Abstract
Most current animal models focus on eosinophil-mediated asthma, despite compelling evidence that a neutrophil-mediated disease occurs in some asthma patients. Using intranasal challenge of mice sensitized either orally or nasally with whole peanut protein extract in the presence of cholera toxin, we developed mouse models of eosinophil- and neutrophil-mediated asthma, respectively. In this study, mice deficient in Th1 (IL-12 and IFN-γ) or Th2 (IL-4 and IL-13) pathways were used to characterized the role played by Th1 and Th2 cytokines during the initial priming phase in the two models.. Antigen-specific Ab responses were controlled primarily by Th2 cytokines in mice sensitized by the oral route, whereas Th1 cytokines appeared to play a predominant role in mice sensitized by the nasal route. Furthermore, the absence of key Th1 or Th2 cytokines during the initial phase of priming reduced lung reactivity in both mouse models of airway inflammation.
Keywords: airway, cholera toxin, hyper-reactivity, inflammation, lung, mucosal, nasal, peanut
Introduction
Asthma is a pulmonary disease characterized by airway inflammation and increased airway responsiveness to a variety of stimuli [1]. Th2 cell cytokines IL-4, IL-5, IL-9 and IL-13 have long been recognized as having a key function in the pathogenesis of asthma and IgE-mediated eosinophilic inflammation of the lungs [2]. There now is increasing evidence that asthma can be associated with Th1 cells, inflammatory cytokines including IL-1, IL-8 and IL-17, and lung neutrophilia [3-5]. The majority of current murine models of asthma include a priming phase, when animals are sensitized by intraperitoneal injection of ovalbumin together with a Th2-skewing adjuvant. The disease subsequently is triggered by intranasal challenge with the antigen [6, 7]. This experimental approach, which promotes IgE Ab, airway hyper-responsiveness and eosinophilia, makes it possible to thoroughly investigate the role of the Th2 pathway in asthma. Despite increasing evidence that Th1 cells could be involved in asthma, only a few studies [8-10] have characterized this pathology in animal models; the mechanisms involved in this process remain to be elucidated.
We recently showed that mice challenged intranasally with peanut antigen developed eosinophil-mediated or neutrophil-mediated asthma depending of the initial nasal or oral route of sensitization [11]. Consistent with previous reports that both Th1 and Th2 cytokines are present in the lungs of asthmatic patients [12], Th1 and Th2 responses were not exclusive in orally sensitized (OS) and nasally sensitized (NS) mice. Other studies have shown that IFN-γ itself [13]], IL-18 [14] or IFN-γ-inducible protein 10 [15] contribute to allergic asthma. Furthermore, the Th1-inducing cytokine IL-12 was shown to stimulate rather than suppress ongoing IgE and Th2-type responses [16, 17]. However, the exact roles played by Th1 and Th2 cytokines during the initial phase of antigen sensitization in the development of eosinophil-mediated and neutrophil-mediated asthma remain unclear. IL-4 and IFN-γ are the key cytokines for the induction and regulation of the Th2 and Th1 pathways, respectively. We examined the profiles of antigen-specific Ab and cytokine responses that occurred after OS or NS induced with PPE and cholera toxin in mice deficient in Th2 (i.e., IL-4 KO and IL-4/IL-13 double KO) or Th1 (IFN-γ KO) pathways and their impact on lung inflammatory responses to subsequent peanut challenge. We also tested IL-12 KO mice in order to evaluate the role of this major product of antigen presenting cells and inducer of Th1-cells in our models of Th1- and Th2-type induced asthma.
Materials and Methods
Mice
Female IL-4 KO, IL-12 KO and IFN-γ KO mice, on a C57BL/6 background, female IL-4/IL-13 double KO (IL-4/13 dKO) mice, on a BALB/c background, and control wild-type (WT) mice were purchased from the Jackson Laboratories (Bar Harbor, ME), the Frederick Cancer Research Facility (NCI, Frederick, MD) or obtained from the UAB Mucosal HIV and Immunobiology Center. Studies were performed in accordance with Institutional guidelines to avoid pain and distress.
