculovirus Expression System
The cDNA encoding full-length myr 5 was assembled from overlapping clones (Reinhard et al., 1995) in pBluescript SK (Stratagene, La Jolla, CA) using suitable restriction sites, yielding pBSKM5. To obtain recombinant baculovirus coding for myr 5, myr 5 cDNA was excised from pBSKM5 by NotI/EcoRV digestion and cloned into the NotI–SmaI sites of the expression vector pVL1392 (PharMingen, San Diego, CA). The myr 5 head expression vector coding for amino acids 1–960 was constructed by cloning a NotI–BamHI fragment of pBSKM5 into the corresponding sites in pVL1392. Because this fragment does not contain a stop codon, 24 additional amino acids encoded by the vector will be added to the C terminus of the myr 5 head. To construct the expression vector coding for amino acids 960-1980 of the myr 5 molecule (myr 5 tail) with a C-terminal influenza hemagglutinin epitope-tag (HA-tag, amino acids YPYDVPDYA), several cloning steps were required. Nucleotides encoding Ser-Leu-HA-tag, stop codon, and SpeI and EcoRV restriction sites were added to the very 3′ region of the myr 5 coding sequence by polymerase chain reaction (PCR). This fragment was subcloned into pUC18 (Sure Clone, Pharmacia, Freiburg, Germany) and excised with BsmI and EcoRV. It was ligated into a myr 5 tail clone in pBSK (starting at nucleotide 3024), which had been linearized with BsmI and EcoRV resulting in plasmid myr 5 tail-HA. A 5′ fragment encompassing a BamHI site and a consensus initiation site followed by nucleotides 3026–3287 of myr 5 was obtained by PCR, digested with BamHI and MluNI, and inserted into the BamHI/MluNI-linearized plasmid myr 5 tail-HA. This, at the 5′ and 3′ regions, modified myr 5 tail fragment was excised with BamHI and EcoRV and cloned into the baculovirus expression vector pVL1393 by using the BamHI site and the EcoRI site, which was made blunt with the Klenow fragment of DNA polymerase I. A myr 5 GAP domain (amino acids 1664–1857) expression vector was constructed by PCR. The 5′ primer introduced a BamHI site followed by a consensus initiation site. The 3′ primer contained two stop codons and an EcoRI site. The resulting fragment was ligated into BamHI/EcoRI-linearized pVL1393 vector. All fragments amplified by PCR were verified by DNA sequencing.
Expression of Recombinant Proteins in the Baculovirus Expression System
Sf9 insect cells were cultured in complete Grace’s insect medium supplemented with lactalbumin hydrolysate, yeastolate (Life Technologies, Gaithersburg, MD), and 10% fetal bovine serum. Recombinant virus was obtained as described by the manufacturer by cotransfecting Sf9 cells with BaculoGold virus DNA and with the gene of interest inserted in the expression vectors pVL1392 or pVL1393 (PharMingen). Recombinant virus was purified by two rounds of endpoint dilution using digoxigenin-labeled probes (Boehringer Mannheim, Mannheim, Germany) for detection. To obtain a high titer, the virus was amplified by two subsequent infections of Sf9 cells with a low multiplicity of infection as described by O‘Reilly et al. (1992).
Plasmid Constructs for Transfection of Mammalian Cells
The cDNA of myr 5 tagged C-terminally with a HA epitope was cloned into the expression vectors pUHD10–3 (Gossen and Bujard, 1992) and pCMV5. myr 5 with a C-terminal HA-tag in pBSK was constructed by replacement of the BamHI–EcoRV tail fragment in pBSKM5 with the corresponding tail fragment from the plasmid myr 5 tail-HA. myr 5-HA was then subcloned in pUHD10–3 by first inserting the EcoRI–SpeI myr 5 3′ fragment into the EcoRI–XbaI sites of pUHD10–3 followed by addition of the myr 5 5′ fragment. To isolate the myr 5 5′ fragment, the myr 5-HA plasmid was first digested with SpeI, blunted with Klenow polymerase, and recut with EcoRI. This fragment was ligated to the blunted SacII and to the EcoRI sites in the pUHD10–3 plasmid containing the myr 5 3′ fragment.
The myr 5-HA sequence was inserted into the pCMV vector in two subsequent steps. The HindIII–EcoRV fragment of myr 5-HA in pBSK was first inserted into the HindIII–SmaI sites of pCMV5. This construct was cut with EcoRI, treated with Klenow polymerase, recut with HindIII, and ligated to the missing myr 5 fragment cut out of myr 5-HA in pBSK with SpeI (made blunt) and HindIII.
The myr 5 GAP domain was subcloned into the pUHD10–3 vector as follows: a cDNA encompassing the myr 5 GAP domain (amino acids 1664–1857) fused to a N-terminal HA-tag was constructed by PCR. The 5′ primer contained an EcoRI site followed by a consensus initiation site, an HA epitope encoding sequence, and nucleotides 5135–5149 of myr 5. The 3′ primer contained nucleotides 5699–5716 of myr 5 cDNA, two stop codons, and XbaI and BamHI sites. The resulting PCR fragment was cloned into the EcoRI and BamHI restriction sites of pBSK+. After sequence verification, it was subcloned into the pUHD10–3 vector via EcoRI–XbaI restriction sites. The arginine in the GAP domain at position 1695 was mutated to a methionine by using the Quick Change mutagenesis kit (Stratagene).
Myc-rhoV14 was generated by PCR. V14 rhoA in pGEX-2T (a kind gift from A. Hall, MRC Laboratory for Molecular Cell Biology, London) was used as a template. The 5′ primer contained an EcoRI site, a consensus initiation sequence, and the sequence encoding the myc epitope tag. The 3′ primer contained a BamHI restriction site. The PCR fragment was cloned in pBSK+ and, after verification of the sequence, was subcloned in pUHD10–3. The mutation N117I was introduced by PCR using rhoA in pGEX-2T as a template. The primers used for amplification and subcloning of myc-rhoV14 were also used for cloning myc-rhoN117I in the pUHD10–3 vector.
Cell Culture and Transient Transfection of HtTA-1 HeLa and NRK Cells
HtTA-1 cells, a HeLa cell clone expressing the tetracycline-repressible transactivating element (Gossen and Bujard, 1992), were a kind gift from H. Bujard (University of Heidelberg, Heidelberg, Germany). They were cultured in DMEM supplemented with 10% fetal calf serum, 2 mM l-glutamine, 0.1 mg/ml streptomycin, 100 U/ml penicillin, and 270 μg/ml active G418 sulfate (Geneticin, Life Technologies). NRK cells were grown under identical conditions except that no G418 was added. Cells were transfected by using the calcium phosphate method (Ausubel et al., 1990). To increase expression levels in NRK cells, 10 mM sodium butyrate was sometimes added 16–18 h before fixation.
Indirect Immunofluorescence
For indirect immunofluorescence, cells were cultured on 12-mm glass coverslips in 24-well tissue culture plates (Greiner, Nürtingen, Germany). Cells were fixed in 4% paraformaldehyde in PBS for 30 min. After quenching in 0.1 M glycine in PBS, cells were permeabilized for 20 min in 0.1% saponin, 5% normal goat serum, in PBS. Primary and secondary antibodies were appropriately diluted in 5% normal goat serum in PBS. The following primary antibodies were used: antibodies Tü 52, Tü 55, and Tü 66 directed against different domains of myr 5 (Reinhard et al., 1995); 9E10.2 anti-myc monoclonal antibody (Evan et al., 1985); rabbit anti-HA polyclonal antibody HA.11 (Berkeley Antibody, Richmond, CA); monoclonal anti-vinculin hVin-1 (Sigma, St. Louis, MO); and anti-phosphotyrosine monoclonal antibody PT-66 (Sigma). Secondary goat anti-rabbit and goat anti-mouse antibodies coupled to 7-amino-4methyl-coumarin-acetate (AMCA), fluorescein isothiocyanate (FITC), lissamine-rhodamine, or Texas Red were purchased from Dianova (Hamburg, Germany). Fluorescein-coupled phalloidin was from Molecular Probes (Eugene, OR). Unbound antibody was removed by rinsing the cells several times with PBS. Finally, the coverslips were inverted and mounted with 15% Mowiol, 30% glycerol, and 2.5% 1,4-diazabicyclo(2.2.2)octane (Sigma) in PBS (pH 9) on microscope slides. Cytosol was removed from cells prior to fixation by permeabilization of the plasma membrane with 1 μg/ml streptolysine-O (gift from Dr. S. Bhakdi, University of Mainz, Mainz, Germany).
