Abstract
Background
Although the efficacy of adjuvant chemotherapy in prolonging survival for women with breast cancer has been well documented, limited population-based information is available on the actual use of chemotherapy.
Objective
To examine the relationship between age and chemotherapy use.
Design
Cohort study.
Setting
New Mexico.
Patients
5101 women 20 years of age or older receiving a diagnosis of stage I, stage II, or stage IIIA breast cancer from 1991 through 1997.
Measurements
Pattern of chemotherapy use by age; logistic regression analysis to generate the odds and probabilities of receiving chemotherapy; and sensitivity analysis to estimate potential effects of unmeasured confounders.
Results
Overall, 29% of women received chemotherapy. The rate of chemotherapy use for women with stage I, stage II, or stage IIIA breast cancer was 11%, 47%, and 68%, respectively. Across all tumor stages, the use of chemotherapy decreased substantially with increasing age (P < 0.001). Overall, 66% of women younger than 45 years of age received chemotherapy compared with 44% of women between 50 and 54 years of age, 31% of women between 55 and 59 years of age, and 18% of women between 60 and 64 years of age. The decreasing pattern of chemotherapy use with age continued after adjustment for prognostic factors and was relatively insensitive to changes in unmeasured factors.
Conclusions
There is considerable discrepancy between the 1990 National Institutes of Health Consensus Conference recommendations for chemotherapy administration in women with breast cancer and the actual use of chemotherapy in the community. The decrease in use with age may relate to the decreasing efficacy of chemotherapy with age, as reported in clinical trials. Outcomes studies should address whether the recommendations are overly aggressive or whether practicing oncologists are too conservative in their use of chemotherapy.
Although the efficacy of chemotherapy in prolonging survival for women with breast cancer has been well documented (1–12), limited population-based information is available on the actual use of chemotherapy. Some hospital-based surveys of breast cancer have examined the use of chemotherapy (13–15), but the completeness of information has been questioned because chemotherapy is frequently administered in outpatient settings.
The evolution of recommendations about the use of adjuvant chemotherapy in women with early breast cancer is illustrated by the National Institutes of Health (NIH) consensus development conferences (1, 3, 12). In 1985, the consensus conference recommended chemotherapy for premenopausal women with lymph node–positive cancer (1). By 1990, the consensus conference recommended chemotherapy for both premenopausal and postmenopausal women with lymph node–positive cancer and for women with cancer confined to the breast but with poor prognostic features, such as large size or negative hormone receptor status (3). The 2000 consensus conference extended the recommendation of chemotherapy to premenopausal and postmenopausal women with node-positive tumors or with node-negative tumors greater than 1 cm in size, regardless of hormone receptor status (12).
Because limited information is available from clinical trials of chemotherapy in women 70 years of age and older, none of the consensus conferences made specific recommendations for that age group, other than to invoke individual decisions based on clinical circumstances and patient preferences. We recently reported on chemotherapy use in women age 65 years and older by using the Surveillance, Epidemiology, and End Results (SEER) tumor registry data linked to Medicare data (16, 17). As expected, chemotherapy use sharply decreased in women older than 70 years of age, and women with higher-stage, larger, or estrogen receptor–negative tumors were more likely to receive chemotherapy (16, 17).
We review the use of chemotherapy in women residing in New Mexico who were 20 years of age or older and received a diagnosis of breast cancer between 1991 and 1997. We hypothesized that chemotherapy use would not vary by age in women younger than 65 years of age who have tumor characteristics for which chemotherapy is generally recommended. Furthermore, we hypothesized that use of chemotherapy would vary by age with highest use in younger women (<45 years of age) among women who had tumors with characteristics for which no clear consensus recommendations had been made.
Methods
Data Source
The New Mexico Tumor Registry is a statewide, population-based tumor registry that was established in 1966; it is one of the seven original members of the SEER registry (18). New Mexico residents who are given a diagnosis and treated at facilities outside the state are identified through data exchange with surrounding state registries in Colorado, Arizona, Utah, and Texas; in addition, information is obtained from the New Mexico Bureau of Vital Records and Health Statistics and from pathology laboratories and hospitals that operate close to New Mexico borders (19).
Patients
We examined data on 5101 patients age 20 years or older with a diagnosis of stage I, stage II, or stage IIIA breast cancer (using the American Joint Committee on Cancer staging system) from 1991 through 1997 (19, 20). We restricted our analyses to these stages because chemotherapy is considered the primary treatment for higher cancer stages rather than an adjuvant treatment. The Institutional Review Boards of the University of Texas Medical Branch and the University of New Mexico approved this study.
Chemotherapy
Information on chemotherapy was coded as follows (20): 0 = none (n = 4093); 1 = chemotherapy, not otherwise specified (n = 158); 2 = chemotherapy, single agent (n = 34); 3 = chemotherapy, multiple agents or combination regimen (n = 1504); 4 to 6 = not used for coding; 7 = patient or patient's guardian declined chemotherapy (n = 81); 8 = chemotherapy recommended but actual administration unknown (n = 77); and 9 = unknown (n = 0). For our analyses, we recoded 1, 2, 3, and 8 as having received chemotherapy; 0 and 7 were recoded as having not received chemotherapy. The pattern of the results did not change if we recoded category 8 as not receiving chemotherapy or if we excluded the 77 cases in category 8 from the analysis.