Mucosal sensitization and nasal challenge
Whole peanut protein extracts (PPE) were obtained as previously described [18]. Mice 8-to-12 weeks of age were sensitized two times a week apart with whole PPE and CT as adjuvant. Anesthetized mice were given 100 μg of PPE and 1 μg of CT in a total volume of 10 μl by intranasal route with 5 μl placed into each nare. This volume of the nasal vaccine is retained in the nasal cavity after nasal administration to anaesthetized mice [19]. For sensitization by the oral route, mice were orally immunized as previously described [20] by intragastric administration of 1 mg of PPE plus 15 μg of CT in 250 μl of PBS. Plasma samples were collected one week after each sensitization on days 7 and 14 for analysis of peanut-specific Ab responses. Mice were anesthetized and challenged intranasally on days 15 and 16 with 200 μg of PPE in a total volume of 100 μl (i.e. 25 μl of PPE per nare, four times at 2-3 min intervals).
Plasma antibody responses
Plasma levels of peanut-specific Abs were measured by ELISA as previously reported [11]. To improve the detection of IgE Abs [21], IgG were removed from plasma samples by overnight incubation of samples at 4°C on protein G coated 96-well plates (Reacti-Bind plates, Pierce, Rockford, IL). Total and antigen-specific IgE levels then were measured by ELISA.
Histology and determination of lung inflammation scores
Lungs were fixed in 10% buffered formaldehyde, paraffin-embedded and cut into 5 μm thick sections. To evaluate the presence of eosinophils, lung sections were incubated for 1 min in 10 mM KCN pH 6.5 before a 15 min incubation with the peroxidase substrate 3,3′-diaminobenzidine and counterstaining with hematoxylin [11].
For quantification of lung inflammation, the slides were coded, and peribronchial and perivascular inflammation were scored in a blinded fashion by two independent investigators. A value of 1 was given when slides showed no sign of inflammation. Slides were graded from 2 to 4 when bronchi were surrounded with a thin layer of inflammatory cells (2: few bronchi; 3: more bronchi: 4: most bronchi). They were graded from 5 to 7 according to the number of bronchi that were surrounded with a thick layer of inflammatory cells (5: few bronchi; 6: more bronchi: 7: most bronchi). Finally, slides were graded 8 or 9 when inflammation spread into the interstitial area (8: severe; 9: extreme).
Flow cytometry
Whole lung tissue was dissociated by digestion with 1 mg/ml collagenase type V (Sigma) as previously described [11], and mononuclear cells were stained with anti-CD3, anti-CD4, anti-B220, anti-CD11c, anti-MAC-1 or anti-MHC class II Abs (BD PharMingen).
Statistics
The results are reported as the mean + one standard deviation (SD). Statistical significance (* p < 0.05) was determined by Student’s t test and by the Mann-Whitney U test of unpaired samples. The results were analyzed using the Instat statistical program (San Diego, CA) for Apple computers.
Results
The lack of endogenous IL-4 more drastically affects peanut-specific Ab responses in mice sensitized by the oral route
Both OS and NS IL-4 KO mice displayed significantly lower levels of peanut-specific IgG1 Abs compared to WT mice (Figure 1). On the other hand, the lack of IL-4 did not affect the levels of peanut-specific IgG2a, IgG2b and IgG3 Abs in NS mice, whereas there was an increase of peanut-specific IgG2a and IgG2b Abs in the OS group. Consistent with the role of IL-4 in IgE class switching, peanut-specific IgE Abs were not detected in IL-4 KO mice after either oral or nasal immunization (results not shown).
Figure 1.
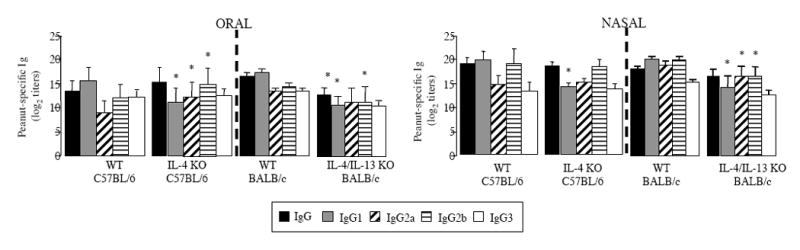
Plasma IgG and IgG-subclass responses of C57BL/6 WT, IL-4 KO, BALB/c WT and IL-4/IL-13 double KO mice. Mice mice were sensitized by oral or nasal route by administration of PPE and CT on days 0 and 7. Plasma samples were collected on day 14 and Ab responses were analyzed by ELISA. Results are expressed as means of individual titles ± SD (5 mice per group). (* p < 0.05).