Determination of GAP Activity
The myr 5 GAP domain (amino acids 1663–1857) was expressed as a glutathione S-transferase (GST) fusion protein in E. coli and, after purification, was cleaved from the GST moiety. Assays with purified GAP domain were carried out essentially as described previously (Reinhard et al., 1995). Briefly, 0.1–0.5 μg of purified GTPases (Ridley et al., 1992) was preloaded with radioactive GTP by incubation with [γ-32P]GTP (6000 Ci/mmol, Du Pont NEN, Bad Homburg, Germany), 1 μl of bovine serum albumin (BSA; 13 mg/ml), 2 μl EDTA (50 mM), and 12.2 μl of binding buffer [20 mM Tris-HCl, pH 7.5, 25 mM NaCl, 0.1 mM dithiothreitol (DTT)] for 10 min at 30°C. The loading reaction was stopped by addition of 5 μl of 100 mM MgCl2. To purified myr 5 GAP in a final volume of 24 μl of incubation buffer (20 mM Tris-HCl, 0.1 mM DTT), 1 μl of unlabeled GTP (30 mM) and 2 μl of BSA (13 mg/ml) were added before the GAP reaction was started by addition of 3 μl of preloaded GTPase. After 0, 5, and 10 min at 30°C, aliquots of 5 μl were removed and pipetted to 1 ml of stop buffer (50 mM Tris-HCl, pH 7.5, 50 mM NaCl, 5 mM MgCl2). The samples were passed immediately over a 0.45-μm (pore size) filter (BA85, Schleicher and Schuell, Dassel, Germany), and washed with 10 ml of stop buffer, and radioactivity remaining on the filter was measured by scintillation counting.
Assays with lysates of Sf9 cells were carried out as described by Settleman and Foster (1995) with minor modifications. Sf9 cells were harvested 48 h after infection with recombinant baculovirus and lysed in a solution containing 25 mM HEPES, pH 7.5, 150 mM NaCl, 0.1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10% glycerol, and protease inhibitors Pefabloc SC (0.1 mg/ml) and aprotinin (7.6 milli trypsin inhibitor units/ml). To remove insoluble material, lysates were centrifuged for 30 min at 15,000 × g at 4°C. Different amounts of supernatants were diluted in lysis buffer to a final volume of 20 μl and added to a reaction mixture containing 5 μl of preloaded GTPase and 25 μl of 20 mM HEPES, pH 7.5, 50 mM NaCl, 2 mM MgCl2, 1 mM DTT, 0.01 mM GTP, and 0.1 mM ATP. The GTPases were preloaded with [γ32P]GTP in an EDTA-facilitated exchange reaction by incubating them with 10 μCi of [γ32P]GTP (6000 Ci/mmol, DuPont New England Nuclear), 20 mM HEPES, pH 7.5, 50 mM NaCl, 1 mg/ml BSA, 1 mM DTT, and 1 mM EDTA in a volume of 100 μl. The loading reaction proceeded at 37°C for 10 min and was then stopped by the addition of MgCl2 to a final concentration of 5 mM and placing the reaction on ice. The GTPase reactions were left for 15 min at 30°C or 25°C (for Rac1) and stopped by the addition of 500 μl of ice-cold stop buffer (20 mM HEPES, pH 7.5, 5 mM MgCl2, 0.1 mM DTT). The remaining bound GTP was determined as described above.
RESULTS
The myr 5 GAP Domain Preferentially Stimulates the GTPase Activity of Rho
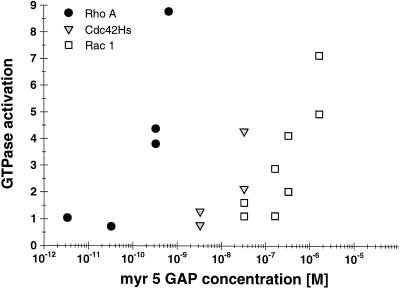
We previously demonstrated that the zinc-GAP domain of myr 5 can stimulate the GTPase activity of several members of the Rho subfamily but not of Ras (Reinhard et al., 1995). This study further suggested that the myr 5 GAP domain was not equally potent in stimulating the GTPase activities of Rho, Rac, and Cdc42. The GTPase activity of Rac1 was shown to be stimulated with lower efficacy than the GTPase activities of RhoA and Cdc42Hs. To obtain more insight into putative functions of myr 5, we analyzed the specificity of its GAP activity in more detail. The concentrations of purified, recombinant myr 5 GAP domain (amino acids 1663–1857) needed to stimulate to a similar extent the GTPase activities of recombinant RhoA, Rac1, and Cdc42Hs were determined (Figure 1). Catalytic amounts of myr 5 GAP domain were sufficient to stimulate RhoA GTPase activity four- to fivefold. In contrast, 100 times or even 1000 times higher concentrations of purified myr 5 GAP domain were needed to stimulate to a similar extent the GTPase activity of Cdc42Hs and Rac1, respectively. This result demonstrates that the isolated myr 5 GAP domain preferentially activates the GTPase activity of RhoA.
Figure 1.
Differential specificity of the myr 5 GAP domain for RhoA, Cdc42Hs, and Rac1. The myr 5 GAP domain was purified as a GST fusion protein from E. coli and subsequently cleaved from GST by thrombin. Recombinant GTPases were preloaded with [γ-32P]GTP and incubated in the absence or presence of different amounts of myr 5 GAP domain. After 0, 5, and 10 min, aliquots of the reactions were removed and the remaining radioactive GTP bound to the GTPases was determined in a filter binding assay. The decrease in radioactivity was used for the calculation of rate constants for the GTPase activity. GTPase activation by increasing amounts of myr 5 GAP domain was determined for RhoA (•), Cdc42Hs (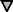 ), and Rac1 (□).
), and Rac1 (□).
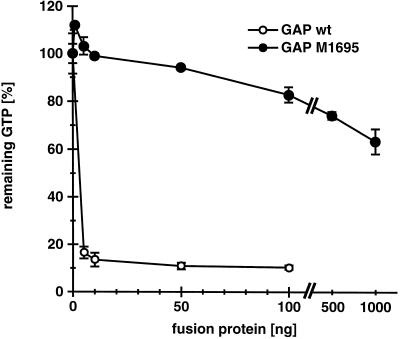
It has been proposed that GAPs could accelerate GTP hydrolysis through the stabilization of the transition state by providing a positive charge to the active site of the G-protein (Mittal et al., 1996). Sequence alignment of various Rho subfamily GAP domains (ABR, L19704; BCR, S98891; β-chimaerin, X69462; n-chimaerin, X67250; p190, M94721; p190-B, U17032; p115, X78817; 3BP-1, X87671; Graf, U36309; Rho-GAP, Z23024; p122, D31962; RLIP76, L42542; rotund, M99702; Bem2p, L33832) revealed a single conserved arginine residue. This arginine residue corresponding to amino acid 1695 in myr 5 was mutated to a methionine residue. Purified recombinant myr 5 GAP domain carrying the M1695 point mutation was at least 500-fold less active in catalyzing RhoA GTPase activity than wild-type myr 5 GAP domain (Figure 2). This result clearly demonstrates the essential role of arginine 1695 in the ability of the myr 5 GAP domain to efficiently stimulate the GTPase activity of RhoA.