Hormone Therapy
Information on hormone therapy was coded as follows (20): 0 = none (n = 3435); 1 = hormones, not otherwise specified, including antihormones (n = 1568); 2 = endocrine surgery or endocrine radiation (n = 5); 3 = combination of 1 and 2 (n = 0); 4 to 6 = not used for coding; 7 = patient or patient's guardian declined hormone therapy (n = 26); 8 = hormone therapy recommended but actual administration unknown (n = 65); and 9 = unknown (n = 2). For our analyses, we recoded 1, 2, 3, 8, and 9 as having received hormone therapy; 0 and 7 were recoded as not having received hormone therapy. The pattern of the results did not change if we recoded 8 and 9 as not having received hormone therapy or if we excluded the 67 cases in categories 8 and 9 from the analysis.
Statistical Analysis
Our analytical strategy had three components. First, we examined the overall and age-specific rate (percentage) of chemotherapy use. The percentage of women receiving chemotherapy was a ratio of the number of women who received chemotherapy to the total number of women with a diagnosis of breast cancer. We used the Mantel–Haenszel chi-square test for trend to obtain the change in use of chemotherapy with age (21). Second, we used multivariable logistic regression analysis to generate the odds ratio of receiving chemotherapy in women with breast cancer and to determine the effect of age (Table 1) on chemotherapy use. In this model, we adjusted for race (white, black, or others), tumor stage (stage I, stage II, or stage IIIA), node status, hormone receptor status (Table 2), whether the patient had received surgery and radiation therapy (categorized as breast-conserving surgery without radiation, breast-conserving surgery with radiation, or mastectomy), and adjuvant hormone therapy use (yes or no).
Table 1. Use of Chemotherapy in Women with Breast Cancer from 1991 through 1997 in New Mexico, by Patient Age and Tumor Stage.
| Age | Cases | Women Receiving Chemotherapy* | |||
|---|---|---|---|---|---|
| Stage I
(n = 2692) |
Stage II
(n = 2235) |
Stage IIIA
(n = 174) |
Total
(n = 5101) |
||
| y | n | ||||
| <45 | 770 | 43.1 | 77.9 | 95.8 | 66.0 |
| 45–49 | 534 | 28.5 | 73.1 | 91.7 | 54.9 |
| 50–54 | 563 | 21.3 | 64.6 | 94.4 | 44.2 |
| 55–59 | 555 | 8.0 | 58.2 | 56.3 | 31.0 |
| 60–64 | 537 | 4.4 | 36.8 | 47.4 | 18.1 |
| 65–69 | 596 | 3.0 | 26.4 | 35.7 | 12.3 |
| 70–74 | 594 | 1.1 | 15.6 | 42.9 | 7.1 |
| ≥75 | 952 | 0.6 | 6.2 | 23.8 | 3.4 |
| Total | 5101 | 11.3 | 46.7 | 68.4 | 28.7 |
| P value for trend with age† | <0.001 | <0.001 | <0.001 | <0.001 | |
Percentage of women receiving chemotherapy was a ratio of the number of women who received chemotherapy to the total number of women with diagnosis of breast cancer in each age and stage subgroup.
The Mantel–Haenszel chi-square test for trend with age groups was used to determine P values.
Table 2. Use of Adjuvant Chemotherapy in Women with Stage I, Stage II, or Stage IIIA Breast Cancer from 1991 through 1997 in New Mexico by Age, Lymph Node Status, and Hormone Receptor Status*.
| Lymph Node and Hormone Receptor Status† | Cases | Women Receiving Chemotherapy by Age | P Value for Trend‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <45 y | 45–49 y | 50–54 y | 55–59 y | 60–64 y | 65–69 y | 70–74 y | ≥75 y | |||
| n | ||||||||||
| Node positive, HR positive | 1030 | 87.2 | 85.3 | 84.6 | 67.0 | 36.6 | 21.7 | 17.5 | 7.3 | <0.001 |
| Node positive, HR negative | 320 | 89.3 | 94.9 | 87.0 | 84.4 | 88.9 | 71.4 | 47.1 | 24.0 | <0.001 |
| Node positive, HR unknown | 150 | 57.7 | 66.7 | 27.8 | 36.4 | 30.0 | 30.8 | 27.3 | 16.2 | <0.001 |
| Node negative, HR positive | 1959 | 44.2 | 33.2 | 18.7 | 9.7 | 4.0 | 3.7 | 1.2 | 1.2 | <0.001 |
| Node negative, HR negative | 490 | 67.9 | 58.3 | 51.3 | 39.0 | 30.2 | 21.7 | 22.6 | 5.3 | <0.001 |
| Node negative, HR unknown | 456 | 36.4 | 29.7 | 10.0 | 2.3 | 2.0 | 1.6 | 0 | 0 | <0.001 |
| Lymph node not examined | 696 | 57.6 | 31.1 | 26.0 | 22.5 | 11.9 | 7.4 | 3.0 | 1.4 | <0.001 |
| Total | 5101 | 66.0 | 54.9 | 44.2 | 31.0 | 18.1 | 12.3 | 7.1 | 3.4 | <0.001 |
HR = hormone receptor.
Hormone receptor positive is defined as estrogen receptor–positive or progesterone receptor–positive tumors, and hormone receptor negative is defined as estrogen receptor–negative and progesterone receptor–negative tumors.
