Abstract
Background
Height, a marker of childhood environmental exposures, is positively associated with prostate cancer risk, perhaps through the insulin-like growth factor system. We investigated the relationship of prostate cancer with height and its components (leg and trunk length) in a nested case-control study and with height in a dose-response meta-analysis.
Methods
We nested a case-control study within a population-based randomized controlled trial evaluating treatments for localized prostate cancer in British men ages 50 to 69 years, including 1,357 cases detected through prostate-specific antigen testing and 7,990 controls (matched on age, general practice, assessment date). Nine bibliographic databases were searched systematically for studies on the height-prostate cancer association that were pooled in a meta-analysis.
Results
Based on the nested case-control, the odds ratio (OR) of prostate-specific antigen-detected prostate cancer per 10 cm increase in height was 1.06 [95% confidence interval (95% CI): 0.97-1.16; ptrend = 0.2]. There was stronger evidence of an association of height with high-grade prostate cancer (OR: 1.23; 95% CI: 1.06-1.43), mainly due to the leg component, but not with low-grade disease (OR: 0.99; 95% CI: 0.90-1.10). In general, associations with leg or trunk length were similar. A meta-analysis of 58 studies found evidence that height is positively associated with prostate cancer (random-effects OR per 10 cm: 1.06; 95% CI: 1.03-1.09), with a stronger effect for prospective studies of more advanced/aggressive cancers (random-effects OR: 1.12; 95% CI: 1.05-1.19).
Conclusion
These data indicate a limited role for childhood environmental exposures—as indexed by adult height—on prostate cancer incidence, while suggesting a greater role for progression, through mechanisms requiring further investigation.
Introduction
A growing body of evidence indicates that greater height—a marker of diet and health throughout the growing years (1)—is positively associated with prostate cancer risk. Many cohort and case-control studies published thus far show an increase in risk of up to 20% to 40% for the top compared with the bottom height quantiles, suggesting the possibility of a long-term influence of childhood nutrition on carcinogenesis (2). Many studies are based on very small numbers, overall findings are inconsistent, and few studies assess the components of height (leg and trunk length) in relation to prostate cancer. Examining the relationship of cancer with leg and trunk length may indicate sensitive periods during childhood growth when risk factors or biological mechanisms underlying height-cancer associations operate (3). It has been shown that prepubertal growth is due more to an increase in leg length rather than trunk length (4), and leg length is sensitive to maternal smoking in pregnancy (5), socioeconomic conditions, nutrition (in particular milk intake; ref. 6), and energy intake in prepubertal children (7). Trunk length reflects factors influencing pubertal growth (7, 8) and is affected by “shrinkage” in late adult life due to compression of the spine (9).
The most plausible biological mechanism for the association of height with prostate cancer is that involving insulin-like growth factor-I (IGF-I) levels in childhood, of which height is a marker (10). In turn, IGF-I levels in adulthood are positively associated with prostate cancer (11).
We investigated the relationship of height and its components to prostate cancer in a case-control study nested within the intensive population-based prostate-specific antigen (PSA) testing phase of the Prostate Testing for Cancer and Treatment (ProtecT) study (12).
In addition, we placed our results in the context of the available evidence by systematically reviewing the published literature on the association between height and prostate cancer and meta-analyzing dose-response estimates derived from retrieved studies. Two previous reviews have been published on this topic (3, 13), but only one included a meta-analysis and was not specifically focused on height as it was part of a broader study on body size and composition (13). Compared with previous studies, our analysis explored in greater detail sources of heterogeneity, including publication bias and the influence of PSA testing on effect estimates (14, 15). We performed additional analysis in the ProtecT study and the meta-analysis by distinguishing between clinical subgroups (defined by grade and/or stage), where possible, to explore whether height could have differing effects on prostate cancer initiation versus progression.
Subjects and Methods
Nested Case-Control Study
Prostate Cancer Detection
ProtecT is an ongoing randomized controlled trial, evaluating the effectiveness of treatment for clinically localized prostate cancer (12). Men aged 50 to 69 years registered with 400 general practices located around nine U.K. cities are invited to a prostate check clinic where PSA testing is carried out. Men with an increased PSA (≥3 ng/mL) are invited for digital rectal examination, repeat PSA test, and transrectal ultrasound-guided biopsy (10 cores).
Men with a normal biopsy are offered repeat biopsy if the PSA is persistently increased or if there is a high index of clinical suspicion (chiefly evidence of high-grade prostatic intraepithelial neoplasia or suspicious features on initial biopsy). Histologic material obtained at biopsy is reviewed by specialized pathologists and given a Gleason score; tumors with a Gleason score ≥7 were considered high grade. Clinical staging used the tumor-node-metastasis staging system (16). Cases with stages T1-T2 and NXM0 were classified as localized cancers; those with T3-T4 were classified as advanced prostate cancers.
Case-Control Selection
This study is nested within the ProtecT PSA-tested cohort. Cases were men aged 50 to 69 years with histologically confirmed prostate cancer, detected among the 59,217 men who attended for PSA testing and had their PSA result recorded between November 2001 and November 2006. Prostate cancers diagnosed over 2 years after the initial PSA test were excluded from this analysis to distinguish “PSA-detected” from possible “incident” cancers. During this period, 6,329 men (11%) had increased PSA levels; of these, 2,022 (32%) had histologically confirmed prostate cancer.
All participants in the ProtecT cohort who had no evidence of prostate cancer were eligible for selection as controls. These included all men with a PSA below 3.0 ng/mL and any men with a PSA above this threshold who were biopsy negative. Cases were frequency matched to up to six controls by age of attendance at the check clinic (5-year age bands) and the general practice from which they were recruited. As the clinics were held and completed in each general practice in turn, matching for general practice automatically matched for the calendar date of recruitment.
All study participants gave fully informed consent for the use of their data for research purposes. The study received ethical approval from Trent MREC.
Exposure Assessment
All participants were asked to complete a health and lifestyle questionnaire after the check clinic but before their PSA result was available. The questionnaire included the following questions on height and leg length: “How tall are you?” (feet and inches) and “What is your inside leg measurement? (If you do not know, please examine a pair of your trousers)” (inches). All measures were converted to centimeters, and trunk length was calculated as total height minus leg length (estimated from inside leg measurement). Self-reported data on current weight, lifestyle, diet, comorbidities, occupation, ethnicity, and early-life factors (including birth weight and number of siblings) were also obtained from the questionnaires.
Overall 1,419 (70% of the total) men with histologically confirmed prostate cancer [1,230 (87%) localized cancers] and 8,343 controls returned the questionnaire and completed the sections on height and leg length. Sixty-two cases and 353 controls were excluded because of implausible values; thus, the analysis was based on 1,357 (67% of the total) cases (1,180 localized) and 7,990 controls.
Statistical Analysis of the ProtecT Nested Case-Control Study
Conditional logistic regression was used to estimate odds ratios (OR) and 95% confidence intervals (95% CI) for the association of height, leg, and trunk length with PSA-detected prostate cancer. As the men were stratum-matched in broad 5-year age bands (5 years), and the risk of prostate cancer increases steeply with age, age as a continuous variable was also entered in all models. ORs were compared across quartiles of height measures, using cutoffs derived from the distribution of controls and the lowest quartile as the reference group. Estimates of dose-response effects were then derived, per 10 cm increase in height and 5 cm increase in leg or trunk length, and per SD increase for all exposures, by fitting models with the exposures as continuous variables. Wald tests were used to test for linear trend across the height distribution. Additional models for leg length were adjusted for trunk length and vice versa, to account for total height and investigate whether the biologically relevant measurement is disproportion or absolute length of the leg or trunk (17). Genetic susceptibility, as indexed by family history of prostate cancer, and early life factors correlated with adult height were adjusted for in multivariable models. These factors included self-reported birth weight, number of siblings, and occupational socioeconomic class (dichotomized as manual and nonmanual, according to their main occupation throughout life). Number of siblings and socioeconomic class may index some of the putative risk factors for prostate cancer that height is a marker for, rather than confound the height-cancer association, so the fully adjusted multivariable models were interpreted with caution.
To investigate whether associations of height with prostate cancer differed for sporadic compared with familial tumors, age, or ethnic groups—which have very different baseline rates of incident and fatal disease—we tested for interactions using likelihood ratio tests.
Associations of cancer risk with height measures were assessed separately by stage, using multinomial logistic regression adjusted for the matching factors, with the outcome variable grouped into three categories: controls, localized cases (stage T1 or T2; NXM0), and advanced cases (stage T3 or T4). Similarly, low-grade (Gleason sum <7) and high-grade (Gleason sum ≥7) cases were compared with controls in multinomial regression models. Heterogeneity in associations of height with localized compared with advanced stage or low-grade compared with high-grade cancers were tested using Wald tests.