IL-13 provides IL-4-independent help for serum Ab responses in mice sensitized to peanut antigen by the nasal route
Our previous studies employed C57BL/6 mice and the Th2-inducer adjuvant cholera toxin. Since IL-4/13 dKO mice were only available on the BALB/c background, we examined control BALB/c mice. The difference observed between OS and NS C57BL/6 mice in IgE levels and IgG1/IgG2a ratio was maintained in BALB/c mice indicating that the BALB/c background also was suitable for our study (data not shown). Figure 1 shows that the IL-4/13 dKO mice also exhibited a pronounced reduction of the magnitude of IgG responses, regardless of the oral or nasal route of sensitization. In addition, IgG1 levels were diminished most dramatically in IL-4/IL-13 double KO mice, making their IgG1/IgG2a ratio significantly lower than that of WT mice (0.99 +/- 0.17 and 1.3 +/- 0.12 respectively, in OS mice; 0.86 +/- 0.06 and 1.07 +/- 0.05 respectively, in NS mice).
Contribution of IL-12 and IFN-γ to serum antibody responses in mice orally or nasally sensitized to peanut antigen
The IL-12p40 KO mice sensitized orally displayed lower levels of peanut-specific IgG3 Abs than WT mice (Figure 2). On the other hand, peanut-specific IgG3 were not affected in NS IL-12p40 KO mice, which displayed lower levels of peanut-specific IgG1 and IgG2b Abs than control WT mice (Figure 2). The IFN-γ KO mice showed no detectable levels of peanut-specific IgG2a Abs after either oral or nasal sensitization (Figure 2) and this finding sets the IFN-γ KO mice apart from the IL-12p40 KO mice. The levels of the other IgG subclasses remained unchanged in OS IFN-γ KO mice suggesting that IFN-γ mostly controls IgG2a Abs after OS. In contrast to OS mice, the NS IFN-γ KO mice exhibited increased levels of peanut-specific IgG3 and decreased IgG2b Abs. Despite the well-described cross-regulatory functions of IL-4 and IFN-γ, we failed to detect enhanced IgE responses in IL-12 or IFN-γ KO mice orally or nasally sensitized to peanut in the presence of cholera toxin (data not shown).
Figure 2.
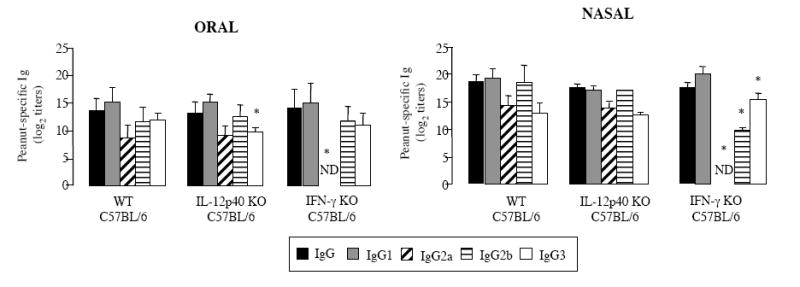
Plasma IgG and IgG-subclass responses of C57BL/6 WT, IL-12p40 KO and IFN-γ KO mice. Mice were sensitized on days 0 and 7. Plasma samples were collected on day 14 and Ab responses were analyzed by ELISA. Results are expressed as means of individual titles ± SD (5 mice per group). (* p < 0.05).
Lung inflammatory responses to antigen challenge in mice lacking Th1 and Th2 signaling and sensitized by the oral or nasal route
One week after the last immunization and before challenge, the cell densities in the lungs were not significantly different between WT and IL-4 KO mice (Figure 3A). In contrast, IL-4/13 dKO mice had higher cell densities in the lungs, regardless of the route of sensitization (Figure 3A). No signs of lung inflammation were seen in OS WT, IL-12 KO or IFN-γ KO mice before nasal challenge with the antigen (Figure 3A). The moderate lung inflammation observed in NS WT mice before challenge also was seen in IL-12 KO mice, but not NS IFN-γ KO mice, suggesting a role for IFN-γ in this pre-challenge inflammation.
Figure 3.