Figure 2.
Mutation of arginine 1695 to a methionine virtually abolishes myr 5 Rho-GAP activity. The wild-type myr 5 Rho-GAP domain and a mutant myr 5 Rho-GAP domain (M1695) in which residue arginine 1695 was replaced by a methionine were purified from E. coli as GST fusion proteins. Different amounts of these fusion proteins (wild-type [wt], ○; M1695, •) were incubated for 15 min with purified RhoA protein preloaded with [γ-32P]GTP. The remaining RhoA-bound radioactive GTP was determined in a filter binding assay. Remaining radioactivity in a control assay for intrinsic GTPase activity of RhoA was set 100%.
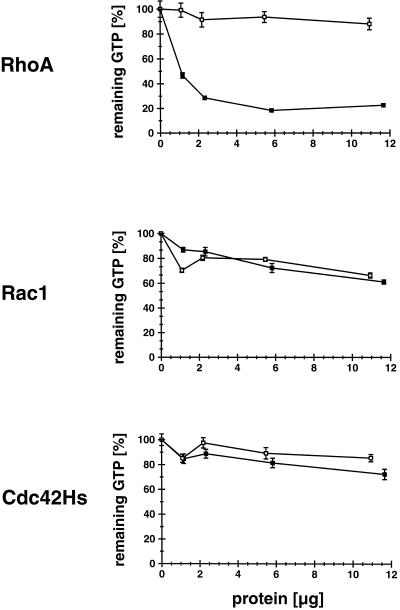
We next determined whether full-length myr 5 also exhibits GAP activity and, if so, whether the GAP activity has a specificity similar to the isolated GAP domain. Intact myr 5 was expressed in Sf9 insect cells by using recombinant baculovirus. Sf9 cells were lysed 48 h after infection with recombinant myr 5 virus and the particulate matter was removed by centrifugation. Different amounts of the lysate were tested for GAP activity toward RhoA, Rac1, and Cdc42Hs and compared with the GAP activity of lysates prepared from uninfected cells (Figure 3). Lysates of cells expressing myr 5 exhibited significant GAP activity for RhoA when compared with lysates from uninfected cells (Figure 3). Aliquots of the same lysates, even at 20–25 times higher concentrations, did not markedly stimulate the GTPase activity of Rac1 and Cdc42Hs. They were indistinguishable in their GAP activity for Rac1 and Cdc42Hs from aliquots of lysates derived from uninfected cells (Figure 3). Therefore, myr 5 does exhibit GAP activity and demonstrates the same preference for RhoA as the isolated myr 5 GAP domain.
Figure 3.
myr 5 expressed in Sf9 insect cells specifically stimulates the in vitro GTP hydrolysis of RhoA but not of Cdc42Hs or Rac1. Sf9 insect cells were infected with a baculovirus encoding myr 5. After 48 h, soluble lysates of the cells were prepared and incubated with purified GTPases preloaded with [γ-32P]GTP. After 15 min at 30°C (RhoA and Cdc42Hs) or 25°C (Rac1), remaining radioactivity was determined in a filter binding assay. Lysate of uninfected cells was used as a control. Remaining radioactive GTP bound to the different GTPases in assays incubated only with lysis buffer was taken as 100% values. Although lysates of uninfected cells (□) and cells infected with virus encoding myr 5 (▪) show no difference in their GAP activity for Cdc42Hs and Rac1, an increased GAP activity for RhoA is detected in lysates of cells infected with myr 5 virus.
Overexpression of myr 5 Rho-GAP Activity Correlates with Morphological Changes in Sf9 Insect Cells
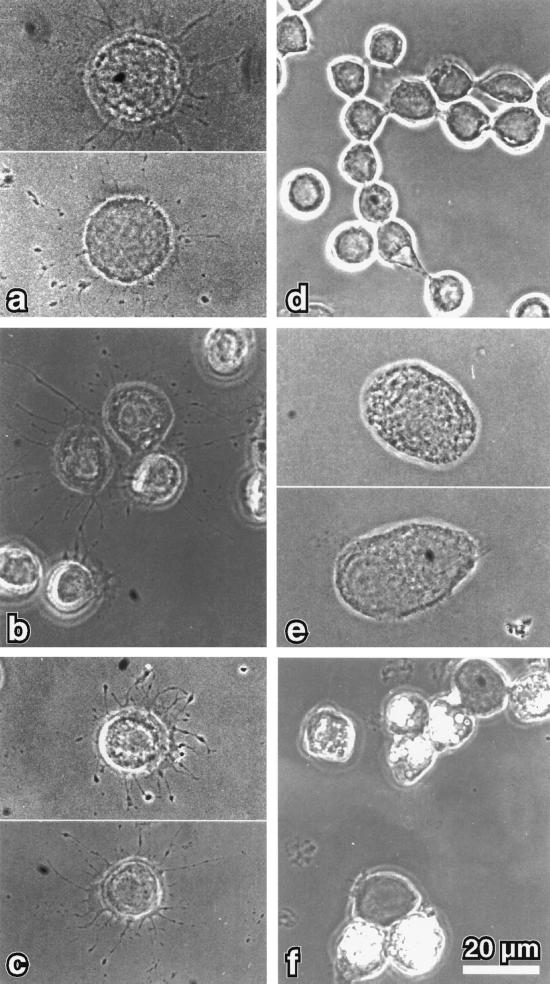
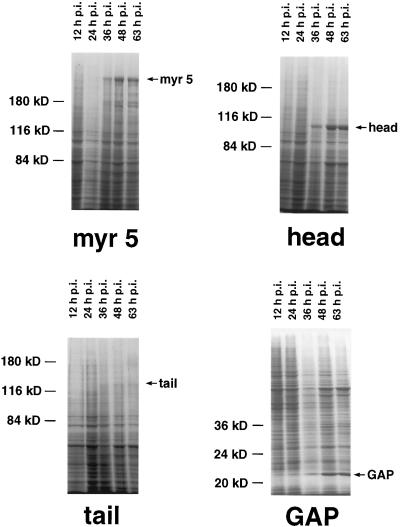
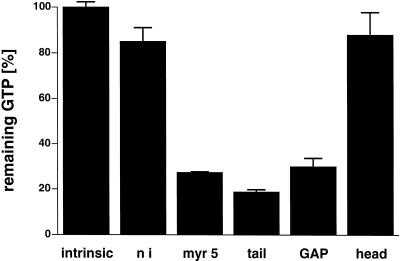
Apart from a well-characterized increase in size, the morphology of the small spherical Sf9 cells is normally not altered by baculovirus infection. However, cells infected with recombinant myr 5 baculovirus started to form numerous thin protrusions 24 h after infection (Figure 4). These protrusions often ended in a varicosity. Such varicosities were also observed along the length of the protrusions. The formation of these protrusions correlated temporally with the expression of the myr 5 molecule in the infected Sf9 cells (Figure 5). These morphological changes were dependent on the expression of myr 5, because uninfected cells or cells infected with wild-type baculovirus virtually never formed such protrusions (Figure 4). To examine whether the protrusion-forming activity depended on the whole myr 5 molecule or could be assigned to a specific myr 5 domain, we generated recombinant baculoviruses encoding the myosin head domain, the neck-tail domain, or just the Rho-GAP domain. The expression of these recombinant proteins was examined at different times after infection (Figure 5). High expression levels of proteins of the expected molecular masses that were cross-reactive with myr 5 antibodies were observed in cells infected with virus encoding myr 5, the myr 5 head domain, and the Rho-GAP domain. The recombinant myr 5 neck-tail protein, however, exhibited a somewhat higher apparent molecular mass (160 kDa as compared with a calculated 120 kDa) than predicted (Figure 5). In addition, in contrast to the other myr 5 constructs, it was readily degraded as an antibody directed against the C terminus of myr 5 recognized a whole ladder of bands after separation by gel electrophoresis. Analysis of the morphology of cells infected either with the neck-tail or Rho-GAP domain encoding viruses revealed that expression of both these myr 5 fragments lead to the formation of thin protrusions (Figure 4). These protrusions were indistinguishable from the ones observed after myr 5 expression. In contrast, infection of cells with baculovirus encoding the myr 5 head domain caused the cells to swell, but no outgrowth of protrusions was observed. Thus, these results strongly suggest that the formation of protrusions can be correlated with increased Rho-GAP activity. Indeed, lysates of cells that express myr 5, myr 5 neck-tail domain, or myr 5 Rho-GAP domain, all of which induce the formation of protrusions, exhibit increased GTPase-stimulatory activity for RhoA in vitro (Figure 6). Lysates of cells expressing the myr 5 head domain that did not induce the formation of protrusions had only minimal GTPase-stimulatory activity for RhoA similar to lysates of uninfected cells (Figure 6). Hence, the formation of numerous thin protrusions by Sf9 cells depends on myr 5 Rho-GAP activity.