A different probability of detecting prostate cancer in taller men compared with shorter men could bias the estimates of height-prostate cancer association. To evaluate the likelihood of this particular detection bias, the mean percentage variation of PSA levels across quartiles of height among controls was estimated by fitting linear regression models to logged PSA values, adjusting for age, center, and date of PSA testing.
Stata 10.1 was used for all statistical analyses (Stata Corp.).
Systematic Review
This review was undertaken as part of a systematic review on the associations of food, nutrition, and physical activity with prostate cancer, funded by the World Cancer Research Fund (18). The review protocol is publicly available (19) and details on searches, inclusion criteria, data extraction procedures, and statistical analysis are available in the Supplementary Methods.
We fitted to the data both fixed-effect and random-effects models; we gave preference to the random-effects meta-analysis, which accounts for between-study heterogeneity.
Sensitivity Analysis
Study Quality
No set of quality criteria on the design and analysis of observational studies is available. It is generally agreed that cohort studies provide higher quality/more robust evidence than case-control studies. Therefore, results from prospective studies (including cohort, case-cohort and nested case-control, and hereafter referred to as “cohort”) were analyzed separately from case-control studies, and further subgroup analyses were done on cohort results only. For the initial (main) pooled analysis, case-control studies were classified based on the selection of controls as stated by the authors. We indicated as “same population” case-control studies those in which the sampled controls were such that they would have become cases (according to the study’s definition of case) had they developed the disease, and all the others as “non-same population,” which we considered to be of lower quality. Differences across study design were formally tested by fitting meta-regression models.
Publication Bias
Most prospective studies collect baseline data on anthropometry. Out of 31 publications from cohort studies included in this meta-analysis, 19 reported on height or anthropometry as (one of) their primary exposure(s) in the abstract: 18 from the systematic review and the ProtecT nested case-control study. However, 12 articles, not mentioning height in the title or abstract, included results on the height-prostate cancer association in the full text and we classified these as “incidental” findings. To assess whether positive results on the height-prostate cancer association were more likely to be published and included in the abstract, we further distinguished between primary versus incidental reporting and tested for the difference in the pooled effect estimates by fitting a linear meta-regression model.
Detection Bias
The advent of widespread use of PSA screening started in the early 1990s and changed the nature of the cases being identified from more advanced to early/small lesions. Choosing 1990 as the temporal cutoff, we did a further subgroup analysis, stratifying on whether the follow-up period was mostly pre-PSA (>50% of follow-up occurring <1990) or during the PSA era. The contribution of the proportion of pre-PSA follow-up time to explaining heterogeneity was explored in a meta-regression analysis.
Biological Significance
Because it is not known whether the mechanisms underlying the association with height act on progression rather than initiation, increasing the risk of poorly rather than well-differentiated lesions, we did subgroup analyses after restricting to data on advanced or aggressive cancers (available from 13 cohort studies). We referred to “advanced or aggressive” cases if the article specified any of the following in their case definition: (a) T stage 3 to 4 on the American Joint Committee on Cancer 1992 classification, (b) advanced cancer, (c) advanced or metastatic cancer, (d) metastatic cancer, (e) stage C or D on the Whitmore/Jewett scale, (f) fatal cancer, (g) “high-stage” or “high-grade,” or (h) Gleason score ≥7.
Due to the poor reporting of ethnicity, no stratified analysis was attempted. Results on subgroups of black men available from three cohort (20, 21) and two same population case-control studies (22, 23) from the United States were presented in narrative form.
Results
ProtecT Nested Case-Control Study
Of the 1,357 PSA-detected cases of prostate cancer included in the analysis, 173 had advanced-stage disease (missing for 4 cases) and 402 had Gleason score ≥7 (missing for 19 cases; ≥8 for 59 cases). The distribution of demographic, anthropometric, and socioeconomic characteristics of the 1,357 cases and 7,990 controls are presented in Table 1.
Table 1. Characteristics of participants included in the analysis—1,357 cases and 7,990 controls, frequency-matched on 5-y age band and general practice.
| Controls |
Cases |
|||
|---|---|---|---|---|
| n * | Mean (SD) or % | n * | Mean (SD) or % | |
| Age (y) | 7,990 | 61.8 (5.0) | 1,357 | 62.2 (4.9) |
| Height (cm) | 7,990 | 176.0 (6.7) | 1,357 | 176.2 (6.8) |
| Leg length (cm) | 7,990 | 76.6 (4.3) | 1,357 | 76.7 (4.4) |
| Trunk length (cm) | 7,990 | 99.3 (4.7) | 1,357 | 99.5 (4.7) |
| BMI (kg/m2) | 7,893 | 27.1 (3.8) | 1,341 | 26.7 (3.5) |
| Birth weight (kg) | 4,305 | 3.47 (0.72) | 684 | 3.46 (0.71) |
| Family history of prostate cancer | 7,990 | 5.2% | 1,357 | 7.4% |
| White ethnic origin | 7,912 | 99.0% | 1,330 | 98.7% |
| 3+ siblings (vs <3) | 7,750 | 33.8% | 1,300 | 31.0% |
| Nonmanual occupation (vs manual) † | 5,519 | 45.8% | 975 | 45.7% |
Abbreviation: BMI, body mass index.
Number with complete data.
Nonmanual occupation includes codes for professional, managerial, nonmanual, and skilled nonmanual occupations. Manual occupation includes codes for manual and skilled manual, semiskilled, and unskilled manual occupations.
There was a 6% (95% CI, −3;-16%) increased risk of PSA-detected prostate cancer per 10 cm increase in height but the statistical evidence supporting this association was weak (ptrend = 0.2). There was no evidence that associations were stronger comparing leg length versus trunk length (Table 2). Comparable results were found for localized and advanced-stage prostate cancer end points, for all height measures (all p for differences in effect estimates by stage ≥0.74; Table 2). Models including simultaneously leg and trunk length yielded very similar effect estimates to the ones from this main analysis (data not shown).
Table 2. Associations of height and height components with PSA-detected prostate cancer, in 1,357 cases and 7,990 controls, frequency-matched on 5-y age band and general practice.
| Quartiles* |
Dose-response† | p trend | p ‡ | ||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||||
| Total prostate cancer§ (n = 1,357) | |||||||
| Height | 1.00 | 1.06 (0.88-1.29) | 1.05 (0.90-1.22) | 1.11 (0.93-1.33) | 1.06 (0.97-1.16) | 0.20 | - |
| Leg length | 1.00 | 1.05 (0.87-1.27) | 1.01 (0.87-1.17) | 1.08 (0.92-1.27) | 1.03 (0.96-1.10) | 0.47 | - |
| Trunk length | 1.00 | 1.07 (0.89-1.28) | 1.01 (0.84-1.22) | 1.11 (0.94-1.32) | 1.04 (0.98-1.10) | 0.24 | - |
| Localized prostate cancer∥ (n = 1,180) | |||||||
| Height | 1.00 | 1.06 (0.85-1.28) | 1.07 (0.91-1.26) | 1.09 (0.90-1.32) | 1.06 (0.96-1.16) | 0.20 | - |
| Leg length | 1.00 | 1.04 (0.85-1.27) | 1.01 (0.86-1.19) | 1.09 (0.92-1.29) | 1.02 (0.95-1.10) | 0.49 | - |
| Trunk length | 1.00 | 1.08 (0.89-1.31) | 1.01 (0.82-1.23) | 1.13 (0.94-1.35) | 1.04 (0.97-1.11) | 0.23 | - |
| Advanced prostate cancer∥ (n = 173) | |||||||
| Height | 1.00 | 1.00 (0.63-1.61) | 0.82 (0.56-1.21) | 1.07 (0.69-1.67) | 1.02 (0.81-1.28) | 0.86 | 0.74 |
| Leg length | 1.00 | 1.02 (0.64-1.64) | 0.88 (0.59-1.31) | 0.99 (0.65-1.49) | 1.00 (0.84-1.19) | 0.99 | 0.78 |
| Trunk length | 1.00 | 0.89 (0.54-1.40) | 0.89 (0.49-1.30) | 0.97 (0.64-1.55) | 1.02 (0.87-1.19) | 0.80 | 0.83 |
| Low-grade prostate cancer∥ (n = 936) | |||||||
| Height | 1.00 | 0.97 (0.78-1.21) | 0.98 (0.82-1.17) | 1.01 (0.82-1.24) | 0.99 (0.90-1.10) | 0.87 | - |
| Leg length | 1.00 | 0.96 (0.77-1.19) | 0.96 (0.81-1.14) | 0.94 (0.78-1.14) | 0.97 (0.90-1.05) | 0.49 | - |
| Trunk length | 1.00 | 1.01 (0.82-1.25) | 1.04 (0.84-1.28) | 1.08 (0.88-1.32) | 1.02 (0.94-1.09) | 0.69 | - |
| High-grade prostate cancer∥ (n = 402) | |||||||
| Height | 1.00 | 1.31 (0.95-1.82) | 1.21 (0.92-1.58) | 1.39 (1.02-1.89) | 1.23 (1.06-1.43) | <0.01 | 0.02 |
| Leg length | 1.00 | 1.19 (0.87-1.65) | 1.13 (0.87-1.47) | 1.41 (1.08-1.84) | 1.14 (1.02-1.28) | 0.02 | 0.02 |
| Trunk length | 1.00 | 1.17 (0.86-1.59) | 0.89 (0.64-1.23) | 1.21 (0.90-1.63) | 1.10 (0.99-1.23) | 0.07 | 0.20 |
Cutoffs based on distribution of controls only. Quartile cutoffs are as follows: height 172.5, 175, 181 cm; leg length 74, 77, 80 cm; trunk length 96, 99, 101.6 cm.