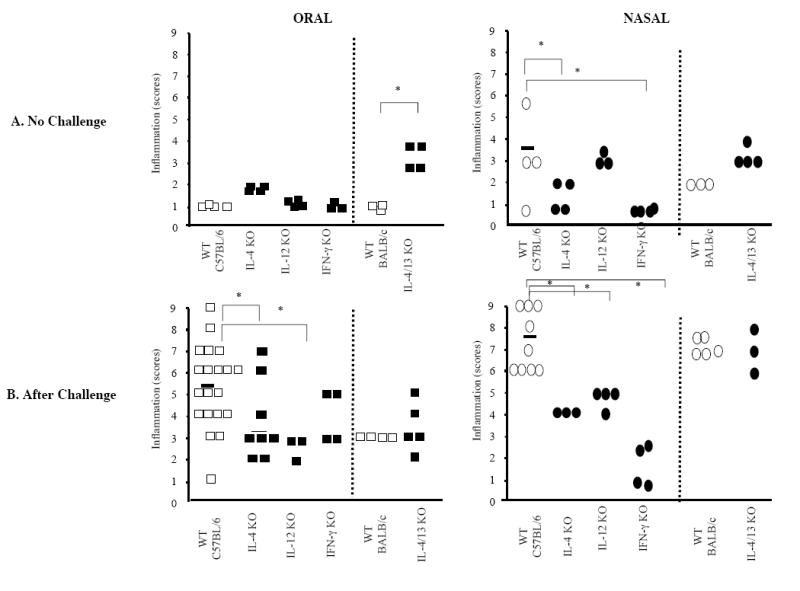
Lung inflammatory responses in WT, and Th1 and Th2 cytokine deficient mice sensitized to peanut by the oral or nasal route. Mice were sensitized on days 0 and 7. Lung tissue was collected on day 17 before (A) or after peanut nasal challenge on days 15 and 16 (B). The density of perivascular and peribronchial infiltrates was determined in a blinded fashion on a subjective 9-point scale (1 = minimal infiltrate; 9 = massive infiltrate). Each point (box or oval) represents an individual mouse. (* p < 0.05).
As previously reported [11]nasal peanut challenge of OS or NS WT mice led to airway hyper-reactivity and lung inflammatory responses characteristic of eosinophil- and neutrophil-mediated asthma (Figure 3B). After intranasal peanut challenge, both OS and NS IL-4 KO mice showed less inflammation than the WT mice (Figure 3B). Interestingly, the lung inflammation was not reduced in IL-4/13 dKO mice (Figure 3B), suggesting t that compensatory mechanisms enhancing pro-inflammatory responses could take place in IL-4/13 dKO mice. The absence of IL-12 substantially reduced airway inflammation in OS mice in response to intranasal antigen challenge (Figure 3B). Reduced, but nonetheless significant, airway inflammation was observed in NS IL-12 KO mice (Figure 3B). Unlike IL-12, the lack of IFN-γ completely abrogated lung inflammation in NS mice, while it was only partially reduced in OS mice.
Flow cytometry analysis of lung mononuclear cell showed that for both C57BL/6 and BALB/c WT mice, macrophages represented the bulk of cells recruited into the lung of after intranasal challenge (Figure 4). As previously seen in C57BL/6 WT mice [11], a higher percentage of lung cells expressed MHC class II molecules (i.e., I-Ab positive) in OS versus NS mice (Figure 4). The most striking effect due to the lack of both IL-4 and IL-13 was the reduction of macrophage recruitment in NS mice after peanut challenge and the considerably lower expression of costimulatory molecules in both OS and NS mice (Figure 4). A similar pattern was seen in mice lacking IFN-γ (data not shown).
Figures 4.