The Mantel–Haenszel chi-square test for trend with age groups was used to determine P values.
Context
National Institutes of Health consensus guidelines recommend adjuvant chemotherapy for premenopausal or post-menopausal women with node-positive tumors or node-negative breast tumors greater than 1 cm, regardless of hormone receptor status. The actual (and age-specific) use of chemotherapy in women with breast cancer is unknown.
Contribution
Using data from the New Mexico Tumor Registry, these investigators show that chemotherapy is used much less frequently than recommended and that frequency decreases sharply with advancing age.
Implications
Since only a minority of postmenopausal women receive adequate treatment for breast cancer, many unnecessary deaths could probably be prevented by following the National Institutes of Health guidelines.
–The Editors
In addition to odds ratios, we generated the probabilities of receiving chemotherapy from the parameters of the logistic regression for women with different ages by holding other factors constant. We used the method described by Hosmer and Lemeshow.
Finally, we performed sensitivity analyses to assess the potential effects of unmeasured confounders on the associations observed between age and chemotherapy use (23). The method developed by Greenland (23) for dichotomous exposure and confounding variable was expanded to accommodate the eight-level exposure variable for age groups. The prevalence of the unmeasured confounding variable was dichotomized at different age cut-points (ages younger than the cut-point had one prevalence rate and ages greater than or equal to the cut-point had another). Over the different prevalence levels, the odds ratio between the unmeasured confounder and chemotherapy ranged from 1.5 to 10.0. We then generated multivariable logistic regression models (which included this unmeasured confounding variable) to determine the effect of unmeasured factors on the result. All computer programming and analyses were done by using SAS software (SAS Institute, Inc., Cary, North Carolina) (21).
Role of the Funding Source
The National Cancer Institute of the National Institutes of Health and the Sealy and Smith Foundation funded this project but had no role in the collection, analysis, and interpretation of the data or in the decision to submit the paper for publication.
Results
Among 5101 women with stage I, stage II, or stage IIIA breast cancer diagnosed from 1991 through 1997 in New Mexico, age at diagnosis ranged from 20 to 98 years; the mean age was 61 years. Table 1 presents the percentage of women receiving chemotherapy by tumor stage and patient age. Overall, 29% of women received chemotherapy, and the rate of chemotherapy use for stage I, stage II, and stage IIIA was 11%, 47%, and 68%, respectively. Across all tumor stages, the use of chemotherapy decreased substantially with increasing age (P < 0.001 for trend). Overall, 66% of women younger than 45 years of age received chemotherapy compared with 44% of women between 50 and 54 years of age, 31% of women between 55 and 59 years of age, and 18% of women between 60 and 64 years of age. Only 12% of women between 65 and 69 years of age and 3% of those older than 75 years of age received chemotherapy.
Table 2 presents the use of chemotherapy by patient age, lymph node status, and hormone receptor status in women with stage I, stage II, or stage IIIA breast cancer. As expected, chemotherapy was used more often in women with node-positive tumors and women with estrogen receptor–negative tumors. However, across all classes of tumor characteristics, use of chemotherapy decreased substantially with age (P < 0.001 for trend). For example, in women with node-positive and hormone receptor–positive tumors, the percentage of women who received chemotherapy decreased as follows: 87% for women younger than 45 years of age, 67% for women 55 to 59 years of age, and 22% for women 65 to 69 years of age. Chemotherapy use was relatively stable in women with node-positive and hormone receptor–negative tumors who were younger than 65 years of age; however, use decreased substantially after age 65 years. In additional analyses that were stratified by node status (positive versus negative) and by tumor size, chemotherapy use decreased with age (data not shown).
Table 3 presents chemotherapy use in relation to use of adjuvant hormone therapy (usually tamoxifen). The percentage of women who received chemotherapy plus hormone therapy or who received chemotherapy alone decreased with advancing age. The percentage of women receiving hormone therapy alone increased with age. However, the overall percentage of women with breast cancer who did not receive adjuvant therapy (neither chemotherapy nor hormone therapy) increased substantially with age.
Table 3. Use of Adjuvant Chemotherapy and Adjuvant Hormone Therapy in Women with Stage I, Stage II, or Stage IIIA Breast Cancer from 1991 through 1997 in New Mexico by Patient Age.
| Age | Women Receiving Adjuvant Therapy | ||||
|---|---|---|---|---|---|
| Chemotherapy plus Hormone Therapy | Chemotherapy Only | Hormone Therapy Only | No Adjuvant Therapy | Total Cases | |
| y | n (%) | ||||
| <45 | 15.8 | 50.1 | 5.1 | 29.0 | 770 (100.0) |
| 45–49 | 17.0 | 37.8 | 9.7 | 35.4 | 534 (100.0) |
| 50–54 | 18.5 | 25.8 | 18.5 | 37.3 | 563 (100.0) |
| 55–59 | 11.7 | 19.3 | 21.6 | 47.4 | 555 (100.0) |
| 60–64 | 8.0 | 10.1 | 31.3 | 50.7 | 537 (100.0) |
| 65–69 | 5.4 | 6.9 | 30.9 | 56.9 | 596 (100.0) |
| 70–74 | 4.0 | 3.0 | 35.2 | 57.7 | 594 (100.0) |
| ≥75 | 0.8 | 2.5 | 28.9 | 67.8 | 952 (100.0) |
| Total | 9.6 | 19.2 | 22.6 | 48.7 | 5101 (100.0) |
| P value for trend* | <0.001 | <0.001 | <0.001 | <0.001 | |
The Mantel–Haenszel chi-square test for trend with age groups was used to determine P values.