Height: per 10 cm increase. Leg length and trunk length: per 5 cm increase.
Test for difference in the effect estimates for localized versus advanced and low-grade versus high-grade prostate cancer.
OR and 95% CI values from conditional logistic regression models, additionally adjusted for age as a continuous variable.
Relative risk ratios and 95% CIs from logistic regression, adjusted for matching factors. For definitions of localized/advanced stage and low/high grade, see Subjects and Methods.
There was no evidence of an increased risk of low-grade tumors with increasing height, leg length, or trunk length. However, for high-grade tumors, we found strong evidence of a 23% increase in risk per 10 cm increase in height (ptrend < 0.01), with a p value of 0.02 for the difference in height associations for low-grade compared with high-grade cancers (Table 2). This seemed to be mainly due to the leg component and in particular to long-for-total-height legs, as in a model including both height components there remained some evidence for an effect of leg length (OR per 5 cm increase, 1.13; 95% CI, 1.01-1.27; ptrend = 0.04), but less so for trunk length (OR per 5 cm increase, 1.09; 95% CI, 0.98-1.21; ptrend = 0.12). As a sensitivity analysis, we reclassified as high grade only those cases with Gleason ≥8 and obtained similar point estimates with wider confidence intervals due to the limited number of cases (n = 59). Results were very similar when ORs were estimated per SD increase in height (SD 6.7 cm), leg length (SD 4.3 cm), and trunk length (SD 4.7 cm; data not shown). Further adjustments for family history of prostate cancer, socioeconomic position, birth weight, or number of siblings did not change the estimates and are thus not reported. There was no evidence of effect modification by family history of prostate cancer, age, or self-reported ethnic origin.
An analysis adjusted for age, center, and date of PSA testing showed evidence that PSA values were on average 9% lower (95% CI, 4-14%, p = 0.001) for controls in the tallest quartile compared with those in the shortest quartile. For both leg and trunk length, the corresponding average PSA difference was 6% (95% CI, 1-10%, p = 0.02).
Systematic Review
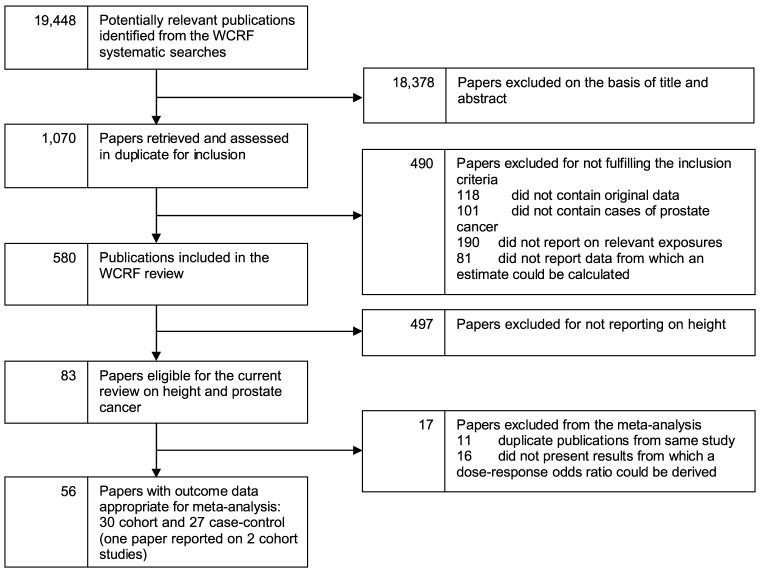
The search strategy for the broad World Cancer Research Fund review yielded 19,448 hits, of which 1,070 were retrieved for full-text screening. From these, results on 30 cohort (20, 21, 24-48) and 27 case-control studies (22, 23, 49-73) could be included in the current meta-analysis (Fig. 1). Table 3 presents the characteristics of studies identified through the systematic searches and included in the meta-analysis. We included 20 of the 21 cohorts and all 18 case-control studies that were in the meta-analysis of MacInnis et al. (13), although the article by Giovannucci et al. (74) was excluded as we included a more recent report from the same study (43). Additionally, we included three more cohorts (36, 42, 75) and five case-control studies (57, 60, 64, 65, 70) published in the period covered by MacInnis’ searches (up to October 31, 2004), and six (26, 35, 38, 39, 46, 47) and four (61, 66, 67, 69) new studies published between November 1, 2004, and July 31, 2007.
Figure 1.
Flow diagram of study selection.
Table 3. Characteristics of studies (from the systematic review) included in the dose-response meta-analysis for height and prostate cancer.
| A. Prospective studies | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| First author (year) | Location | Ethnicity | Reporting (incidental or primary) | Age at baseline (y)* | Dates of recruitment and follow-up | % Follow-up <1990 | Exposure assessment | Outcome | No. cases |
| Greenwald (1974; ref. 31) | Harvard (USA) | White | Incidental | NS | 1880-1916 to 1967 | 100 | Mixed | Mortality | 268 |
| Albanes (1988; ref. 24) | USA | Multiethnic | Primary | (52) | 1971-1975 to 1982-84 | 100 | Measured | Incidence | 95 |
| Le Marchand (1994; ref. 37) | Hawaii (USA) | Multiethnic | Incidental | 45-90 | 1975-1980 to 1989 | 100 | Self-report | Incidence | 198 |
| Thune (1994; ref. 75) | Norway | NS | Incidental | 35-54 | 1972-1978 to 1991 | 94 | Measured | Incidence | 220 |
| Tulinius (1997; ref. 48) | Iceland | NS | Primary | 23-60 | 1967-1995 to 1995 | 64 | Measured | Incidence | 524 |
| Hebert (1997; ref. 33) | USA | NS | Primary | 40-84 | 1982 to 1995 | 62 | Self-report | Incidence | 1,047 |
| Andersson (1997; ref. 25) | Sweden | NS | Primary | 40+ | 1971-1975 to 1991 | 94 | Measured | Incidence | 2,368 |
| Mortality | 708 | ||||||||
| Cerhan (1997; ref. 27) | Iowa (USA) | NS | Incidental | 65-101 | 1982 to 1993 | 73 | Self-report | Incidence | 71 |
| Veierod (1997; ref. 93) | Norway | NS | Incidental | 16-56 | 1977-1983 to 1992 | 83 | Measured | Incidence | 72 |
| Nilsen (1999; ref. 41) | Norway | NS | Primary | (59) | 1984-1986 to 1997 | 42 | Measured | I+M | 642 |
| Davey-Smith (2000; ref. 28) | Scotland (UK) | NS | Primary | 45-64 | 1972-1976 to 1996 | 73 | Measured | Mortality | 59 |
| Habel (2000; ref. 20) | USA | Multiethnic | Primary | 18-84 | 1964-1973 to 1996 | 78 | Measured | Incidence | 2,079 |
| Putnam (2000; ref. 44) | Iowa (USA) | White | Primary | 40-86 | 1986-1989 to 1995 | 33 | Self-report | Incidence | 101 |
| Schuurman (2000; ref. 45) | The Netherlands | NS | Primary | 55-69 | 1986 to 1992 | 67 | Self-report | Incidence | 681 |
| Rodriguez (2001; ref. 21) | USA (CPS I) | Multiethnic | Primary | (52) | 1959 to 1972 | 100 | Self-report | Mortality | 1,590 |
| Rodriguez (2001; ref. 21) | USA (CPS II) | Multiethnic | Primary | (57) | 1982 to 1996 | 57 | Self-report | Mortality | 3,622 |
| Freeman (2001; ref. 30) | USA | Multiethnic | Primary | 50+ | 1986 to 1994 | 50 | Self-report | Mortality | 633 |
| Jonsson (2003; ref. 34) | Sweden | NS | Incidental | 44-83 | 1961-1967 to 1997 | 79 | Self-report | I+M | 631 |
| Engeland (2003; ref. 29) | Norway | NS | Primary | 20-74 | 1963 to 2001 | 71 | Measured | I+M | 33,314 |
| MacInnis (2003; ref. 40) | Australia | NS | Incidental | 27-75 | 1990-1994 to 2002 | 0 | Measured | Incidence | 477 |
| Gunnell (2003; ref. 32) | South Wales (UK) | NS | Primary | 45-59 | 1979-1983 to 2003 | 41 | Measured | Incidence | 33 |