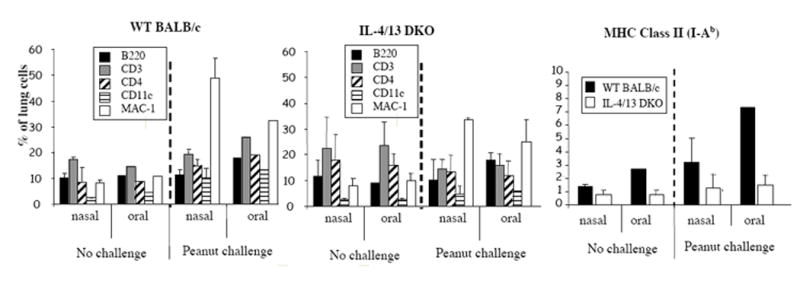
Phenotype of lung mononuclear cells in after nasal peanut challenge.. Lung tissues were collected on day 17 from mice orally or nasally immunized with PPE and either not challenged or challenged intranasally with PPE on days 15 and 16. Results are expressed as means ± SD (3-5 mice per group)
Discussion
Most current animal models mimic the Th2-mediated eosinophilic asthma. The mechanisms underlying Th1- [8-10] and neutrophil-associated [22] airway inflammation, as well as the reciprocal regulation of Th1 and Th2 subsets in eosinophil- and neutrophil-mediated asthma, remain poorly understood. Our study demonstrated a differential requirement for Th2 (IL-4 and IL-13) and Th1 (IL-12 and IFN-γ) cytokines for Ab responses induced by oral versus nasal sensitization. We also found that both Th1 and Th2 cytokines are required in both models for optimal lung reactivity to nasal peanut challenge.
It is welll established that IL-4 is a major inducer of IgE and IgG1 producing cells in mice [23, 24]. In line with many previous reports [25, 26], no IgE Abs were detected in IL-4 KO or IL-4/13 dKO mice. Deficiency in endogenous IL-4 was reported to reduce IgG1 response, while enhancing IgG2a and IgG2b Abs [26, 27]. We observed such an effect in OS mice. In contrast with previous study where Ag-specific IgG1 levels were completely abrogated in IL-4 and IL-13 double KO [28], IgG1 Abs were not reduced in IL-4/13 dKO mice after either oral or nasal sensitization.
The IL-4 KO mice were reported to both develop less peribronchial inflammation and eosinophilia than their WT counterparts [29, 30], and more severe eosinophilic inflammation [31, 32]. Conflicting results also were reported about the role of IL-13 in the development of allergic asthma [33, 34]. In our studies, the attenuated recruitment of cells into the lungs of IL-4 KO mice confirms a key role for IL-4 in inflammatory responses of OS mice, but also argue for a crucial role of IL-4 in inflammatory responses of NS mice, despite a predominantly Th1 environment. We were most surprised by the enhanced lung inflammation seen in IL-4/13 dKO mice. This observation also supports the hypothesis of compensatory mechanisms promoting Th1 and proinflammatory responses in IL-4/13 dKO mice.
Our results undeniably demonstrate that the Th1 pathway is required for full lung reactivity in both eosinophil- and neutrophil-associated asthma. The lack of peanut-specific IgG2a Abs in IFN-γ KO mice was in line with the known role of this cytokine for B-cell switch to IgG2a production [35]. More interesting was our observation that IFN-γ deficiency reduces antigen-specific IgG2b and increased IgG3, but only in NS mice, which suggests a limited role for Th1 cells in antigen priming by the GI tract. Nonetheless, IFN-γ appear to play a role during secondary Ag exposures, regardless of the initial mucosal route of sensitization. Both enhanced [36] and reduced [37] eosinophil recruitment into the airways were reported in IL-12 KO mice. The latter study also reported that antigen-specific IgE, IgG1 and IgG2a Ab levels were not affected in IL-12 KO mice. In line with this report, no compensatory Th2 responses were seen IL-12 KO mice, suggesting a sustained IFN-γ and Th1 component supported by IL-18 or other members of the IL-12 family. It is worth noting that IL-12p40 KO mice in our model of eosinophilic lung inflammation displayed significantly reduced cell infiltration into the lung. In this regard, IL-12p40 recently was shown to contribute to the generation of a Th2-type environment in a mouse model of allergic diarrhea [38]. Finally, the fact that lung inflammation was reduced in OS IFN-γ KO mice and NS IL-4 KO mice, which did not display generalized alteration of serum Ab responses, suggests that Th1 and Th2 cytokines are important during both the initial sensitization and the challenge phase.
Acknowledgments
The authors thank Mrs. Annette Pitts for technical assistance with histology.
