Abstract
We described, for the first time, the metal enhanced fluorescence from the CdTe nanocrystals spin coated on silver island films (SIFs). CdTe nanocrystals show ∼5 fold increase in fluorescence intensity, 3-fold decrease in lifetimes and reduction in blinking on SIFs surfaces that can be observed by both ensemble and single molecule fluorescence studies. The single molecule study also provides further insight on the heterogeneity in the fluorescence enhancement and lifetimes of the CdTe nanocrystals on both glass and SIFs surfaces, which is otherwise not possible to observe using ensemble measurements.
Signal detection is a challenging task in chemistry and biology. Most often background noise hinders the detection of the signal of interest. This can be prevailed if one has a signal enhancing and/or directing technique. In this regard, Metal-Enhanced Fluorescence (MEF) is a newly recognized technology, where we study the interactions of fluorophores with metallic colloids or surfaces, which provides fluorescence enhancement.1 Along with signal enhancement, MEF also provides several other important spectral changes such as increased photostability, decreased lifetime due to increased rates of radiative decay, and increased distance for fluorescence resonance energy transfer (RET).1c Because of this exceptional improvement in signal detectability provided by the MEF, we were able to increase the intrinsic DNA fluorescence, which is otherwise impractical to perceive.1d Accordingly we have made considerable progress with MEF studies where we used wide varieties of fluorophores that can be potentially used in different applications.2
Single-molecule fluorescence spectroscopy provides several advantages over ensemble measurements.3 For instance, it eliminates averaging of the spectral properties over all members of ensemble and can reveal fundamental features otherwise masked in ensemble exp eriments. Accordingly, using single-molecule fluorescence studies we anticipate that the metal-fluorophore interactions in the MEF studies can be better revealed, especially at single molecule level. For this, we choose CdTe nanocrystals (QDots) as fluorophores. QDots having few nanometer sizes, tunable-absorption and fluorescence spectral properties are widely used as probes in various fields including biological labeling and imaging.4 Potential applications of QDots are rapidly increasing. Also single QDot fluorescence spectroscopy has been accomplished successfully for several colloidal semiconductor materials including CdSe,5a CdS,5b CdTe,5c Si5d and InP.5e Also quantum dots fluorescence spectral properties are reported in the presence of metallic nanoparticles at the ensemble level with little details.6,7
In this communication, we describe the ensemble and single-molecule fluorescence spectral properties of CdTe nanocrystals spin coated in PVA matrix on glass and silver island films (SIFs). CdTe nanocrystals were prepared using a modified Weller method (for further details see Supporting Information).8 Figure 1 shows the fluorescence emission spectra and corresponding intensity decays of CdTe nanocrystals on glass and SIFs surface. Interestingly, the emission spectra from metalized and that from non-metalized area are completely overlapped on each other with a band maxima at about 660 nm (Figure 1A inset). As seen from the figure, a significant enhancement (of ∼ 5-fold) in fluorescence intensity is observed from the CdTe nanocrystals on SIF surface as compared to that on the glass. On the other hand, the fluorescence intensity decay of CdTe nanocrystals on SIFs show a faster decay with ∼ 3-fold decrease (from 4.52 ns on glass to 1.45 ns on SIFs) in average fluorescence lifetime. These anomalous fluorescence properties i.e. increase in fluorescence intensity and decrease in lifetime of CdTe nanocrystals near metallic surface can be reasonably explained as below.
Figure 1.
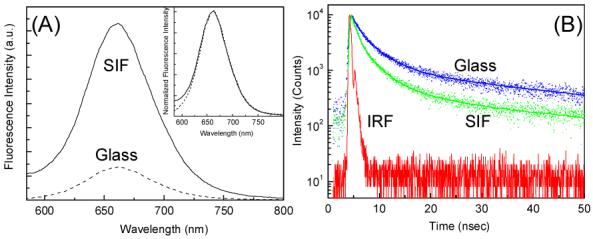
(A) Fluorescence spectra of CdTe on glass and SIF; Inset shows the normalized emission spectra. (B) Intensity time decay of CdTe on glass and SIF. Instrument response function (IRF) is also included.
The emission of fluorophores near the silver nanostructures is dependent on at least two factors: an enhanced local field and an increase in the intrinsic decay rate of the fluorophore, both due to MEF. The first factor provides stronger excitation rates but does not modify the fluorescence lifetime of the molecules. The second factor changes the quantum yield and lifetime of the fluorophore.1 Results shown in Figure 1 are in accordance with these arguments. The radiative decay rate modification of the CdTe nanocrystals in close proximity to the silver nanostructures is consistent with the previous reports on the MEF phenomenon and due to the proximity of the fluorophores to the silver nanostructures.
Single CdTe nanocrystal fluorescence measurements were obtained using a scanning confocal microscope (Picoquant MicroTime 200). The excitation laser was reflected by a dichroic mirror to a high numerical aperture (NA) oil objective (100x, NA 1.3) and focused to a diffraction limited spot (∼ 300 nm) on the sample surface. CdTe fluorescence was collected by an avalanche photodiode through the dichroic beam splitter and a bandpass (650-720 nm, Chroma) filter. Integration times of 3 msec per pixel were used to obtain 512 × 512 pixel raster scanned 20 × 20 μm images. The samples were excited with a typical power density of ∼ 55 W/cm2 from a 470 nm solid state laser. Intensity-time trajectories and intensity-time decays were obtained by positioning the excitation beam above the individual CdTe.