| Lamharzi (2003; ref. 36) | USA | Multiethnic | Incidental | 45-69 | 1985-1994 to 1998 | 6 | Measured | Incidence | 300 |
| Platz (2004; ref. 43) | USA | White | Incidental | 40-75 | 1993-1995 to 1998 | 0 | Self-report | I+M | 460 |
| Platz (2004; ref. 42) | Maryland (USA) | White | Incidental | (65) | 1989 to 2002 | 8 | Self-report | Incidence | 264 |
| Stattin (2004; ref. 47) | Sweden | NS | Incidental | 40+ | 1985-1994 to 2001 | 4 | Measured | Incidence | 265 |
| Batty (2006; ref. 26) | London (UK) | NS | Primary | 40-64 | 1967-1970 to 2002 | 64 | Measured | Mortality | 434 |
| Lund Haheim (2006; ref. 39) | Norway | NS | Incidental | 40-49 | 1972-1973 to 1998 | 70 | Measured | Incidence | 507 |
| Kurahashi (2006; ref. 35) | Japan | NS | Primary | 40-69 | 1990-1993 to 2003 | 0 | Self-report | Incidence | 311 |
| Sequoia (2006; ref. 46) | Finland | White | Primary | 50-69 | 1985-1988 to 1993 | 54 | Measured | Incidence | 1,346 |
| Littman (2007; ref. 38) | Washington State (USA) | Multiethnic | Primary | 50-76 | 2000-2002 to 2004 | 0 | Self-report | Incidence | 832 |
| B. Case-control studies | ||||||
|---|---|---|---|---|---|---|
| First author (year) | Location | Ethnicity | Mean age (cases, controls) | Exposure assessment | Outcome | No. cases/controls |
| Same-Population case-control studies | ||||||
| Kolonel (1988; ref. 63) | Hawaii (USA) | Multiethnic | NS | NS | I+P | 452/899 |
| Fincham (1990; ref. 52) | Canada | NS | NS | Self-report | Incidence | 382/NS |
| Walker (1992; ref. 72) | South Africa | Black | (69.2, 69.6) | Measured | PP | 166/166 |
| Whittemore (1995; ref. 23) | USA | Multiethnic | (70.8, 70.2) | Mixed | Prevalence | 1,655/1,645 |
| Andersson (1995; ref. 49) | Sweden | NS | (70.0, 69.8) | Measured | Incidence | 256/252 |
| Key (1997; ref. 62) | UK | White | (68.1, 68.1) | NS | Incidence | 328/267 |
| Villeneuve (1999; ref. 71) | Canada | Multiethnic | NS | Self-report | I+P | 1,623/1,623 |
| Hayes (1999; ref. 22) | USA | Multiethnic | 40-79† | Mixed | Incidence | 934/1,201 |
| Norrish (2000; ref. 68) | New Zealand | NS | 40-80† | Self-report | Incidence | 317/480 |
| Hsing (2000; ref. 59) | Shangai (China) | NS | (72.7, 73.1) | Mixed | Incidence | 238/471 |
| Giles (2003; ref. 55) | Australia | NS | (60.3, 60.6) | Self-report | Incidence | 1,476/1,409 |
| Lightfoot (2004; ref. 66) | Canada | White | (68.0, 67.8) | Self-report | PP | 760/1,632 |
| Friedenreich (2004; ref. 53) | Canada | NS | NS | Mixed | Incidence | 988/1,063 |
| John (2005; ref. 61) | San Francisco (USA) | Non-Hispanic white | (64.0, 65.0) | Measured | Incidence | 450/455 |
| Porter (2005; ref. 69) | Washington State (USA) | Multiethnic | (58.0, 58.0) | Self-report | Incidence | 753/703 |
| Non-same-population case-control studies | ||||||
| Koppel (1967; ref. 64) | Philadelphia (USA) | Multiethnic | (72.2, 71.5) | Measured | PP | 83/83 |
| Wynder (1971; ref. 73) | NYC (USA) | Multiethnic | (65.2, 64.4) | NS | PP | 300/400 |
| Hill (1982; ref. 57) | South Africa | Black | (70.0, 66.0) | Measured | PP | 21/6 |
| La Vecchia (1990; ref. 65) | Milan (Italy) | NS | (65.0, 55.0) | Self-report | PP | 80/1971 |
| Hayes (1992; ref. 56) | The Netherlands | NS | (63.1, 60.7) | Self-report | PP | 100/113 |
| Denmark-Wahnefried (1997; ref. 51) | USA | Multiethnic | NS | Measured | Incidence | 159/156 |
| Furuya (1998; ref. 54) | Chiba (Japan) | NS | (72.8, 69.5) | NS | Incidence | 329/190 |
| Rao (1999; ref. 70) | Toronto (Canada) | NS | (62.0, 65.1) | NS | PP | 12/12 |
| Hsieh (1999; ref. 58) | Athens (Greece) | NS | (71.2, 70.4) | Self-report | Incidence | 320/246 |
| Huang (2003; ref. 60) | Taiwan | NS | (71.5, 71.7) | NS | Incidence | 66/104 |
| Dal Maso (2004; ref. 50) | Italy | NS | (66.0, 63.0) | Self-report | Incidence | 1,294/1,451 |
| Liu (2005; ref. 67) | USA | Multiethnic | (61.0, 63.0) | Self-report | Prevalence | 439/479 |
Abbreviations: NS, not stated; I+M, incidence and mortality; I+P, incidence and prevalence; PP, presumed prevalence.
Range or mean (if in brackets).
Age range of both cases and controls.
Overall, the meta-analysis pooling adjusted results from all 58 studies found evidence of a modest increase in risk of prostate cancer per 10 cm increase in height (random-effects OR, 1.06; 95% CI, 1.03-1.09; Fig. 2).
Figure 2.
Association of height and prostate cancer—random-effects meta-analysis of adjusted risk ratio (RR) per 10 cm increase in height, plotted on the log scale, stratified by study design [cohort, same-population (SP) and non-same population (NSP) case-control studies]. I-V, inverse-probability weighting model (fixed-effect); D+L, DerSimonian and Laird model (random effects).
Results from the Egger test showed no evidence of small study effects for case-control studies, but some for prospective studies (cohorts, p = 0.013), which was not confirmed by Begg’s test (cohorts: p = 0.424). There was no clear indication of asymmetry from the funnel plot, for any of the study designs (Supplementary Fig. S1).
Sensitivity Analysis
Study Quality
There was evidence of heterogeneity across study design (pheterogeneity = 0.001; Fig. 2). Evidence for, and the magnitude of, the effect of height on prostate cancer were strongest for cohorts (OR, 1.09; 95% CI, 1.06-1.12, n = 31) and heterogeneity was lower (I2 = 23%) compared with all case-control studies. Only weak evidence of an association of height with prostate cancer was found pooling same-population case-control studies (OR, 1.03; 95% CI, 0.97-1.10, I2 = 36%, n = 15), and there was no evidence of a height-prostate cancer association for non-same population studies (OR, 0.98; 95% CI, 0.86-1.10; I2 = 49%, n = 12; Fig. 2).
Publication Bias
There was evidence that pooled estimates differed according to whether the study reported on height as (one of) the primary exposure(s) or only incidentally (pheterogeneity = 0.002; Table 4). Based on data from the 19 publications focused on height-cancer associations, there was evidence of an 11% increase in prostate cancer risk per 10 cm increase in height (95% CI, 9-13%; I2 = 7%). In contrast, the 12 studies reporting results as incidental findings yielded an OR of 1.01 (95% CI, 0.95-1.07; I2 = 0%; Table 4).