Grants support: NIH Grants AI 18958, AI 43197 and DC 04976, and the French Ministry of Education.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maddox L, Schwartz DA. The pathophysiology of asthma. Annu Rev Med. 2002;53:477–498. doi: 10.1146/annurev.med.53.082901.103921. [DOI] [PubMed] [Google Scholar]
- 2.Romagnani S. Cytokines and chemoattractants in allergic inflammation. Mol Immunol. 2002;38:881–885. doi: 10.1016/s0161-5890(02)00013-5. [DOI] [PubMed] [Google Scholar]
- 3.Douwes J, Gibson P, Pekkanen J, Pearce N. Non-eosinophilic asthma: importance and possible mechanisms. Thorax. 2002;57:643–648. doi: 10.1136/thorax.57.7.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linden A. Role of interleukin-17 and the neutrophil in asthma. Int Arch Allergy Immunol. 2001;126:179–184. doi: 10.1159/000049511. [DOI] [PubMed] [Google Scholar]
- 5.Wenzel SE, Schwartz LB, Langmack EL, Halliday JL, Trudeau JB, Gibbs RL, Chu HW. Evidence that severe asthma can be divided pathologically into two inflammatory subtypes with distinct physiologic and clinical characteristics. Am J Respir Crit Care Med. 1999;160:1001–1008. doi: 10.1164/ajrccm.160.3.9812110. [DOI] [PubMed] [Google Scholar]
- 6.Herz U, Renz H, Wiedermann U. Animal models of type I allergy using recombinant allergens. Methods. 2004;32:271–280. doi: 10.1016/j.ymeth.2003.08.013. [DOI] [PubMed] [Google Scholar]
- 7.Kips JC, Anderson GP, Fredberg JJ, Herz U, Inman MD, Jordana M, Kemeny DM, Lotvall J, Pauwels RA, Plopper CG, Schmidt D, Sterk PJ, Van Oosterhout AJ, Vargaftig BB, Chung KF. Murine models of asthma. Eur Respir J. 2003;22:374–382. doi: 10.1183/09031936.03.00026403. [DOI] [PubMed] [Google Scholar]
- 8.Huang TJ, MacAry PA, Eynott P, Moussavi A, Daniel KC, Askenase PW, Kemeny DM, Chung KF. Allergen-specific Th1 cells counteract efferent Th2 cell-dependent bronchial hyperresponsiveness and eosinophilic inflammation partly via IFN-gamma. J Immunol. 2001;166:207–217. doi: 10.4049/jimmunol.166.1.207. [DOI] [PubMed] [Google Scholar]
- 9.Randolph DA, Carruthers CJ, Szabo SJ, Murphy KM, Chaplin DD. Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma. J Immunol. 1999;162:2375–2383. [PubMed] [Google Scholar]
- 10.Randolph DA, Stephens R, Carruthers CJ, Chaplin DD. Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation. J Clin Invest. 1999;104:1021–1029. doi: 10.1172/JCI7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer R, McGhee JR, Vu HL, Atkinson TP, Jackson RJ, Tome D, Boyaka PN. Oral and nasal sensitization promote distinct immune responses and lung reactivity in a mouse model of peanut allergy. Am J Pathol. 2005;167:1621–1630. doi: 10.1016/S0002-9440(10)61246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bodey KJ, Semper AE, Redington AE, Madden J, Teran LM, Holgate ST, Frew AJ. Cytokine profiles of BAL T cells and T-cell clones obtained from human asthmatic airways after local allergen challenge. Allergy. 1999;54:1083–1093. doi: 10.1034/j.1398-9995.1999.00889.x. [DOI] [PubMed] [Google Scholar]
- 13.Ford JG, Rennick D, Donaldson DD, Venkayya R, McArthur C, Hansell E, Kurup VP, Warnock M, Grunig G. Il-13 and IFN-gamma: interactions in lung inflammation. J Immunol. 2001;167:1769–1777. doi: 10.4049/jimmunol.167.3.1769. [DOI] [PubMed] [Google Scholar]
- 14.Wild JS, Sigounas A, Sur N, Siddiqui MS, Alam R, Kurimoto M, Sur S. IFN-gammainducing factor (IL-18) increases allergic sensitization, serum IgE, Th2 cytokines, and airway eosinophilia in a mouse model of allergic asthma. J Immunol. 2000;164:2701–2710. doi: 10.4049/jimmunol.164.5.2701. [DOI] [PubMed] [Google Scholar]