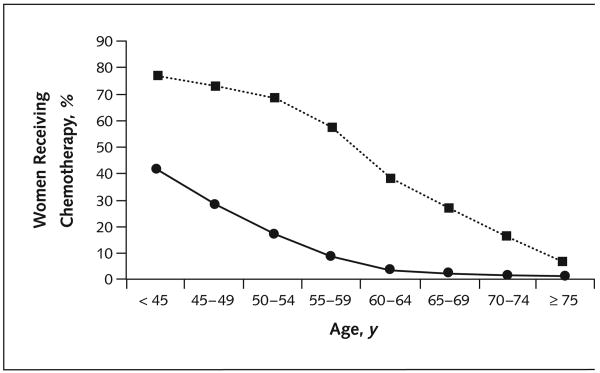
The Figure presents the use of chemotherapy in women with breast cancer as a function of two variables: age and whether use of chemotherapy was clearly recommended in the 1990 NIH Consensus Conference (based on tumor characteristics). We examined women younger than 70 years of age with 1) node-positive tumors, 2) node-negative, hormone receptor–negative tumors larger than 1.0 cm in size, or 3) node-negative tumors of any hormone receptor status that were greater than 3.0 cm in size. Any other tumors were categorized as having no clear consensus recommendation, based on the 1990 Consensus Conference. As hypothesized, the use of chemotherapy in women with tumors for which no clear chemotherapy guidelines existed decreased sharply with age, falling to less than 10% for women 55 years of age and older. However, we observed an almost parallel decrease in the use of chemotherapy with age in women with tumors for which chemotherapy was generally recommended. In these women, 77.4% of those who were younger than 45 years of age received chemotherapy. In contrast, chemotherapy was given to only 58.1% of those 55 to 59 years of age, only 37.7% of those 60 to 64 years of age, and only 25.0% of those 65 to 69 years of age.
Figure. Receipt of adjuvant chemotherapy as a function of age and tumor characteristics in women with breast cancer.
Women with stage I, stage II, or stage IIIA breast cancer are categorized into two groups based on their tumor characteristics—characteristics for which the 1990 National Institute of Health (NIH) Consensus Conference (3) generally recommended chemotherapy (n = 2486; dotted line) and characteristics for which the consensus conference recommended decisions on an individual, discretionary basis (n = 2615; solid line). The 2000 NIH Consensus Conference recommended chemotherapy for all breast tumors 1.0 cm or larger in size; however, these recommendations were made after we conducted our study. For women 70 to 74 years of age and women 75 years of age and older, the NIH Consensus Conference made no specific consensus recommendations on treatment with chemotherapy.
Table 4 presents a multivariable analysis of the effect of age on the adjusted odds of receiving chemotherapy in women with breast cancer. We then converted the parameters used to generate odds ratios and their confidence intervals to probabilities (22). We obtained the probability of receiving chemotherapy for each age group, holding constant race, tumor stage, node status and hormone receptor status, surgery and radiation therapy status, and adjuvant hormone therapy use across age groups. We observed the same pattern seen in the bivariate analyses of declining chemotherapy use with age (Tables 1, 2, and 3 and Figure). Younger women with breast cancer had a greater chance of receiving adjuvant chemotherapy, and, as expected, women older than 70 years of age were least likely to be given chemotherapy.
Table 4. Multivariable Analysis for the Odds or Probability of Receiving Chemotherapy in Women with Stage I, Stage II, or Stage IIIA Breast Cancer from 1991 through 1997 in New Mexico.
| Age | Cases
(Total, n = 5101) |
Women Receiving Chemotherapy | Odds Ratio of Receiving Chemotherapy (95% CI)* | Probability of Receiving Chemotherapy (95% CI)† |
|---|---|---|---|---|
| y | n | % | ||
| <45 | 770 | 66.0 | 1.00 (reference) | 0.51 (0.39–0.63) |
| 45–49 | 534 | 54.9 | 0.71 (0.54–0.93) | 0.43 (0.31–0.55) |
| 50–54 | 563 | 44.2 | 0.40 (0.30–0.52) | 0.29 (0.21–0.40) |
| 55–59 | 555 | 31.0 | 0.21 (0.16–0.29) | 0.18 (0.12–0.27) |
| 60–64 | 537 | 18.1 | 0.10 (0.07–0.13) | 0.09 (0.06–0.15) |
| 65–69 | 596 | 12.3 | 0.06 (0.04–0.08) | 0.05 (0.03–0.09) |
| 70–74 | 594 | 7.1 | 0.03 (0.02–0.05) | 0.03 (0.02–0.05) |
| ≥75 | 952 | 3.4 | 0.02 (0.01–0.02) | 0.02 (0.01–0.03) |
Odds ratios of having received adjuvant chemotherapy in women within various age groups compared with women younger than 45 years of age were generated from the logistic regression model and adjusted for race, tumor stage, lymph node and hormone receptor status, and other treatments received (see Methods section for categorization).