Figure 4.
Phase-contrast micrographs of Sf9 cells infected for 48 h with recombinant baculovirus encoding different myr 5 constructs. Sf9 cells that overexpress myr 5 (a) show numerous thin extensions that often contain varicosities. Similar extensions were noticed for cells overexpressing either myr 5 tail domain (b) or just the myr 5 GAP domain (c). However, no extensions were observed in Sf9 cells that overexpress the myr 5 head domain (e), were left uninfected (d), or were infected with wild-type baculovirus (f). Bar, 20 μm.
Figure 5.
Expression of the different myr 5 constructs in baculovirus-infected Sf9 cells. Sf9 cells were infected with recombinant baculovirus encoding full-length myr 5 (myr 5), the myr 5 head domain (head), the myr 5 tail domain (tail), or the myr 5 Rho-GAP domain (GAP). Cells were lysed 12, 24, 36, 48, and 63 h after infection, respectively, and prepared for SDS-PAGE. Coomassie blue-stained gels are shown. Molecular mass markers are indicated on the left; time after infection is indicated above each lane and bands corresponding to the respective myr 5 constructs are marked on the right.
Figure 6.
Rho-GAP activity in lysates of cells infected with recombinant baculovirus encoding different myr 5 constructs. Purified RhoA was preloaded with radioactive GTP and incubated at 30°C with 6 μg of protein from lysates of cells infected for 48 h. After 15 min, the remaining GTP was determined in a filter-binding assay. As a control, RhoA was incubated with lysis buffer alone (intrinsic) and the remaining GTP was set 100%. Lysates of cells expressing myr 5 (myr 5), myr 5 tail domain (tail), or the myr 5 Rho-GAP domain (GAP) exhibit increased Rho-GAP activity as compared with lysates of cells expressing the myr 5 head domain (head) or uninfected cells (ni).
Overexpression of myr 5 in NRK and HeLa Cells Inactivates Rho, Causing a Loss of Actin Stress Fibers and Focal Contacts
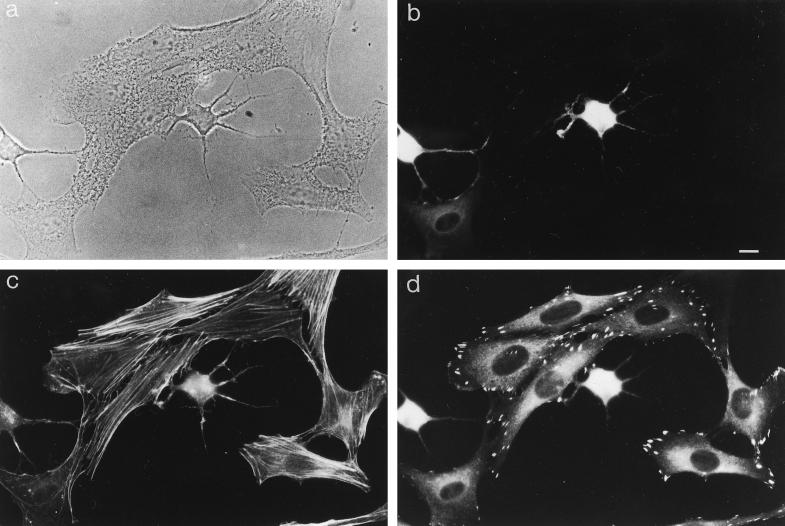
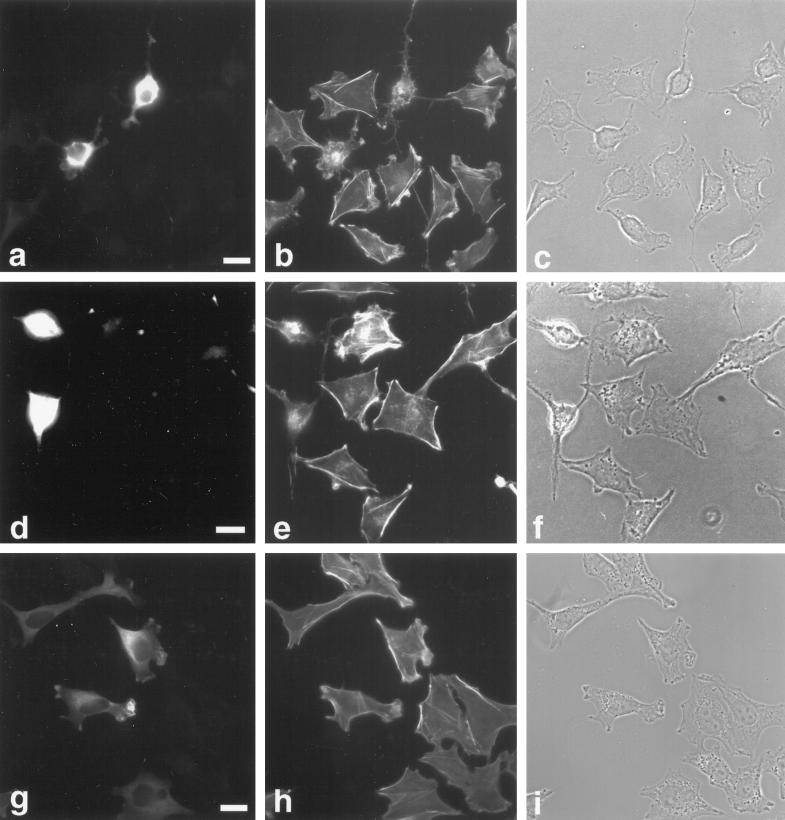
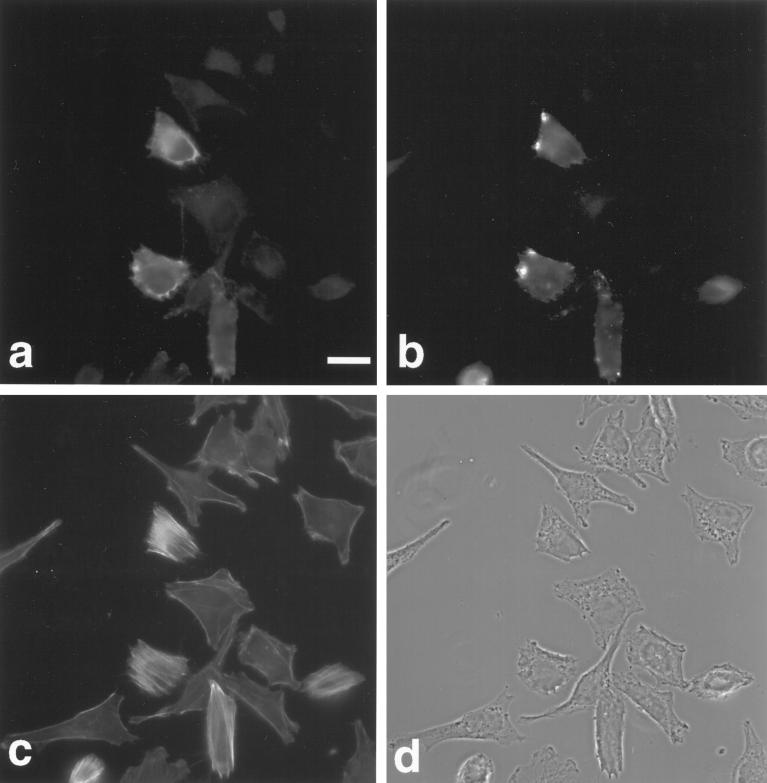
To investigate the potential role of myr 5 in mammalian cells in vivo, we overexpressed an epitope-tagged version of myr 5 in NRK and HtTA-1 HeLa cells. Both NRK and HtTA-1 HeLa cells transiently transfected with myr 5 showed a dramatic alteration in morphology (Figures 7 and 8). They became more refractile, rounded up, and exhibited extensions that frequently contacted neighboring cells or even crossed over them (Figures 7 and 8). These morphological alterations are caused by changes in cytoskeletal organization and cell adhesion. Immunofluorescence staining for filamentous actin (F-actin) revealed that stress fibers were either absent or strongly reduced in transfected cells depending on the expression level of myr 5 (Figures 7 and 8). However, transfected cells still exhibited F-actin staining in cortical regions, in lamellipodia, and in the cytoplasm. Focal adhesions as detected by antibodies against vinculin and phosphotyrosine were either absent or strongly reduced in number and size (Figure 7). The observed loss of stress fibers and focal contacts that ultimately led to alterations in cell morphology is very reminiscent of results obtained by specific inactivation of Rho with the bacterial toxin C3 