Table 4. Pooled-effect estimates for the associations of height and prostate cancer from prospective studies only, by selected study characteristics.
| No. studies | Pooled dose-response* | Heterogeneity (I2), % | p † | |
|---|---|---|---|---|
| All cohorts | 31 | 1.09 (1.06-1.12) | 23.4 | |
| Advanced/aggressive/fatal prostate cancers only | 13 | 1.12 (1.05-1.19) | 47.3 | |
| Incidental reporting of height findings | 12 | 1.01 (0.95-1.07) | 0.0 | <0.01 |
| Primary reporting of height findings | 19 | 1.11 (1.09-1.13) | 6.6 | |
| Pre-PSA era (>50% of follow-up occurring <1990) | 19 | 1.10 (1.06-1.13) | 28.7 | 0.12 |
| PSA era (≤50% of follow-up occurring <1990) | 12 | 1.07 (1.02-1.12) | 4.3 |
Pooled-effect estimates per 10 cm increase in height, and 95% CI, from random-effects models using adjusted results.
P value for heterogeneity between strata, from linear meta-regression models.
Detection Bias
When stratifying on whether the follow-up period was mostly in the pre-PSA era (>50% of follow-up occurring <1990) or during the PSA era, we found similar effects (pheterogeneity = 0.12), with the former studies carrying more heterogeneity (I2 = 29% versus 4%; Table 4).
Stage and Grade
Results based on advanced or aggressive cancer outcomes, including fatal disease, were available from 13 cohorts (12 from the review and the current ProtecT data) and showed marked between-study heterogeneity (I2 = 47%; Table 4). The pooled estimate was higher than for the all-prostate cancer analysis based on prospective studies (OR, 1.12; 95% CI, 1.05-1.19).
Ethnicity
None of the five studies (all from the United States) presenting ethnicity-specific results found evidence of a height-prostate cancer association among high-risk ethnic groups. Habel et al. (20) reported a hazard ratio of 0.75 (95% CI, 0.42-1.31) comparing the top versus bottom quintile of height among black men in a cohort study. Results on black men were included in a publication with results from rounds I and II of the Cancer Prevention Study: the hazard ratio for prostate cancer comparing black men ≥70 versus ≤66 inches were 0.63 (95% CI, 0.32-1.25) and 0.89 (95% CI, 0.66-1.21), respectively (21). One case-control study did not find any difference in mean height comparing black cases and controls (23). Another presented an OR of 0.9 (95% CI, 0.6-1.5) comparing black men who were tall in childhood versus those who were short (22).
Discussion
Based on a meta-analysis of 58 studies, including new results from a nested case-control of PSA-detected prostate cancer (ProtecT), we found evidence that greater stature is associated with an increased prostate cancer risk. The overall magnitude of the effect was modest and varied with study design, yet results from cohort studies were compatible with a 6% to 12% increase in risk per 10 cm increase in height, and a 5% to 19% increase in risk for more advanced and/or aggressive cancers. Between-study heterogeneity was higher for case-control studies and for those cohorts including higher proportions of clinically detected, more advanced, or aggressive cancers. Results from the ProtecT nested case-control study were in line with the meta-analysis, with a stronger association for high-grade cancers.
This article benefits both from the strengths offered by analyzing original data and from the power of a meta-analysis based on inclusive systematic searches. The ProtecT case-control series is a population-based study that allowed us to investigate the separate effects of different height components on cancer risk, split by both stage and grade (76). These data are unlikely to be affected by detection bias for two reasons. First, all men had been invited to PSA testing and therefore the likelihood of cancer detection should be unrelated to confounding factors associated with access to or take up of PSA testing. Second, we showed that PSA levels among controls were not positively associated with height, ruling out PSA-mediated access to biopsy as a potential explanation for our findings. Others also found a lower probability of detecting prostate cancer following PSA testing in taller men (77-79). Histologic confirmation of all cancers guarantees high specificity, and the 10-core biopsy protocol standardized the diagnosis of prostate cancer.
Strengths of the meta-analysis include its large power, derived from pooling results on 68,133 cases overall (54,152 from cohorts and 13,981 from case-control studies) and using dose-response models to assess trend effects per 10 cm increase in height. The extensive effort of the searches allowed us to minimize the effect of search and publication bias and to explore the pattern of reporting of the height-prostate cancer associations in prospective studies, as well as other potential sources of between-study heterogeneity that we had specified a priori. One of these was detection bias, potentially introduced by opportunistic use of PSA testing starting from the 1990s and leading to over-diagnosis of microscopic lesions that could have otherwise remained asymptomatic (80, 81). The effect of height could be artifactually overestimated, if taller people, often more educated and better off (82), were also more likely to undergo screening (81, 83). In the absence of data on national PSA screening practices and coverage over time for the countries included in the meta-analysis, we compared studies whose case ascertainment occurred mainly before 1990 (including predominantly clinically presenting cases) with those conducted subsequently (including increasing proportions of asymptomatic cases) and found a modestly increased effect of height for the former, against the suggestion of detection bias.
The dose-dependent nature of the observed height-cancer association adds strength to the evidence of an effect. However, whether this is causal should be assessed in light of the limitations of the studies included in this meta-analysis. Marked differences were observed across study designs, which could be explained by different biases affecting them, mainly selection bias from choice of control population or differential participation in case-control studies. More detailed investigation of heterogeneity across cohort studies suggested that positive results were more likely to be highlighted in the title or abstract of a publication, whereas those null findings that were published were usually only found in the body of any article. However, we found no evidence of a small-study effect, suggesting that such positive results may not be spurious.
None of the well-established risk factors are likely to explain the association with height. Age, which was adjusted for in all prospective studies but one, is expected to be inversely associated with height, if anything (because of marked cohort effects on height). Adjustment for family history of prostate cancer, carried out by some studies including this one, did not appreciably alter the effect estimates. Ethnicity did not seem to play a role in height-cancer associations, its relationship with stature not being obvious and ethnic-specific results for height-prostate cancer generally being similar to those from multiethnic populations. There are reports of an increased risk of more advanced/fatal prostate cancer associated with obesity (84); however, adjustment for body mass index did not change the observed effect estimates in our nested case-control study.
The present nested case-control and most of the studies included in the meta-analysis used self-reported data on height (and leg length), which are subject to measurement error. However, a validation carried out on data from the pilot phase of the study based on 4,708 men found a correlation of 0.96 for measured versus self-reported height, and no systematic misreporting (85), in line with the literature (86, 87). Data on measured and self-reported (inside leg) leg length were available for 3,673 men and showed that inside leg is a shorter measure of leg length (by around 6 cm). However, the difference was consistent across the range of leg lengths (85); thus, it would not result in a biased estimate of the leg length-prostate cancer association. Moreover, any error is likely to be nondifferential, because both exposures were reported before knowledge of prostate cancer diagnosis. Such error would attenuate rather than explain the observed associations. The same is true for trunk length, derived from total height and leg length, which is likely to have produced a large measurement error. The ProtecT and other studies included in the meta-analysis recorded height of subjects late in life. However, shrinkage in middle-aged men could produce a dilution of the effect (especially for the trunk component) but should not affect cancer incidence or mortality (88).
The case definition in the ProtecT nested case-control only included men with screen-detected cancers. Although the association of height with prostate cancer is unlikely to be seen only in PSA-diagnosed men, this was done to ensure homogeneity in case definition. In any case, only two men in total were excluded who developed “incident” prostate cancer and the results are not altered by their inclusion in our analyses. It is possible that among our group of men with localized, low-grade prostate cancers, some would have been recently initiated and, despite aggressive potential in some, might not have had time to become more advanced. This heterogeneity in the localized, low-grade group may have attenuated any differences in associations between height and localized versus advanced cancers in the ProtecT study.
Between-study heterogeneity was investigated among the prospective studies. If height, as a marker of early environmental factors, had a different effect on various clinical subtypes of prostate cancer, then heterogeneity of findings could be due to differing proportions (or definitions) of the clinical subtypes. This was suggested by our ProtecT results for low-grade versus high-grade cancers and by the pooled estimate from cohorts reporting on advanced/aggressive cancers, consistently showing stronger effects compared with localized or well-differentiated cancers. It was also supported by the modest difference between estimates from pre-PSA and PSA era studies, the latter likely to include a higher proportion of smaller asymptomatic tumors. Therefore, factors associated with height could be risk factors for progression to fatal prostate cancer, rather than for the initiation of a well-differentiated tumor (76).
A plausible mechanism to explain the association of height with breast and prostate cancer risk involves dietary programming of the IGF-I system (89, 90). IGF-I plays an important role in the regulation of postnatal growth, and there is evidence that its levels in adulthood can be influenced (“programmed”) by dietary manipulation in early childhood (91). IGF-I levels are also associated with prepubertal growth in height (10), although no stronger association was found for leg versus trunk length in the current study and in three published cohort studies (24, 32, 92). In line with our height findings, there is some evidence that associations of IGF-I with prostate cancer are stronger for advanced versus local disease (11). It is therefore possible that variations in the IGF-I system may underlie associations of height with prostate cancers that are more likely to progress (11).