Probabilities were converted from the logistic regression model by using the method described by Hosmer and Lemeshow (22). These probabilities were standardized to the following variables: white race, stage I tumors, having received breast-conserving surgery without radiation, node-positive and hormone receptor–positive tumor status and having received adjuvant hormone therapy.
We also conducted sensitivity analyses to estimate the potential effect of unknown confounders on the study results. These analyses demonstrated that the relationship between age and chemotherapy use in our large population-based setting was relatively insensitive to confounding by unmeasured factors. For example, let us examine the following scenario: The prevalence of an unmeasured confounding variable is 90% in women younger than 50 years of age and 50% in women 50 years of age or older. The odds ratio for receipt of chemotherapy associated with this unmeasured confounder is 2.0. Thus, the adjusted odds ratio of receiving chemotherapy for the women in the 55- to 59-year-old age group relative to women younger than 45 years of age would be 0.25 (95% CI, 0.19 to 0.34) instead of 0.21 (CI, 0.16 to 0.29), as shown in Table 4. However, under extreme conditions, the effect of age on chemotherapy use could be affected. If the prevalence of an unmeasured confounding variable were 90% in women younger than 50 years of age and 10% in women 50 years of age and older and if the odds ratio for receiving chemotherapy associated with this unmeasured confounder were 5.0, the adjusted odds ratio of receiving chemotherapy for the 50- to 54-year-old age group relative to the group younger than 45 years of age would become insignificant (0.79 [CI, 0.58 to 1.07]). Even under such extreme circumstances, the age groups 55 to 59 years of age and 60 to 64 years of age relative to the group younger than 45 years of age are still significantly associated with decreasing use of chemotherapy. However, the significance is reduced to 0.43 (CI, 0.31 to 0.59) for women 55 to 59 years of age and reduced to 0.19 (CI, 0.14 to 0.27) for women 60 to 64 years of age compared with women younger than 45 years of age. In addition, we used two different approaches for a small number of women with missing information on chemotherapy and hormone therapy use: We excluded these women from the total number of women or recorded them as not having received therapy. The results showed little difference in the relationship between age and chemotherapy use, although overall rate of chemotherapy use was slightly altered (data not shown).
Discussion
Our study describes the patterns of adjuvant chemotherapy use in women 20 years of age or older with stage I, stage II, or stage IIIA breast cancer in New Mexico from 1991 through 1997. The rates of chemotherapy use for stage I, stage II, and stage IIIA were 11%, 47%, and 68%, respectively. Across all tumor stages, the use of chemotherapy decreased substantially with increasing age (P < 0.001 for trend). Overall, 66% of women younger than 45 years of age received chemotherapy compared with 44% of women 50 to 54 years of age, 31% of women 55 to 59 years of age, and 18% of women 60 to 64 years of age. In women age 65 to 69 years and those older than 75 years, only 12% and 3%, respectively, received chemotherapy. The decreasing pattern of chemotherapy use with age was maintained after adjustment for such prognostic factors as node status, hormone receptor status, and other treatments received.
Although numerous studies have shown that age older than 65 years is a risk factor for inadequate treatment of breast cancer (13–15, 24–35), few studies addressed chemotherapy (13–15). In the hospital-based reports, the rates of chemotherapy use did not vary much by age in women younger than 65 years of age. A striking finding of our population-based study is that, even in women younger than 65 years of age, the use of adjuvant chemotherapy dramatically decreased with age.
Several factors may explain the decreasing use of chemotherapy with age. First, the efficacy of adjuvant chemotherapy has been shown to decrease with age. A systematic overview of the 47 randomized trials of early-stage breast cancer by the Early Breast Cancer Trialists' Collaborative Group showed a proportional reduction in 10-year mortality with chemotherapy of 27% for women younger than 50 years of age, 14% for women 50 to 59 years of age, and 8% for women 60 to 69 years of age (6). The result is an absolute benefit in 10-year survival of 7% to 11% for women younger than 50 years of age and 2% to 3% for women 50 to 69 years of age (6). A similar pattern was seen for reduction in risk for recurrence by age groups (2, 4, 6). These meta-analyses found no benefit of chemotherapy in women age 70 years and older. On the other hand, the National Surgical Adjuvant Breast and Bowel Project (NSABP) showed efficacy of chemotherapy in older women, including those age 70 years and older (5, 10, 36); however, the effect size was smaller in older women than in younger women.
Second, physicians and older patients who are considering chemotherapy may be concerned about the possibility of toxicity increasing with age. However, several studies have shown that women of different ages whose general health is otherwise satisfactory experience similar toxicity profiles when treated with commonly used chemotherapy regimens (37–41).
Regardless of the reasons for decreased chemotherapy use with age, the result is that only a small number of postmenopausal women are receiving chemotherapy in compliance with consensus recommendations. The decrease in efficacy of chemotherapy with age is not reflected in the consensus recommendations, which do not vary greatly by age for women younger than 70 years of age (1, 3, 7–9, 11, 12). The development and dissemination of clinical practice guidelines have been recognized as a major advance in improving quality and decreasing variations in medical practice (42–44). Nevertheless, concerns have been raised about the limitations of such guidelines, including how quickly they may become outdated (42, 45). Evidence-based guidelines rely on the results of clinical trials, but participants in clinical trials do not always represent a cross-section of patients in the community. This is especially true for trials of cancer therapies (34, 46). However, information comparing the effectiveness of chemotherapy in the community with efficacy in trials is very limited. It seems that community oncologists and their postmenopausal patients with breast cancer often conclude that the clinical guidelines should be ignored because of the risk–benefit profile for chemotherapy use.