transferase (Paterson et al., 1990; Miura et al., 1993). To test whether the cellular changes that occur upon overexpression of myr 5 can be attributed to the inactivation of Rho by its Rho-GAP activity, we constructed an expression plasmid encoding only the Rho-GAP domain of myr 5 tagged with an HA-epitope. HtTA-1 HeLa cells transiently transfected with this GAP plasmid showed alterations in morphology that were very similar to cells transfected with myr 5 (Figure 8). They rounded up and became more refractile displaying several extensions that could be of considerable length. Actin stress fibers were absent, although there was still noticeable F-actin staining (Figure 8). Cells expressing the myr 5 GAP domain were also devoid of focal contacts as seen by staining with antibodies against vinculin or phosphotyrosine (our unpublished observations). These findings argue that the cellular changes after myr 5 overexpression are due to its GAP activity.
Figure 7.
Overexpression of myr 5 causes the loss of actin stress fibers and focal adhesions in NRK cells. NRK cells were transiently transfected with myr 5-HA expression vector (pCMV5 myr 5-HA). Cells were processed for triple immunofluorescence 40 h after transfection. They were viewed in phase-contrast microscopy (a). myr 5 was visualized with antibody Tü 66 followed by AMCA-coupled goat anti-rabbit antibody (b), F-actin was visualized with FITC-phalloidin (c), and vinculin was visualized with antibody hVin-1 followed by lissamine rhodamine-coupled goat anti-mouse antibody (d). Note the cell in the lower left corner of the images that expresses only small levels of myr 5. F-actin stress fibers are decreased and focal contact size is reduced. Bar, 10 μm.
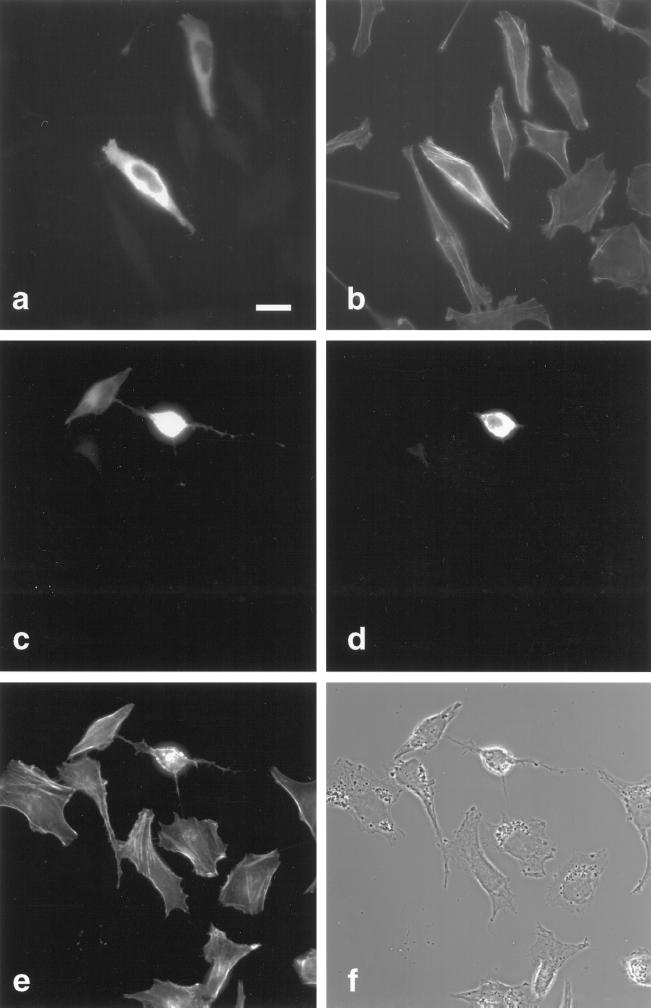
Figure 8.
Rho-GAP activity of myr 5 is responsible for the observed alterations in cytoskeletal organization. HtTA-1 HeLa cells were transfected with plasmids pUHD10–3 myr 5-HA (a–c), pUHD10–3 HA-myr 5 Rho-GAP domain (d–f), and pUHD10–3 myr 5-HA M1695 (g–i). The cells were stained 40 h after transfection for myr 5 with antibody Tü 66 (a and g) or hemagglutinin antibody HA.11 (d) followed by a secondary antibody coupled to lissamine rhodamine and for F-actin (b, e, and h) by using FITC-phalloidin. The identical fields of cells are also shown in phase-contrast optics (c, f, and i). Bars, 20 μm.
To further analyze whether the cellular effects noticed after myr 5 overexpression were solely due to its GAP activity, we transfected cells with myr 5 carrying the M1695 point mutation that virtually abolishes GAP activity in vitro. In contrast to wild-type myr 5, overexpression of myr 5 M1695 did not cause any obvious morphological changes in HtTA-1 HeLa cells (Figure 8). Furthermore, no obvious alterations in the F-actin organization as compared with nontransfected cells were apparent (Figure 8). Similar results were also obtained upon overexpression of a truncated myr 5 that had residues 1668–1688 deleted (our unpublished results). These findings further indicate that mutation of arginine 1695 in myr 5 to a methionine also abolishes GAP activity in vivo and that the observed changes in actin organization and adhesion upon myr 5 overexpression are brought about by the myr 5 Rho-GAP activity.