- 15.Medoff BD, Sauty A, Tager AM, Maclean JA, Smith RN, Mathew A, Dufour JH, Luster AD. IFN-gamma-inducible protein 10 (CXCL10) contributes to airway hyperreactivity and airway inflammation in a mouse model of asthma. J Immunol. 2002;168:5278–5286. doi: 10.4049/jimmunol.168.10.5278. [DOI] [PubMed] [Google Scholar]
- 16.Marinaro M, Boyaka PN, Jackson RJ, Finkelman FD, Kiyono H, Jirillo E, McGhee JR. Use of intranasal IL-12 to target predominantly Th1 responses to nasal and Th2 responses to oral vaccines given with cholera toxin. J Immunol. 1999;162:114–121. [PubMed] [Google Scholar]
- 17.Wynn TA, Jankovic D, Hieny S, Zioncheck K, Jardieu P, Cheever AW, Sher A. IL-12 exacerbates rather than suppresses T helper 2-dependent pathology in the absence of endogenous IFN-gamma. J Immunol. 1995;154:3999–4009. [PubMed] [Google Scholar]
- 18.Bernhisel-Broadbent J, Sampson HA. Cross-allergenicity in the legume botanical family in children with food hypersensitivity. J Allergy Clin Immunol. 1989;83:435–440. doi: 10.1016/0091-6749(89)90130-9. [DOI] [PubMed] [Google Scholar]
- 19.Visweswaraiah A, Novotny LA, Hjemdahl-Monsen EJ, Bakaletz LO, Thanavala Y. Tracking the tissue distribution of marker dye following intranasal delivery in mice and chinchillas: a multifactorial analysis of parameters affecting nasal retention. Vaccine. 2002;20:3209–3220. doi: 10.1016/s0264-410x(02)00247-5. [DOI] [PubMed] [Google Scholar]
- 20.Marinaro M, Staats HF, Hiroi T, Jackson RJ, Coste M, Boyaka PN, Okahashi N, Yamamoto M, Kiyono H, Bluethmann H, Fujihashi K, McGhee JR. Mucosal adjuvant effect of cholera toxin in mice results from induction of T helper 2 (Th2) cells and IL-4. J Immunol. 1995;155:4621–4629. [PubMed] [Google Scholar]
- 21.Lehrer SB, Reish R, Fernandes J, Gaudry P, Dai G, Reese G. Enhancement of murine IgE antibody detection by IgG removal. J Immunol Methods. 2004;284:1–6. doi: 10.1016/j.jim.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 22.Takaoka A, Tanaka Y, Tsuji T, Jinushi T, Hoshino A, Asakura Y, Mita Y, Watanabe K, Nakaike S, Togashi Y, Koda T, Matsushima K, Nishimura T. A critical role for mouse CXC chemokine(s) in pulmonary neutrophilia during Th type 1-dependent airway inflammation. J Immunol. 2001;167:2349–2353. doi: 10.4049/jimmunol.167.4.2349. [DOI] [PubMed] [Google Scholar]
- 23.Bergstedt-Lindqvist S, Moon HB, Persson U, Moller G, Heusser C, Severinson E. Interleukin 4 instructs uncommitted B lymphocytes to switch to IgG1 and IgE. Eur J Immunol. 1988;18:1073–1077. doi: 10.1002/eji.1830180716. [DOI] [PubMed] [Google Scholar]
- 24.Finkelman FD, Katona IM, Urban JF, Jr, Holmes J, Ohara J, Tung AS, Sample JV, Paul WE. IL-4 is required to generate and sustain in vivo IgE responses. J Immunol. 1988;141:2335–2341. [PubMed] [Google Scholar]
- 25.Kuhn R, Rajewsky K, Muller W. Generation and analysis of interleukin-4 deficient mice. Science. 1991;254:707–710. doi: 10.1126/science.1948049. [DOI] [PubMed] [Google Scholar]