Our study has several limitations. One concern is whether the information on chemotherapy from the SEER tumor registries is valid and complete. This information may be incomplete because SEER does not require all medical oncologists' offices to be checked for chemotherapy administration by the SEER data collectors (47). The SEER information may also be incomplete because patient follow-up may not have been long enough to obtain complete chemotherapy treatment information (20).
On the other hand, the validity of the registry data regarding chemotherapy was supported by several internal consistencies of the data. For example, patients who might be expected to use chemotherapy often include younger women with advanced tumor stage and women with hormone receptor–negative tumors. The registry data confirmed this expectation. Another example of the internal consistency of the registry data is that 96% of women younger than 45 years of age with stage IIIA breast cancer were recorded as having received chemotherapy (Table 1). More than 71% of women 65 to 69 years of age with node-positive and hormone receptor–negative tumors received chemotherapy; only 4% of women categorized into these same age groups who had node-negative and hormone receptor–positive tumors received chemotherapy (Table 2). Such internal consistency supports the validity of the information on chemotherapy use in the New Mexico Tumor Registry. In addition, even if the tumor registry information on chemotherapy were incomplete, it is difficult to envision how this limitation would produce a marked decrease in chemotherapy use with age.
Another limitation of our study is that we did not investigate whether older women with breast cancer who received chemotherapy fared any better than those who did not. In addition, we did not have information on comorbidity. However, although comorbidity becomes an important issue in the choice of therapy for women older than 65 years of age, it is unlikely that the increase in comorbidity associated with age in women younger than 65 years of age is sufficient to explain the decrease in chemotherapy use. Furthermore, we did not have information on patient preferences and physician attitudes and preferences toward chemotherapy use in women of different ages. We also do not have data on variation in the choice of chemotherapy for breast cancer at the level of the provider. A sensitivity analysis suggested that the relationship between age and chemotherapy use in our large population-based setting was relatively insensitive to changes in these unmeasured factors.
In conclusion, chemotherapy administration was substantially influenced by patient age and tumor stage. Chemotherapy use declined substantially with age in women younger than 70 years of age who had tumors with characteristics for which chemotherapy was recommended by the NIH Consensus Conference. In other words, there is a clear divergence between consensus recommendations and clinical practice. Given the decrease in efficacy of chemotherapy with age in the clinical trials, one could argue that our findings represent appropriate responses of oncologists and their patients to the less favorable benefit–risk profile in older women with breast cancer. The question of whether the recommendations are overly aggressive or whether practicing oncologists are too conservative in chemotherapy use should be addressed in population-based outcome studies and in studies that examine how oncologists and their postmenopausal patients with breast cancer deal with the benefits and risks of chemotherapy.
Acknowledgments
Grant Support: By grants from the National Cancer Institute (R01-CA90626 and R01-CA871773) and the Sealy and Smith Foundation, Galveston, Texas.
Footnotes
Author Contributions: Conception and design: X.L. Du, C.R. Key, J.S. Goodwin.
Analysis and interpretation of the data: X.L. Du, J.D. Mahnken, J.S. Goodwin.
Drafting of the article: X.L. Du.
Critical revision of the article for important intellectual content: X.L. Du, C. Osborne, J.D. Mahnken, J.S. Goodwin.
Final approval of the article: X.L. Du, C.R. Key, C. Osborne, J.D. Mahnken, J.S. Goodwin.
Provision of study materials or patients: X.L. Du, C.R. Key.
Statistical expertise: X.L. Du, J.D. Mahnken.
Obtaining of funding: X.L. Du, C.R. Key, J.S. Goodwin.
Administrative, technical, or logistic support: X.L. Du, C.R. Key.
Collection and assembly of data: X.L. Du, C.R. Key.
One thing worse than rigidity without skepsis is skepsis without rigor. For skepsis is either a virtue of the intellect or a disorder of the emotions. It is impossible to skate on ice that is either too warm or too cold. Skating depends on the fact that ice is not too cold to be melted by pressure (which permits control) but remains cold enough to be slippery (which facilitates movement). Skepsis can—almost by definition—serve no useful purpose unless it favors just such a controllable dynamic stability, thus avoiding the several perils of rigidity, stagnation, and chaos. The principal use of skepsis is to refine. The principal abuse is to destroy.
Edmond A. Murphy, Skepsis, Dogma, and Belief, Baltimore and London: Johns Hopkins Univ Pr; 1981.