We conclude that there is evidence that height is positively associated with prostate cancer risk, but the magnitude of the effect is modest and the literature is influenced by publication bias. Overall, these data indicate only a small role for childhood environmental exposures—as indexed by adult height—on prostate cancer incidence. However, the positive association with high-grade cancers in ProtecT was consistent with the evidence on advanced or aggressive cancers from the meta-analysis, suggesting that early life environmental factors may play some role in the development and/or progression of neoplasia with a worse prognosis. Mechanisms that could underlie a height effect on the more aggressive forms of the disease now need to be elucidated.
Supplementary Material
Acknowledgments
We thank the tremendous contribution of all members of the ProtecT study research group and especially the following who were involved in this research: Prasad Bollina, Sue Bonnington, Debbie Cooper, Andrew Doble, Alan Doherty, Emma Elliott, David Gillatt, Pippa Herbert, Peter Holding, Joanne Howson, Mandy Jones, Roger Kockelbergh, Howard Kynaston, Teresa Lennon, Norma Lyons, Hilary Moody, Philip Powell, Stephen Prescott, Liz Salter, and Pauline Thompson. We thank Prof. Jonathan Sterne, Dr. Trudy Bekkering, Margaret Burke, and Dr. Anne-Marie Mayer for their help and assistance at different stages of the systematic review.
Grant support: The ProtecT trial is funded by the National Health Service Health Technology Assessment Program (projects 96/20/06, 96/20/99). The database and analytical support for epidemiological studies was supported by a project grant from the National Cancer Research Institute (administered by the Medical Research Council), which provided funding through ProMPT (Prostate Mechanisms of Progression and Treatment), and grants from Cancer Research UK (project grant C18281/A7062) and the World Cancer Research Fund (grants 2006/15 and 2007/07). The systematic review was funded by the World Cancer Research Fund. L. Zuccolo was partially funded by a fellowship from the Cancer Epidemiology Unit, CeRMS and CPO Piemonte, University of Turin, Turin (Italy), and partly by a Medical Research Council Special Training Fellowship. The sponsors had no role in the analysis, the interpretation of the results, the preparation of the manuscript, or the decision to submit the manuscript for publication.
Footnotes
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Department of Health disclaimer: The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Department of Health.
References
- 1.Tanner JM. Foetus into man: physical growth from conception to maturity. Ware (United Kingdom): Castlemead Publications; 1989. [Google Scholar]
- 2.Gunnell D. Can adult anthropometry be used as a ‘biomarker’ for prenatal and childhood exposures? Int J Epidemiol. 2002;31:390–4. [PubMed] [Google Scholar]
- 3.Gunnell D, Okasha M, Smith GD, Oliver SE, Sandhu J, Holly JMP. Height, leg length, and cancer risk: a systematic review. Epidemiol Rev. 2001;23:313–42. doi: 10.1093/oxfordjournals.epirev.a000809. [DOI] [PubMed] [Google Scholar]
- 4.Leitch I. Growth and health. Br J Nutr. 1951;5:142–51. doi: 10.1079/bjn19510017. [DOI] [PubMed] [Google Scholar]
- 5.Leary S, Smith GD, Ness A. Smoking during pregnancy and components of stature in offspring. Am J Hum Biol. 2006;18:502–12. doi: 10.1002/ajhb.20518. [DOI] [PubMed] [Google Scholar]
- 6.Rogers I, Emmett P, Gunnell D, Dunger D, Holly J. Milk as a food for growth? The insulin-like growth factors link. Public Health Nutr. 2006;9:359–68. doi: 10.1079/phn2006853. [DOI] [PubMed] [Google Scholar]
- 7.Gunnell DJ, Smith GD, Frankel SJ, Kemp M, Peters TJ. Socio-economic and dietary influences on leg length and trunk length in childhood: a reanalysis of the Carnegie (Boyd Orr) survey of diet and health in prewar Britain (1937-39) Paediatr Perinat Epidemiol. 1998;12(Suppl 1):96–113. doi: 10.1046/j.1365-3016.1998.0120s1096.x. [DOI] [PubMed] [Google Scholar]
- 8.Wadsworth ME, Hardy RJ, Paul AA, Marshall SF, Cole TJ. Leg and trunk length at 43 years in relation to childhood health, diet and family circumstances; evidence from the 1946 national birth cohort. Int J Epidemiol. 2002;31:383–90. [PubMed] [Google Scholar]
- 9.Chandler PJ, Bock RD. Age changes in adult stature: trend estimation from mixed longitudinal data. Ann Hum Biol. 1991;18:433–40. doi: 10.1080/03014469100001732. [DOI] [PubMed] [Google Scholar]
- 10.Rogers I, Metcalfe C, Gunnell D, Emmett P, Dunger D, Holly J. Insulin-like growth factor-I and growth in height, leg length, and trunk length between ages 5 and 10 years. J Clin Endocrinol Metabol. 2006;91:2514–9. doi: 10.1210/jc.2006-0388. [DOI] [PubMed] [Google Scholar]
- 11.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–53. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 12.Donovan J, Mills N, Smith M, et al. Quality improvement report: improving design and conduct of randomised trials by embedding them in qualitative research: ProtecT (prostate testing for cancer and treatment) study. BMJ. 2002;325:766–70. doi: 10.1136/bmj.325.7367.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacInnis R, English D. Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control. 2006;17:989–1003. doi: 10.1007/s10552-006-0049-z. [DOI] [PubMed] [Google Scholar]
- 14.Hankey BF, Feuer EJ, Clegg LX, et al. Cancer surveillance series: interpreting trends in prostate cancer-part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017–24. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- 15.Kvale R, Auvinen A, Adami HO, et al. Interpreting trends in prostate cancer incidence and mortality in the five Nordic countries. J Natl Cancer Inst. 2007;99:1881–7. doi: 10.1093/jnci/djm249. [DOI] [PubMed] [Google Scholar]
- 16.Ohori M, Wheeler TM, Scardino PT. The new American Joint Committee on Cancer and International Union Against Cancer TNM classification of prostate cancer. Cancer. 1994;73:104–14. doi: 10.1002/1097-0142(19940701)74:1<104::aid-cncr2820740119>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 17.Gunnell D. Can adult anthropometry be used as a biomarker for prenatal and childhood exposures? Int J Epidemiol. 2002;31:390–4. [PubMed] [Google Scholar]
- 18.American Institute for Cancer Research and World Cancer Research Fund . Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington DC: AICR; 2007. [Google Scholar]
- 19.WCRF Systematic literature review protocol. [Accessed 2008 Jan 23]. WCRF Web site 2008 January 23. Available from http://www.wcrf.org/research/research_pdfs/prostate_protocol.pdf.