Our in vitro measurements of myr 5 GAP activity demonstrated a high specificity for RhoA. In agreement with these in vitro measurements, overexpression of myr 5 had effects on cells that were very similar to those reported for C3 transferase from Clostridium botulinum, which specifically inactivates Rho. Furthermore, activation of Rho has been shown to be responsible for formation of stress fibers and focal contacts, structures that are lost upon myr 5 overexpression, arguing for inactivation of Rho by myr 5. If myr 5 inactivates Rho, then a dominant active form of RhoA, RhoA V14, that is no longer able to hydrolyze GTP should be able to block the myr 5 effects. Expression of myc-epitope–tagged RhoV14 in HtTA-1 HeLa cells induced in accordance with previous observations the formation of abundant actin stress fibers and the cells adopted a less irregular shape. When HtTA-1 HeLa cells were cotransfected with RhoA V14 and myr 5, they exhibited prominent actin stress fibers and a less irregular shape just as cells did that expressed only dominant active RhoA V14 (Figure 9). Specifically, the cells that expressed both proteins did not lose their stress fibers and did not round up and display extensions as is typical for cells expressing only myr 5. Identical results were obtained when the myr 5 GAP domain was cotransfected with RhoA V14 (our unpublished observations). These results demonstrate that dominant active RhoA V14 is able to inhibit the responses elicited by overexpression of myr 5 GAP activity.
Figure 9.
Dominant active RhoA V14 abrogates the cytoskeletal alterations induced by myr 5 expression. HtTA-1 HeLa cells were cotransfected with plasmids pUHD10–3 myc-RhoA V14 and pUHD10–3 myr 5-HA. Cells were processed for triple immunofluorescence 40 h after transfection. (a) myr 5 was stained with antibody Tü 66 followed by a secondary antibody coupled to AMCA. (b) Myc-RhoA V14 was stained with antibody 9E10 followed by a secondary antibody coupled to lissamine rhodamine. (c) F-actin was stained with FITC-phalloidin. (d) The same field of cells was also viewed by phase-contrast microscopy. Bar, 20 μm.
We next tested whether dominant active RhoA I117, which in contrast to RhoA V14 is still able to hydrolyze GTP, can modify the cellular responses caused by myr 5 overexpression. The analogous mutation in Ras and Rac1 (replacement of asparagine 116 or 115 by isoleucine, respectively) increases the GDP/GTP exchange rate and renders them more active (Der et al., 1988; Koshravi-Far et al., 1995). HtTA-1 HeLa cells transiently transfected with myc-tagged RhoA I117 did not show any obvious morphological changes and only occasionally exhibited increased F-actin staining and stress fiber formation (Figure 10). HtTA-1 HeLa cells that were cotransfected with both RhoA I117 and myr 5 exhibited a phenotype very similar to cells transfected with myr 5 alone (Figure 10). They lost their stress fibers, rounded up, and showed prominent extensions. Only in cells that expressed high levels of RhoA I117 and relatively low levels of myr 5 was an attenuated myr 5 phenotype observed. In cotransfections of RhoA I117 and just the GAP domain of myr 5, the myr 5 GAP phenotype was even more prevalent (our unpublished observations). Thus, myr 5 does inactivate RhoA I117 in vivo. Thus, these results demonstrate that myr 5 functions as a Rho-GAP in vivo.
Figure 10.
Dominant active RhoA I117 does not abrogate the cytoskeletal alterations induced by myr 5 expression. HtTA-1 HeLa cells were transfected with pUHD10–3 myc-RhoA I117 alone (a and b) or together with pUHD10–3 myr 5-HA (c–f). Cells were processed for immunofluorescence 40 h after transfection. Double immunofluorescence staining for myc-RhoA I117 (a) with antibody 9E10 followed by a secondary antibody coupled to lissamine rhodamine and F-actin (b) with FITC-phalloidin was performed in cells transfected with myc-RhoA I117. Triple immunofluorescence staining was performed for cells cotransfected with myc-RhoA I117 and myr 5-HA. Myc-RhoA I117 (c) was stained with antibody 9E10 followed by a secondary antibody coupled to lissamine rhodamine; myr 5-HA (d) was stained with antibody Tü 66 followed by a secondary antibody coupled to AMCA; and F-actin (e) was stained with FITC-phalloidin. (f) Cells are shown in phase-contrast optics. Bar, 20 μm.
Subcellular Localization of myr 5 in NRK Cells
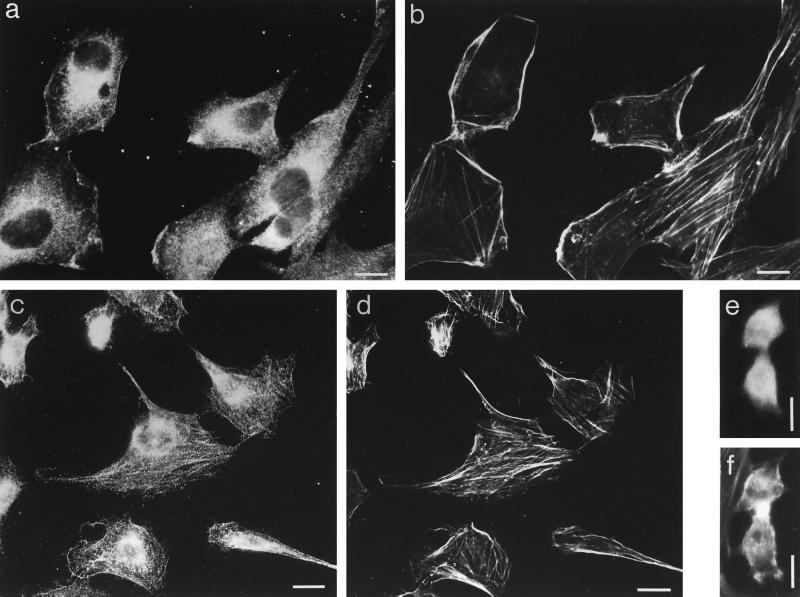
NRK cells express myr 5 as detected by immunoblotting. Upon homogenization of NRK cells, myr 5 was recovered partly in the soluble fraction and partly in the particulate fraction. In immunofluorescence experiments, myr 5 was seen to be distributed throughout the cytoplasm (Figure 11). Furthermore, specific staining for myr 5 was noticed in dynamic regions of the cell periphery, such as ruffles and lamellipodia, and in the perinuclear region in the area of the Golgi apparatus where it most likely is associated with membranous organelles. Punctate staining was occasionally observed along actin stress fibers. Staining of these structures was seen more clearly in NRK cells devoid of their cytosol due to permeabilization with streptolysine-O prior to fixation (Figure 11). A similar localization was observed for overexpressed myr 5 M1695 in HeLa cells (see Figure 8). myr 5 M1695 was localized in membrane ruffles and in the perinuclear region. HeLa cells, unlike NRK cells, exhibit only few actin stress fibers precluding the verification of an occasional punctate myr 5 M1695 colocalization with actin stress fibers. During cytokinesis, endogenous myr 5 in NRK cells was not concentrated in the cleavage furrow (Figure 11, e and f).
Figure 11.
Localization of endogenous myr 5 in NRK cells. NRK cells were fixed directly (a, b, e, and f) or after permeabilization with Streptolysine-O (c and d) to remove cytosol and processed for double immunofluorescence. myr 5 was stained with antibody Tü 66 (a and e) or antibody Tü 52 (c) followed by a secondary antibody coupled to Texas red and F-actin (b, d, and f) was stained with FITC-phalloidin. The cell in e and f is undergoing cytokinesis. Bars, 10 μm.
DISCUSSION
We demonstrate in this report that the unconventional myosin myr 5 exhibits GAP activity for Rho that is critically dependent on an arginine residue at position 1695. Although a large number of GAPs for the Rho subfamily have been identified, at present the specificity with which they act on different members of the Rho subfamily cannot be predicted on the basis of sequence comparison. To elucidate the determinants of specificity, a detailed analysis of the specificity of the individual GAPs is needed. The isolated myr 5 GAP domain is most specific for RhoA. A 100-fold and 1000-fold higher concentration of myr 5 GAP was needed to achieve similar activation of Cdc42Hs and Rac1, respectively. The myr 5 GAP domain is totally inactive toward H-Ras (Reinhard et al., 1995). myr 5 expressed in insect cells exhibited the same specificity for RhoA as the isolated GAP domain, demonstrating that the determinants for the specificity reside exclusively in the GAP domain. The additional domains present in myr 5 may regulate the accessibility of the Rho protein to the GAP domain by targeting myr 5 to specific intracellular locations (see below) or by allosteric modulation.