Submitted by: Thomas W. Simpson, MD, John Hopkins University, Baltimore, MD 21210
Submissions from readers are welcomed. If the quotation is published, the sender's name will be acknowledged. Please include a complete citation (along with page number on which the quotation was found), as done for any reference.–The Editor
References
- 1.Consensus conference. Adjuvant chemotherapy for breast cancer. JAMA. 1985;254:3461–3. [PubMed] [Google Scholar]
- 2.Effects of adjuvant tamoxifen and of cytotoxic therapy on mortality in early breast cancer. An overview of 61 randomized trials among 28,896 women. Early Breast Cancer Trialists' Collaborative Group. N Engl J Med. 1988;319:1681–92. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 3.NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–5. [PubMed] [Google Scholar]
- 4.Systemic treatment of early breast cancer by hormonal, cytotoxic or immune therapy. 133 randomised trials involving 31,000 recurrences and 24,000 deaths among 75,000 women. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1992;339:1–15. [PubMed] [Google Scholar]
- 5.Fisher B, Dignam J, Wolmark N, DeCillis A, Emir B, Wickerham DL, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89:1673–82. doi: 10.1093/jnci/89.22.1673. [DOI] [PubMed] [Google Scholar]
- 6.Polychemotherapy for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998;352:930–42. [PubMed] [Google Scholar]
- 7.Adjuvant systemic therapy for women with node-negative breast cancer. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. CMAJ. 1998;158 3:S43–S51. [PubMed] [Google Scholar]
- 8.Adjuvant systemic therapy for women with node-positive breast cancer. The Steering Committee on Clinical Practice Guidelines for the Care and Treatment of Breast Cancer. CMAJ. 1998;158 3:S52–S64. [PubMed] [Google Scholar]
- 9.Hortobagyi GN. Treatment of breast cancer. N Engl J Med. 1998;339:974–84. doi: 10.1056/NEJM199810013391407. [DOI] [PubMed] [Google Scholar]
- 10.Fisher B. Highlights from recent National Surgical Adjuvant Breast and Bowel Project studies in the treatment and prevention of breast cancer. CA Cancer J Clin. 1999;49:159–77. doi: 10.3322/canjclin.49.3.159. [DOI] [PubMed] [Google Scholar]
- 11.Carlson RW, Anderson BO, Bensinger W, Cox CE, Davidson NE, Edge SB, et al. NCCN Practice Guidelines for Breast Cancer. Oncology (Huntingt) 2000;14:33–49. [PubMed] [Google Scholar]
- 12.Eifel P, Axelson JA, Costa J, Crowley J, Curran WJ, Jr, Deshler A, et al. National Institutes of Health Consensus Development Conference Statement: adjuvant therapy for breast cancer, November 1-3, 2000. J Natl Cancer Inst. 2001;93:979–89. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 13.Chu J, Diehr P, Feigl P, Glaefke G, Begg C, Glicksman A, et al. The effect of age on the care of women with breast cancer in community hospitals. J Gerontol. 1987;42:185–90. doi: 10.1093/geronj/42.2.185. [DOI] [PubMed] [Google Scholar]
- 14.Osteen RT, Cady B, Chmiel JS, Clive RE, Doggett RL, Friedman MA, et al. 1991 national survey of carcinoma of the breast by the Commission on Cancer. J Am Coll Surg. 1994;178:213–9. [PubMed] [Google Scholar]
- 15.Wilson RE, Donegan WL, Mettlin C, Natarajan N, Smart CR, Murphy GP. The 1982 national survey of carcinoma of the breast in the United States by the American College of Surgeons. Surg Gynecol Obstet. 1984;159:309–18. [PubMed] [Google Scholar]
- 16.Du X, Goodwin JS. Patterns of use of chemotherapy for breast cancer in older women: findings from Medicare claims data. J Clin Oncol. 2001;19:1455–61. doi: 10.1200/JCO.2001.19.5.1455. [DOI] [PubMed] [Google Scholar]
- 17.Du X, Goodwin JS. Increase of chemotherapy use in older women with breast carcinoma from 1991 to 1996. Cancer. 2001;92:730–7. doi: 10.1002/1097-0142(20010815)92:4<730::aid-cncr1376>3.0.co;2-p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ries LA, Kosary CL, Hankey BF, Miller BA, Harras A, Edwards BK, editors. SEER Cancer Statistics Review, 1973-1994. Bethesda, MD: National Cancer Institute; 1997. NIH publication no. 97-2789. [Google Scholar]
- 19.The New Mexico Tumor Registry. About the New Mexico Tumor Registry. [18 November 2002]; Accessed at http://hsc.unm.edu/epiccpro/nmtr.html.