- 20.Habel LA, Van Den Eeden SK, Friedman GD. Body size, age at shaving initiation, and prostate cancer in a large, multiracial cohort. Prostate. 2000;43:136–43. doi: 10.1002/(sici)1097-0045(20000501)43:2<136::aid-pros8>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 21.Rodriguez C, Patel AV, Calle EE, Jacobs EJ, Chao A, Thun MJ. Body mass index, height, and prostate cancer mortality in two large cohorts of adult men in the United States. Cancer Epidemiol Biomarkers Prev. 2001;10:345–53. [PubMed] [Google Scholar]
- 22.Hayes RB, Ziegler RG, Gridley G, et al. Dietary factors and risks for prostate cancer among blacks and whites in the United States. Cancer Epidemiol Biomarkers Prev. 1999;8:25–34. [PubMed] [Google Scholar]
- 23.Whittemore AS, Kolonel LN, Wu AH, et al. Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. J. 1995;87:652–61. doi: 10.1093/jnci/87.9.652. [see comment] [DOI] [PubMed] [Google Scholar]
- 24.Albanes D, Jones DY, Schatzkin A, Micozzi MS, Taylor PR. Adult stature and risk of cancer. Cancer Res. 1988;48:1658–62. [PubMed] [Google Scholar]
- 25.Andersson SO, Wolk A, Bergstrom R, et al. Body size and prostate cancer: a 20-year follow-up study among 135006 Swedish construction workers. J. 1997;89:385–9. doi: 10.1093/jnci/89.5.385. [DOI] [PubMed] [Google Scholar]
- 26.Batty GD, Shipley MJ, Langenberg C, Marmot MG, Davey Smith G. Adult height in relation to mortality from 14 cancer sites in men in London (UK): evidence from the original Whitehall study. Ann Oncol. 2006;17:157–66. doi: 10.1093/annonc/mdj018. [DOI] [PubMed] [Google Scholar]
- 27.Cerhan JR, Torner JC, Lynch CF, et al. Association of smoking, body mass, and physical activity with risk of prostate cancer in the Iowa 65+ Rural Health Study (United States) Cancer Causes Control. 1997;8:229–38. doi: 10.1023/a:1018428531619. [DOI] [PubMed] [Google Scholar]
- 28.Davey-Smith G, Hart C, Upton M, et al. Height and risk of death among men and women: aetiological implications of associations with cardiorespiratory disease and cancer mortality. J Epidemiol Community Health. 2000;54:97–103. doi: 10.1136/jech.54.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Engeland A, Tretli S, Bjorge T. Height, body mass index, and prostate cancer: a follow-up of 950000 Norwegian men. Br J Cancer. 2003;89:1237–42. doi: 10.1038/sj.bjc.6601206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Freeman VL, Liao Y, Durazo-Arvizu R, Cooper RS. Height and risk of fatal prostate cancer: findings from the National Health Interview Survey (1986 to 1994) Ann Epidemiol. 2001;11:22–7. doi: 10.1016/s1047-2797(00)00172-1. [DOI] [PubMed] [Google Scholar]
- 31.Greenwald P, Damon A, Kirmss V, Polan AK. Physical and demographic features of men before developing cancer of the prostate. J. 1974;53:341–6. doi: 10.1093/jnci/53.2.341. [DOI] [PubMed] [Google Scholar]
- 32.Gunnell D, May M, Ben-Shlomo Y, Yarnell J, Smith GD. Height, leg length, and cancer: the Caerphilly Study. Nutr Cancer. 2003;47:34–9. doi: 10.1207/s15327914nc4701_4. [DOI] [PubMed] [Google Scholar]
- 33.Hebert PR, Ajani U, Cook NR, Lee IM, Chan KS, Hennekens CH. Adult height and incidence of cancer in male physicians (United States) Cancer Causes Control. 1997;8:591–7. doi: 10.1023/a:1018442329319. [DOI] [PubMed] [Google Scholar]
- 34.Jonsson F, Wolk A, Pedersen NL, et al. Obesity and hormone-dependent tumors: cohort and co-twin control studies based on the Swedish Twin Registry. Int J Cancer. 2003;106:594–9. doi: 10.1002/ijc.11266. [DOI] [PubMed] [Google Scholar]
- 35.Kurahashi N, Iwasaki M, Sasazuki S, Otani T, Inoue M, Tsugane S. Association of body mass index and height with risk of prostate cancer among middle-aged Japanese men. Br J Cancer. 2006;94:740–2. doi: 10.1038/sj.bjc.6602983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lamharzi N, Johnson MM, Goodman G, et al. Polymorphic markers in the 5A-reductase type II gene and the incidence of prostate cancer. Int J Cancer. 2003;105:480–3. doi: 10.1002/ijc.11126. [DOI] [PubMed] [Google Scholar]
- 37.Le Marchand L, Kolonel LN, Wilkens LR, Myers BC, Hirohata T. Animal fat consumption and prostate cancer: a prospective study in Hawaii. Epidemiology. 1994;5:276–82. doi: 10.1097/00001648-199405000-00004. [see comment] [DOI] [PubMed] [Google Scholar]
- 38.Littman AJ, White E, Kristal AR. Anthropometrics and prostate cancer risk. Am J Epidemiol. 2007;165:1271–9. doi: 10.1093/aje/kwm013. [DOI] [PubMed] [Google Scholar]
- 39.Lund HL, Wisloff TF, Holme I, Nafstad P. Metabolic syndrome predicts prostate cancer in a cohort of middle-aged Norwegian men followed for 27 years. Am J Epidemiol. 2006;164:769–74. doi: 10.1093/aje/kwj284. [DOI] [PubMed] [Google Scholar]
- 40.MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG. Body Size and composition and prostate cancer risk. Cancer Epidemiol Biomarkers Prev. 2003;12:1417–21. [PubMed] [Google Scholar]
- 41.Nilsen TI, Vatten LJ. Anthropometry and prostate cancer risk: a prospective study of 22,248 Norwegian men. Cancer Causes Control. 1999;10:269–75. doi: 10.1023/a:1008967330619. [DOI] [PubMed] [Google Scholar]
- 42.Platz EA, De Marzo AM, Erlinger TP, et al. No association between pre-diagnostic plasma C-reactive protein concentration and subsequent prostate cancer. Prostate. 2004;59:393–400. doi: 10.1002/pros.10368. [DOI] [PubMed] [Google Scholar]
- 43.Platz EA, Leitzmann MF, Hollis BW, Willett WC, Giovannucci E. Plasma 1,25-dihydroxy- and 25-hydroxyvitamin D and subsequent risk of prostate cancer. Cancer Causes Control. 2004;15:255–65. doi: 10.1023/B:CACO.0000024245.24880.8a. [DOI] [PubMed] [Google Scholar]
- 44.Putnam SD, Cerhan JR, Parker AS, et al. Lifestyle and anthropometric risk factors for prostate cancer in a cohort of Iowa men. Ann Epidemiol. 2000;10:361–9. doi: 10.1016/s1047-2797(00)00057-0. [DOI] [PubMed] [Google Scholar]
- 45.Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA. Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol. 2000;151:541–9. doi: 10.1093/oxfordjournals.aje.a010241. [see comment] [DOI] [PubMed] [Google Scholar]
- 46.Sequoia JSP, Wright ME, McCarron P, et al. A Prospective Investigation of Height and Prostate Cancer Risk. Cancer Epidemiol Biomarkers Prev. 2006;15:2174–8. doi: 10.1158/1055-9965.EPI-06-0467. [DOI] [PubMed] [Google Scholar]
- 47.Stattin P, Bylund A, Biessy C, Kaaks R, Hallmans G, Adlercreutz H. Prospective study of plasma enterolactone and prostate cancer risk (Sweden) Cancer Causes Control. 2004;15:1095–102. doi: 10.1007/s10552-004-1480-7. [DOI] [PubMed] [Google Scholar]
- 48.Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6:863–73. [PubMed] [Google Scholar]
- 49.Andersson SO, Baron J, Wolk A, Lindgren C, Bergstrom R, Adami HO. Early life risk factors for prostate cancer: a population-based case-control study in Sweden. Cancer Epidemiol Biomarkers Prev. 1995;4:187–92. [PubMed] [Google Scholar]
- 50.Dal ML, Zucchetto A, la VC, et al. Prostate cancer and body size at different ages: an Italian multicentre case-control study. Br J Cancer. 2004;90:2176–80. doi: 10.1038/sj.bjc.6601859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demark-Wahnefried W, Conaway MR, Robertson CN, Mathias BJ, Anderson EE, Paulson DF. Anthropometric risk factors for prostate cancer. Nutr Cancer. 1997;28:302–7. doi: 10.1080/01635589709514591. [DOI] [PubMed] [Google Scholar]
- 52.Fincham SM, Hill GB, Hanson J, Wijayasinghe C. Epidemiology of prostatic cancer: a case-control study. Prostate. 1990;17:189–206. doi: 10.1002/pros.2990170303. [DOI] [PubMed] [Google Scholar]
- 53.Friedenreich CM, McGregor SE, Courneya KS, Angyalfi SJ, Elliott FG. Case-control study of anthropometric measures and prostate cancer risk. Int J Cancer. 2004;110:278–83. doi: 10.1002/ijc.20110. [DOI] [PubMed] [Google Scholar]
- 54.Furuya Y, Akimoto S, Akakura K, Ito H. Smoking and obesity in relation to the etiology and disease progression of prostate cancer in Japan. Int J Urol. 1998;5:134–7. doi: 10.1111/j.1442-2042.1998.tb00261.x. [DOI] [PubMed] [Google Scholar]
- 55.Giles GG, Severi G, English DR, et al. Early growth, adult body size and prostate cancer risk. Int J Cancer. 2003;103:241–5. doi: 10.1002/ijc.10810. [DOI] [PubMed] [Google Scholar]