- 26.Okahashi N, Yamamoto M, Vancott JL, Chatfield SN, Roberts M, Bluethmann H, Hiroi T, Kiyono H, McGhee JR. Oral immunization of interleukin-4 (IL-4) knockout mice with a recombinant Salmonella strain or cholera toxin reveals that CD4+ Th2 cells producing IL-6 and IL-10 are associated with mucosal immunoglobulin A responses. Infect Immun. 1996;64:1516–1525. doi: 10.1128/iai.64.5.1516-1525.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vajdy M, Kosco-Vilbois MH, Kopf M, Kohler G, Lycke N. Impaired mucosal immune responses in interleukin 4-targeted mice. J Exp Med. 1995;181:41–53. doi: 10.1084/jem.181.1.41. [DOI] [PubMed] [Google Scholar]
- 28.McKenzie GJ, Fallon PG, Emson CL, Grencis RK, McKenzie AN. Simultaneous disruption of interleukin (IL)-4 and IL-13 defines individual roles in T helper cell type 2-mediated responses. J Exp Med. 1999;189:1565–1572. doi: 10.1084/jem.189.10.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brusselle GG, Kips JC, Tavernier JH, van der Heyden JG, Cuvelier CA, Pauwels RA, Bluethmann H. Attenuation of allergic airway inflammation in IL-4 deficient mice. Clin Exp Allergy. 1994;24:73–80. doi: 10.1111/j.1365-2222.1994.tb00920.x. [DOI] [PubMed] [Google Scholar]
- 30.Lukacs NW, Strieter RM, Chensue SW, Kunkel SL. Interleukin-4-dependent pulmonary eosinophil infiltration in a murine model of asthma. Am J Respir Cell Mol Biol. 1994;10:526–532. doi: 10.1165/ajrcmb.10.5.8179915. [DOI] [PubMed] [Google Scholar]
- 31.Hogan SP, Mould A, Kikutani H, Ramsay AJ, Foster PS. Aeroallergen-induced eosinophilic inflammation, lung damage, and airways hyperreactivity in mice can occur independently of IL-4 and allergen-specific immunoglobulins. J Clin Invest. 1997;99:1329–1339. doi: 10.1172/JCI119292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okano M, Satoskar AR, Abe M, Harn DA, Jr, Okano M, Nishizaki K, Takeda Y, Yoshino T, Brombacher F, Satoskar AA. Interleukin-4-independent production of Th2 cytokines by nasal lymphocytes and nasal eosinophilia in murine allergic rhinitis. Allergy. 2000;55:723–731. doi: 10.1034/j.1398-9995.2000.00429.x. [DOI] [PubMed] [Google Scholar]
- 33.Walter DM, McIntire JJ, Berry G, McKenzie AN, Donaldson DD, DeKruyff RH, Umetsu DT. Critical role for IL-13 in the development of allergen-induced airway hyperreactivity. J Immunol. 2001;167:4668–4675. doi: 10.4049/jimmunol.167.8.4668. [DOI] [PubMed] [Google Scholar]
- 34.Webb DC, McKenzie AN, Koskinen AM, Yang M, Mattes J, Foster PS. Integrated signals between IL-13, IL-4, and IL-5 regulate airways hyperreactivity. J Immunol. 2000;165:108–113. doi: 10.4049/jimmunol.165.1.108. [DOI] [PubMed] [Google Scholar]
- 35.Finkelman FD, Holmes J, Katona IM, Urban JF, Jr, Beckmann MP, Park LS, Schooley KA, Coffman RL, Mosmann TR, Paul WE. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. doi: 10.1146/annurev.iy.08.040190.001511. [DOI] [PubMed] [Google Scholar]
- 36.Zhao LL, Linden A, Sjostrand M, Cui ZH, Lotvall J, Jordana M. IL-12 regulates bone marrow eosinophilia and airway eotaxin levels induced by airway allergen exposure. Allergy. 2000;55:749–756. doi: 10.1034/j.1398-9995.2000.00583.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang S, Fan Y, Han X, Yang J, Bilenki L, Yang X. IL-12-dependent vascular cell adhesion molecule-1 expression contributes to airway eosinophilic inflammation in a mouse model of asthma-like reaction. J Immunol. 2001;166:2741–2749. doi: 10.4049/jimmunol.166.4.2741. [DOI] [PubMed] [Google Scholar]
- 38.Hino A, Kweon MN, Fujihashi K, McGhee JR, Kiyono H. Pathological role of large intestinal IL-12p40 for the induction of Th2-type allergic diarrhea. Am J Pathol. 2004;164:1327–1335. doi: 10.1016/S0002-9440(10)63219-1. [DOI] [PMC free article] [PubMed] [Google Scholar]