- 20.The SEER Program Code Manual. Revised. Bethesda, MD: National Cancer Institute; 1994. NIH publication no. 94-1999. [Google Scholar]
- 21.Stokes ME, Davis CS, Koch GG. Categorical data analysis using the SAS system. Cary, NC: SAS Institute; 1997. [Google Scholar]
- 22.Hosmer DW, Lemeshow S. Applied Logistic Regression. 2nd. New York: J Wiley; 2000. pp. 17–21. [Google Scholar]
- 23.Greenland S. Basic methods for sensitivity analysis and external adjustment. In: Rothman KJ, Greenland S, editors. Modern Epidemiology. 2nd. Philadelphia: Lippincott-Raven; 1998. pp. 343–57. [Google Scholar]
- 24.Mann BA, Samet JM, Hunt WC, Key CR, Goodwin JM, Goodwin JS. Changing treatment of breast cancer in New Mexico from 1969 through 1985. JAMA. 1988;259:3413–7. [PubMed] [Google Scholar]
- 25.Samet J, Hunt WC, Key C, Humble CG, Goodwin JS. Choice of cancer therapy varies with age of patient. JAMA. 1986;255:3385–90. [PubMed] [Google Scholar]
- 26.Lazovich DA, White E, Thomas DB, Moe RE. Underutilization of breast-conserving surgery and radiation therapy among women with stage I or II breast cancer. JAMA. 1991;266:3433–8. [PubMed] [Google Scholar]
- 27.Goodwin JS, Samet JM, Hunt WC. Determinants of survival in older cancer patients. J Natl Cancer Inst. 1996;88:1031–8. doi: 10.1093/jnci/88.15.1031. [DOI] [PubMed] [Google Scholar]
- 28.Du XL, Freeman JL, Freeman DH, Syblik DA, Goodwin JS. Temporal and regional variation in the use of breast-conserving surgery and radiotherapy for older women with early-stage breast cancer from 1983 to 1995. J Gerontol A Biol Sci Med Sci. 1999;54:M474–8. doi: 10.1093/gerona/54.9.m474. [DOI] [PubMed] [Google Scholar]
- 29.Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J Natl Cancer Inst. 2000;92:269–71. doi: 10.1093/jnci/92.3.269. [DOI] [PubMed] [Google Scholar]
- 30.Du X, Freeman JL, Goodwin JS. Information on radiation treatment in patients with breast cancer: the advantages of the linked medicare and SEER data. Surveillance, Epidemiology and End Results. J Clin Epidemiol. 1999;52:463–70. doi: 10.1016/s0895-4356(99)00011-6. [DOI] [PubMed] [Google Scholar]
- 31.Samet J, Hunt WC, Key C, Humble CG, Goodwin JS. Choice of cancer therapy varies with age of patient. JAMA. 1986;255:3385–90. [PubMed] [Google Scholar]
- 32.Greenfield S, Blanco DM, Elashoff RM, Ganz PA. Patterns of care related to age of breast cancer patients. JAMA. 1987;257:2766–70. [PubMed] [Google Scholar]
- 33.Wetle T. Age as a risk factor for inadequate treatment [Editorial] JAMA. 1987;258:516. doi: 10.1001/jama.1987.03400040114035. [DOI] [PubMed] [Google Scholar]
- 34.Goodwin JS, Hunt WC, Humble CG, Key CR, Samet JM. Cancer treatment protocols. Who gets chosen? Arch Intern Med. 1988;148:2258–60. [PubMed] [Google Scholar]
- 35.Goodwin JS, Hunt WC, Samet JM. Determinants of cancer therapy in elderly patients. Cancer. 1993;72:594–601. doi: 10.1002/1097-0142(19930715)72:2<594::aid-cncr2820720243>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 36.Fisher B, Jeong JH, Dignam J, Anderson S, Mamounas E, Wickerham DL, et al. Findings from recent National Surgical Adjuvant Breast and Bowel Project adjuvant studies in stage I breast cancer. J Natl Cancer Inst Monogr. 2001:62–6. doi: 10.1093/oxfordjournals.jncimonographs.a003463. [DOI] [PubMed] [Google Scholar]
- 37.Muss HB. The role of chemotherapy and adjuvant therapy in the management of breast cancer in older women. Cancer. 1994;74:2165–71. doi: 10.1002/1097-0142(19941001)74:7+<2165::aid-cncr2820741727>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 38.Muss HB. Chemotherapy of breast cancer in the older patient. Semin Oncol. 1995;22:14–6. [PubMed] [Google Scholar]
- 39.Christman K, Muss HB, Case LD, Stanley V. Chemotherapy of metastatic breast cancer in the elderly. The Piedmont Oncology Association experience [see comment] JAMA. 1992;268:57–62. [PubMed] [Google Scholar]
- 40.Nerenz DR, Love RR, Leventhal H, Easterling DV. Psychosocial consequences of cancer chemotherapy for elderly patients. Health Serv Res. 1986;20:961–76. [PMC free article] [PubMed] [Google Scholar]
- 41.Du XL, Osborne C, Goodwin JS. Population-Based Assessment of Hospitalizations for Toxicity from Chemotherapy in Older Women with Breast Cancer. J Clin Oncol. 2002;20:4636–42. doi: 10.1200/JCO.2002.05.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shekelle PG, Ortiz E, Rhodes S, Morton SC, Eccles MP, Grimshaw JM, et al. Validity of the Agency for Healthcare Research and Quality clinical practice guidelines: how quickly do guidelines become outdated? JAMA. 2001;286:1461–7. doi: 10.1001/jama.286.12.1461. [DOI] [PubMed] [Google Scholar]
- 43.Field MJ, Lohr KN. Guidelines for Clinical Practice: From Development to Use. Washington, DC: National Academy Pr; 1992. [PubMed] [Google Scholar]
- 44.Woolf SH, Grol R, Hutchinson A, Eccles M, Grimshaw J. Clinical guidelines: potential benefits, limitations, and harms of clinical guidelines. BMJ. 1999;318:527–30. doi: 10.1136/bmj.318.7182.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shekelle P, Eccles MP, Grimshaw JM, Woolf SH. When should clinical guidelines be updated? BMJ. 2001;323:155–7. doi: 10.1136/bmj.323.7305.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Under-representation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 47.Potosky AL, Riley GF, Lubitz JD, Mentnech RM, Kessler LG. Potential for cancer related health services research using a linked Medicare-tumor registry database. Med Care. 1993;31:732–48. [PubMed] [Google Scholar]