The mechanism by which GAPs stimulate the GTP-hydrolysis reaction on monomeric G-proteins may either involve the formation of a GTPase-competent state in the G-protein or the supply of amino acid residues that participate in catalysis to the active site in the G-protein (Scheffzek et al., 1996). Experimental support for the second model has been obtained for Ras-GAPs. Mutation of an arginine residue (R903) in p120 Ras-GAP results in a protein defective in catalysis but not in binding (Brownbridge et al., 1993). A presumed transition-state analogue of the GTPase reaction was formed in the presence of Ras in its GDP-bound form, AlF4−, and stoichiometric amounts of p120-GAP or neurofibromin. This result suggests that Ras-GAPs may accelerate GTP hydrolysis by stabilizing the increasing negative charge that develops on the β–γ bridging oxygen during bond cleavage. Ras-GAPs contain two totally conserved arginine residues and one lysine residue, and mutation of either of these reduces GAP activity by one or two orders of magnitude (Mittal et al., 1996). Inspection of sequence alignments of Rho-subfamily GAPs revealed a single invariant arginine and lysine residue, respectively. Because in Gα proteins and in adenylate kinases arginine residues are involved in stabilizing the transition state (Coleman et al., 1994; Sondek et al., 1994), we have mutated the invariant arginine in the GAP domain of myr 5 (arginine 1695). Mutation of this arginine to a methionine virtually abolished myr 5 GAP activity both in vitro and in vivo. This result demonstrates that arginine 1695 of myr 5 is essential for the stimulation of GTP hydrolysis by Rho. Furthermore, it is compatible with the notion that this arginine residue stabilizes the transition state. Mutation of this arginine residue does not abolish binding of the GAP domain to RhoA because the M1695 mutation in the myr 5 GAP domain did not noticeably weaken the low binding affinity of the myr 5 GAP domain for RhoA V14 (our unpublished observations). Similarly, deletion in the Rac-GAP n-chimaerin of the three residues Tyr-Arg-Val, including the invariant arginine, did not abolish binding of n-chimaerin to Rac (Ahmed et al., 1994). Deletion of the conserved lysine residue and three additional residues (Leu-Lys-Leu-Tyr) in n-chimaerin also abolished GTPase stimulation on Rac by n-chimaerin without completely destroying binding (Ahmed et al., 1994). On the basis of our myr 5 GAP mutation M1695 and the recently determined crystal structures of the GTPase-activating domain from p50rhoGAP (Barrett et al., 1997) and phosphatidylinositol 3-kinase p85α subunit (Musacchio et al., 1996), and the very similar structure of p120rasGAP (Scheffzek et al., 1996), it can be deduced that this conserved lysine residue in Rho-subfamily GAPs (analogous to R903 in p120GAP) stabilizes the orientation of the conserved arginine (arginine 85 in p50rhoGAP and arginine 789 in p120GAP) that is likely used for catalysis by stabilizing the transition state.
Overexpression of myr 5 or fragments of myr 5 that include the Rho-GAP domain caused dramatic changes in the morphology of Sf9 insect cells and mammalian NRK and HtTa-1 HeLa cells. The spherical Sf9 insect cells formed long thin processes upon expression of myr 5. The formation of these processes correlated with increased Rho-GAP activity in cell lysates. The inactivation of Rho in Sf9 cells might lead to a weakening of cortical tension by affecting the F-actin organization. The weakening of cortical tension might be sufficient to allow process formation as has been reported for astrocytes (Baorto et al., 1992). Interestingly, a similar phenotype was observed in Sf9 cells expressing the C-terminal domain of ezrin, a protein involved in the regulated interaction of the actin cytoskeleton with the plasma membrane (Martin et al., 1995). Recently obtained evidence in mammalian cells has actually implicated Rho and ezrin in a common pathway controlling actin organization (Hirao et al., 1996). In addition, a colocalization of Rho and ezrin at the plasma membrane–actin cytoskeleton interface has been reported (Takaishi et al., 1995).
In NRK and HtTA-1 HeLa cells, the overexpression of myr 5 or just the myr 5 Rho-GAP domain alone caused a rounding up of cells and the appearance of numerous long processes. These cell-shape changes were due to the fragmentation of actin filaments and the loss of focal adhesions. Expression of a myr 5 in which the myr 5 Rho-GAP activity was inactivated by mutation of arginine 1695 to a methionine did not cause obvious morphological changes in HtTa-1 HeLa cells. Expression of dominant active RhoV14 that is not able to hydrolyze GTP and to a much lesser extent RhoI117 that presumably has a higher exchange rate of GDP for GTP induced the opposite phenotype, namely, the formation of actin stress fibers and focal contacts. When coexpressed with myr 5, RhoV14 blocked the morphological changes induced by overexpression of myr 5, whereas RhoI117 at best attenuated them. Morphological changes similar to the ones induced by the overexpression of myr 5 have been observed in cells treated with the bacterial exoenzyme C3 that specifically inactivates Rho (Chardin et al., 1989; Miura et al., 1993). The cumulative evidence lets us conclude that overexpression of myr 5 inactivates Rho, which in turn is responsible for the observed morphological changes.
Because myr 5 not only contains a Rho-GAP domain but also an actin-binding myosin domain (Reinhard et al., 1995), it was speculated that myr 5 could serve an effector function of Rho participating in the Rho-dependent reorganization of the actin cytoskeleton. However, no evidence for such an effector function of myr 5 was obtained. On the basis of our results, it seems more likely that the myr 5 myosin domain participates in controlling the intracellular localization of myr 5 and thereby contributes to spatially and temporally coordinated signaling of Rho. myr 5 was localized in dynamic actin-containing regions of the plasma membrane, in the perinuclear region, in the cytosol, and occasionally along stress fibers. These myr 5 localization data suggest that myr 5 shuttles between different compartments and are compatible with the notion that the myosin domain might participate in determining the cellular localization of myr 5. A role of the myosin domain in maintaining a proper subcellular localization has been reported for other unconventional myosin molecules (Porter and Montell, 1993; Ruppert et al., 1995). The demonstration that myr 5 is a specific Rho-GAP that can be inactivated by mutation of a conserved arginine residue that participates in catalysis of GTP hydrolysis provides a means to address the role that individual myr 5 domains, including the myosin domain, play in subcellular targeting of myr 5 and in the regulation of the myr 5 Rho-GAP activity.
ACKNOWLEDGMENTS
We thank Dr. A. Hall (London) for the gift of plasmids, Dr. H.Bujard (Heidelberg) for cells and plasmids, Dr. S. Bhakdi (Mainz) for purified streptolysine-O, and Iris Kehrer for excellent technical assistance. Barbara Wiechers kindly performed the immunofluorescence experiments with streptolysine-O permeabilization. We acknowledge the support of Dr. M. Schliwa, the Max-Planck-Society, the Deutsche Forschungs Gemeinschaft (Ba 1354/1–2), and the European Commission (contract CHRX-CT94–0652).