- 56.Hayes RB, de Jong FH, Raatgever J, et al. Physical characteristics and factors related to sexual development and behaviour and the risk for prostatic cancer. Eur J Cancer Prev. 1992;1:239–45. doi: 10.1097/00008469-199204000-00005. [DOI] [PubMed] [Google Scholar]
- 57.Hill P, Wynder EL, Garbaczewski L, Walker AR. Effect of diet on plasma and urinary hormones in South African black men with prostatic cancer. Cancer Res. 1982;42:3864–9. [PubMed] [Google Scholar]
- 58.Hsieh CC, Thanos A, Mitropoulos D, Deliveliotis C, Mantzoros CS, Trichopoulos D. Risk factors for prostate cancer: a case-control study in Greece. Int J Cancer. 1999;80:699–703. doi: 10.1002/(sici)1097-0215(19990301)80:5<699::aid-ijc12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 59.Hsing AW, Deng J, Sesterhenn IA, et al. Body size and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomarkers Prev. 2000;9:1335–41. [PubMed] [Google Scholar]
- 60.Huang SP, Chou YH, Chang WS, et al. Androgen receptor gene polymorphism and prostate cancer in Taiwan. J Formos Med Assoc. 2003;102:680–6. [PubMed] [Google Scholar]
- 61.John EM, Schwartz GG, Koo J, Van Den BD, Ingles SA. Sun exposure, vitamin D receptor gene polymorphisms, and risk of advanced prostate cancer. Cancer Res. 2005;65:5470–9. doi: 10.1158/0008-5472.CAN-04-3134. [DOI] [PubMed] [Google Scholar]
- 62.Key TJ, Silcocks PB, Davey GK, Appleby PN, Bishop DT. A case-control study of diet and prostate cancer. Br J Cancer. 1997;76:678–87. doi: 10.1038/bjc.1997.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kolonel LN, Yoshizawa CN, Hankin JH. Diet and prostatic cancer: a case-control study in Hawaii. Am J Epidemiol. 1988;127:999–1012. doi: 10.1093/oxfordjournals.aje.a114903. [DOI] [PubMed] [Google Scholar]
- 64.Koppel M, Meranze DR, Shimkin MB. Characteristics of patients with prostatic carcinoma: a control case study on 83 autopsy pairs. J Urol. 1967;98:229–33. doi: 10.1016/S0022-5347(17)62861-1. [DOI] [PubMed] [Google Scholar]
- 65.La Vecchia C, Negri E, Parazzini F, et al. Height and cancer risk in a network of case-control studies from Northern Italy. Int J Cancer. 1990;45:275–9. doi: 10.1002/ijc.2910450212. [DOI] [PubMed] [Google Scholar]
- 66.Lightfoot N, Conlon M, Kreiger N, Sass-Kortsak A, Purdham J, Darlington G. Medical history, sexual, and maturational factors and prostate cancer risk. Ann Epidemiol. 2004;14:655–62. doi: 10.1016/j.annepidem.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 67.Liu X, Rybicki BA, Casey G, Witte JS. Relationship between body size and prostate cancer in a sibling based case-control study. J Urol. 2005;174:2169–73. doi: 10.1097/01.ju.0000181207.02213.06. [DOI] [PubMed] [Google Scholar]
- 68.Norrish AE, McRae CU, Holdaway IM, Jackson RT. Height-related risk factors for prostate cancer. Br J Cancer. 2000;82:241–5. doi: 10.1054/bjoc.1999.0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Porter MP, Stanford JL. Obesity and the risk of prostate cancer. Prostate. 2005;62:316–21. doi: 10.1002/pros.20121. [DOI] [PubMed] [Google Scholar]
- 70.Rao AV, Fleshner N, Garwal S. Serum and tissue lycopene and biomarkers of oxidation in prostate cancer patients: a case-control study. Nutr Cancer. 1999;33:159–64. doi: 10.1207/S15327914NC330207. [DOI] [PubMed] [Google Scholar]
- 71.Villeneuve PJ, Johnson KC, Kreiger N, Mao Y, The Canadian Cancer Registries Epidemiology Research Group Risk factors for prostate cancer: results from the Canadian National Enhanced Cancer Surveillance System. Cancer Causes Control. 1999;10:355–67. doi: 10.1023/a:1008958103865. [DOI] [PubMed] [Google Scholar]
- 72.Walker AR, Walker BF, Tsotetsi NG, Sebitso C, Siwedi D, Walker AJ. Case-control study of prostate cancer in black patients in Soweto, South Africa. Br J Cancer. 1992;65:438–41. doi: 10.1038/bjc.1992.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wynder EL, Mabuchi K, Whitmore WF., Jr. Epidemiology of cancer of the prostate. Cancer. 1971;28:344–60. doi: 10.1002/1097-0142(197108)28:2<344::aid-cncr2820280214>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 74.Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC. Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomarkers Prev. 1997;6:557–63. [PubMed] [Google Scholar]
- 75.Thune I, Lund E. Physical activity and the risk of prostate and testicular cancer: a cohort study of 53,000 Norwegian men. Cancer Causes Control. 1994;5:549–56. doi: 10.1007/BF01831383. [DOI] [PubMed] [Google Scholar]
- 76.Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC. Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer. 2007;121:1571–8. doi: 10.1002/ijc.22788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fowke JH, Signorello LB, Chang SS, et al. Effects of obesity and height on prostate-specific antigen (PSA) and percentage of free PSA levels among African-American and Caucasian men. Cancer. 2006;107:2361–7. doi: 10.1002/cncr.22249. [DOI] [PubMed] [Google Scholar]
- 78.Werny DM, Thompson T, Saraiya M, et al. Obesity is negatively associated with prostate-specific antigen in U.S. men, 2001-2004. Cancer Epidemiol Biomarkers Prev. 2007;16:70–6. doi: 10.1158/1055-9965.EPI-06-0588. [DOI] [PubMed] [Google Scholar]
- 79.Fowke JH, Motley SS, Cookson MS, et al. The association between body size, prostate volume and prostate-specific antigen. Prostate Cancer Prostatic Dis. 2006;10:137–42. doi: 10.1038/sj.pcan.4500924. [DOI] [PubMed] [Google Scholar]
- 80.Draisma G, Boer R, Otto SJ, et al. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 81.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–90. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 82.Batty GD, Leon DA. Socio-economic position and coronary heart disease risk factors in children and young people: evidence from UK epidemiological studies. Eur J Public Health. 2002;12:263–72. doi: 10.1093/eurpub/12.4.263. [DOI] [PubMed] [Google Scholar]
- 83.Spencer BA, Babey SH, Etzioni DA, et al. A population-based survey of prostate-specific antigen testing among California men at higher risk for prostate carcinoma. Cancer. 2006;106:765–74. doi: 10.1002/cncr.21673. [DOI] [PubMed] [Google Scholar]
- 84.Freedland SJ, Platz EA. Obesity and prostate cancer: making sense out of apparently conflicting data. Epidemiol Rev. 2007;29:88–97. doi: 10.1093/epirev/mxm006. [DOI] [PubMed] [Google Scholar]
- 85.Oliver SE. PhD Thesis. Department of Social Medicine, University of Bristol; 2002. [Google Scholar]
- 86.Gunnell D, Berney L, Holland P, et al. How accurately are height, weight and leg length reported by the elderly, and how closely are they related to measurements recorded in childhood? Int J Epidemiol. 2000;29:456–64. [PubMed] [Google Scholar]
- 87.Spencer EA, Appleby PN, Davey GK, Key TJ. Validity of self-reported height and weight in 4808 EPIC-Oxford participants. Public Health Nutr. 2002;5:561–5. doi: 10.1079/PHN2001322. [DOI] [PubMed] [Google Scholar]
- 88.Wannamethee SG, Shaper AG, Lennon L, Whincup PH. Height loss in older men: associations with total mortality and incidence of cardiovascular disease. Arch Intern Med. 2006;166:2546–52. doi: 10.1001/archinte.166.22.2546. [DOI] [PubMed] [Google Scholar]
- 89.Chan JM, Stampfer MJ, Giovannucci E, et al. Plasma insulin-like growth factor I and prostate cancer risk: A prospective study. Science. 1998;279:563–6. doi: 10.1126/science.279.5350.563. [DOI] [PubMed] [Google Scholar]
- 90.Vatten LJ, Kvinnsland S. Body height and risk of breast cancer. A prospective study of 23,831 Norwegian women. Br J Cancer. 1990;61:881–5. doi: 10.1038/bjc.1990.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ben-Shlomo Y, Holly J, McCarthy A, Savage P, Davies D, Davey Smith G. Pre and post natal milk supplementation and adult insulin-like growth factor I: long term follow-up of a randomised controlled trial. Cancer Epidemiol Biomarkers Prev. 2005;14:1336–9. doi: 10.1158/1055-9965.EPI-04-0908. [DOI] [PubMed] [Google Scholar]
- 92.Chyou PH, Nomura AM, Stemmermann GN. A prospective study of weight, body mass index and other anthropometric measurements in relation to site-specific cancers. Int J Cancer. 1994;57:313–7. doi: 10.1002/ijc.2910570304. [DOI] [PubMed] [Google Scholar]
- 93.Veierod MB, Laake P, Thelle DS. Dietary fat intake and risk of prostate cancer: a prospective study of 25,708 Norwegian men. Int J Cancer. 1997;73:634–8. doi: 10.1002/(sici)1097-0215(19971127)73:5<634::aid-ijc4>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.