REFERENCES
- Agnel M, Röder L, Vola C, Griffin-Shea R. A Drosophila rotund transcript expressed during spermatogenesis and imaginal disc morphogenesis encodes a protein which is similar to human rac GTPase-activating (racGAP) proteins. Mol Cell Biol. 1992;12:5111–5122. doi: 10.1128/mcb.12.11.5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Lee J, Wen L-P, Zhao Z, Ho J, Best A, Kozma R, Lim L. Breakpoint cluster region gene product-related domain of n-chimaerin. J Biol Chem. 1994;269:17642–17648. [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley & Sons; 1990. pp. 9.1.1.–9.1.3.. [Google Scholar]
- Bähler M. Myosins on the move to signal transduction. Curr Opin Cell Biol. 1996;8:18–22. doi: 10.1016/s0955-0674(96)80043-3. [DOI] [PubMed] [Google Scholar]
- Baorto DM, Mellado W, Shelanski ML. Astrocyte process growth induction by actin breakdown. J Cell Biol. 1992;117:357–367. doi: 10.1083/jcb.117.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett T, Xiao B, Dodson EJ, Dodson G, Ludbrook SB, Nurmahomed K, Gamblin SJ, Musacchio A, Smerdon SJ, Eccleston JF. The structure of the GTPase-activating domain from p50rhoGAP. Nature. 1997;385:458–461. doi: 10.1038/385458a0. [DOI] [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;349:117–121. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Brownbridge GG, Lowe PN, Moore KJM, Skinner RH, Webb MR. Interaction of GTPase activating proteins (GAPs) with p21ras measured by a novel fluorescence anisotropy method. J Biol Chem. 1993;268:10914–10919. [PubMed] [Google Scholar]
- Chardin P, Boquet P, Madaule P, Popoff MR, Rubin EJ, Gill DM. The mammalian G protein rhoC is ADP-ribosylated by Clostridium botulinum exoenzyme C3 and affects actin microfilaments in Vero cells. EMBO J. 1989;8:1087–1092. doi: 10.1002/j.1460-2075.1989.tb03477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheney RE, Riley MA, Mooseker MS. Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton. 1993;24:215–223. doi: 10.1002/cm.970240402. [DOI] [PubMed] [Google Scholar]
- Coleman DE, Berghuis AM, Lee E, Linder ME, Gilman AG, Sprang SR. Structures of active conformations of Giα1 and the mechanism of GTP hydrolysis. Science. 1994;265:1405–1412. doi: 10.1126/science.8073283. [DOI] [PubMed] [Google Scholar]
- Der CJ, Weissmann B, MacDonald MJ. Altered guanine nucleotide binding and H-ras transforming and differentiating activities. Oncogene. 1988;3:105–112. [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop MJ. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson HV, Spudich JA. Molecular evolution of the myosin family: Relationships derived from comparisons of amino acid sequences. Proc Natl Acad Sci USA. 1993;90:659–663. doi: 10.1073/pnas.90.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Monfries C, Smith P, Lim H-H, Kozma R, Ahmed S, Vanniasingham V, Leung T, Lim L. Novel human brain cDNA encoding a 34,000 Mr protein n-chimaerin, related to both the regulatory domain of protein kinase C and BCR, the product of breakpoint of cluster region gene. J Mol Biol. 1990;211:11–16. doi: 10.1016/0022-2836(90)90006-8. [DOI] [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita Sh, Tsukita S. Regulation mechanism of ERM (ezrin/radixin/moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshravi-Far R, Solski PA, Clark GJ, Kinch MS, Der CJ. Activation of Rac1, RhoA, and mitogen-activated protein kinases is required for Ras transformation. Mol Cell Biol. 1995;15:6443–6453. doi: 10.1128/mcb.15.11.6443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamarche N, Hall A. GAPs for rho-related GTPases. Trends Genet. 1994;12:436–440. doi: 10.1016/0168-9525(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Martin M, Andreoli C, Sahuquet A, Montcurrier P, Algrain M, Mangeat P. Ezrin NH2-terminal domain inhibits the cell extension activity of the COOH-terminal domain. J Cell Biol. 1995;128:1081–1093. doi: 10.1083/jcb.128.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R, Ahmadian MR, Goody RS, Wittinghofer A. Formation of a transition-state analog of the Ras GTPase reaction by Ras·GDP, tetrafluoroaluminate, and GTPase-activating proteins. Science. 1996;273:115–117. doi: 10.1126/science.273.5271.115. [DOI] [PubMed] [Google Scholar]
- Miura Y, Kikuchi A, Musha T, Kuroda S, Yaku H, Sasaki T, Takai Y. Regulation of morphology by rho p21 and its inhibitory GDP/GTP exchange protein (rhoGDI) in Swiss 3T3 Cells. J Biol Chem. 1993;268:510–515. [PubMed] [Google Scholar]
- Mooseker MS, Cheney RE. Unconventional myosins. Annu Rev Cell Dev Biol. 1995;11:633–675. doi: 10.1146/annurev.cb.11.110195.003221. [DOI] [PubMed] [Google Scholar]
- Musacchio A, Cantley LC, Harrison SC. Crystal structure of the breakpoint cluster region-homology domain from phosphoinositide 3-kinase p85α subunit. Proc Natl Acad Sci USA. 1996;93:14373–14378. doi: 10.1073/pnas.93.25.14373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac and Cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- O’Reilly DR, Miller LK, Luckow VA. Baculovirus Expression Vectors. New York: W.H. Freeman; 1992. pp. 128–130. [Google Scholar]
- Paterson HF, Self AJ, Garrett MD, Just I, Aktories KK, Hall A. Microinjection of recombinant p21rho induces rapid changes in morphology. J Cell Biol. 1990;111:1001–1007. doi: 10.1083/jcb.111.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JA, Montell C. Distinct roles of the Drosophila ninaC kinase and myosin domains revealed by systematic mutagenesis. J Cell Biol. 1993;122:601–612. doi: 10.1083/jcb.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard J, Scheel AA, Diekmann D, Hall A, Ruppert C, Bähler M. A novel type of myosin implicated in signalling by rho family GTPases. EMBO J. 1995;14:697–704. doi: 10.1002/j.1460-2075.1995.tb07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Paterson H-F, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ruppert C, Godel J, Müller RT, Kroschewski R, Reinhard J, Bähler M. Localization of the rat myosin I molecules myr 1 and myr 2 and in vivo targeting of their tail domains. J Cell Sci. 1995;108:3775–3786. doi: 10.1242/jcs.108.12.3775. [DOI] [PubMed] [Google Scholar]
- Scheffzek K, Lautwein A, Kabsch W, Ahmadian MA, Wittinghofer A. Crystal structure of the GTPase-activating domain of human p120GAP and implications for the interaction with Ras. Nature. 1996;384:591–596. doi: 10.1038/384591a0. [DOI] [PubMed] [Google Scholar]
- Settleman J, Foster R. Purification and GTPase-activating protein activity of baculovirus-expressed p190. Methods Enzymol. 1995;256:105–113. doi: 10.1016/0076-6879(95)56015-7. [DOI] [PubMed] [Google Scholar]
- Sondek J, Lambright DG, Noel JP, Hamm HE, Sigler PB. GTPase mechanism of G proteins from the 1.7-Å crystal structure of transducin α·GDP·AlF4−. Nature. 1994;372:276–279. doi: 10.1038/372276a0. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kamayama T, Tsukita S, Tsukita Sh, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Whitehead I, Kirk H, Tognon C, Trigo-Gonzalez G, Kay R. Expression cloning of lfc, a novel oncogene with structural similarities to guanine nucleotide exchange factors and to the regulatory region of protein kinase C. J Biol Chem. 1995;270:18388–18395. doi: 10.1074/jbc.270.31.18388. [DOI] [PubMed] [Google Scholar]
- Wirth JA, Jensen KA, Post PL, Bement WM, Mooseker MS. Human myosin-IXb, an unconventional myosin with a chimaerin-like Rho/Rac GTPase-activating protein domain in its tail. J Cell Sci. 1996;109:653–661. doi: 10.1242/jcs.109.3.653. [DOI] [PubMed] [Google Scholar]