Figure 2 shows representative scanning confocal images of CdTe nanocrystals spin coated on glass and SIF surfaces. Well separated bright spots represent fluorescence emission from the single CdTe nanocrystals. The significant differences in the peak intensities of the two images are immediately evident from the figure (See the Scale bar). For CdTe nanocrystals on the glass and SIF surfaces, the average values of peak intensities are ∼ 25 and 125 counts/bin, indicating single CdTe nanocrystal on SIF surface are ∼5-fold brighter than that on the glass. This brightness of the spots can be ascribed to our so-called MEF.1 Also it is pertinent to note the heterogeneity in the spots brightness. On glass surface, the observed heterogeneity in the brightness could be ascribed simply to the size-dependent emission efficiencies of CdTe nanocrystals, which is inherent property of QDots.5c But explaining this on SIFs surface is complex, where the metal fluorophore interactions also play a dominant role (vide infra).
Figure 2.
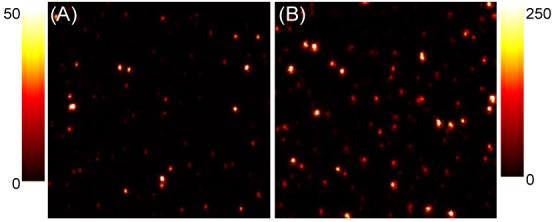
Scanning confocal images (20×20 μm) of CdTe on (A) glass and (B) SIF. Scale bar shows the intensity counts in 3 msec bin.
In order to more quantitatively compare the collected single molecule count rates and explore the changes in underlying photophysics of individual nanocrystals on glass and SIF surfaces, we monitored the fluorescence intensity of individual nanocrystals as a function of time, while under continuous excitation. Accordingly, we monitored fluorescence intensities of over 100 CdTe nanocrystals on both glass and SIFs surfaces. Representative intensity-time trajectories collected for CdTe nanocrystals on glass and SIFs surfaces are shown in Figure 3, Top panel. From these intensity-time trajectories one can deduce various spectral features. At first, the fluorescence intensity of individual CdTe nanocrystals on SIFs is several-fold higher than that on the glass. Secondly, the blinking phenomenon of the CdTe nanocrystals: the CdTe nanocrystals show characteristic blinking phenomenon on glass surface (Figure 3A).4,5c On the other hand, the blinking is almost eliminated on SIFs surface, which results in a more constant brightness of the CdTe nanocrystals on SIFs surface. These results suggest that the CdTe nanocrystals show reduced blinking on SIFs surface. Figure 3 (middle panel) shows the fluorescence intensity decays of single CdTe nanocrystal on both surfaces studied. Similar to that was observed in ensemble measurements, the CdTe nanocrystals show longer decay times on glass surface compared to that on the SIFs surface.
Figure 3.
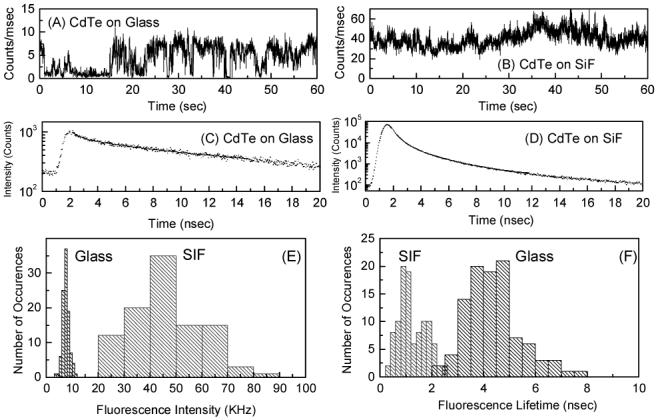
Intensity-time trajectories (A&B), intensity decays (C&D) of individual CdTe on glass and SIFs. Fluorescence intensity (count rate) (E) and average fluorescence lifetime (F) histograms of CdTe on glass & SIFs surfaces.
Figure 3E and F shows the CdTe fluorescence intensity and lifetime histograms collected on glass and SIFs surfaces. With in the same experimental conditions, the fluorescence intensities of single CdTe nanocrystals on glass are ∼8 kHz and that on SIFs surface are ∼45 kHz, which imply an about 5-fold increase in fluorescence intensity on SIFs that is similar to that was observed in ensemble measurements (Figure 1). From these histograms, it is clear that the fluorescence intensities as well as lifetimes of individual CdTe nanocrystals on SIFs surfaces are more heterogeneously distributed than that on glass surfaces. Such a distribution phenomenon is impossible to observe with ensemble measurements. CdTe fluorescence intensity distributions on glass surface show relatively low standard deviation of 16 % where as the corresponding value on SIFs is ∼30 %. This is because, the heterogeneity in the fluorescence intensity on glass surface largely depend on deviation in CdTe nanocrystals size, whereas for SIFs, additional complexity arises from the varied metal-fluorophore interactions that may be due to the heterogeneous surface of SIFs. A similar heterogeneous distribution for the CdTe lifetimes is also observed (Figure 3F) on SIFs (std dev ∼ 43%) compared to that on glass (std dev ∼ 24%).
In conclusion, in this communication we reported, for the first time, the metal enhanced fluorescence from the CdTe nanocrystals spin coated on SIFs surface. CdTe nanocrystals show ∼5 fold increase in fluorescence intensity, 3-fold decrease in lifetimes and reduction in blinking on SIFs surfaces that can be observed by both ensemble and single molecule fluorescence studies. The single molecule study also provides further insight on the heterogeneity in the fluorescence enhancement and lifetimes of the CdTe nanocrystals on both glass and SIFs surfaces, which is otherwise not possible to observe using ensemble measurements. We believe single molecule study can reveal further information on the metal-fluorophore interactions to better understand the MEF when we use more defined sample and surface conditions. Further work in this direction is under consideration.
Supplementary Material
Acknowledgment
The present work was supported by NIH, NCRR (RR-08119, NHGRI HG-02655) and NIBIB (EB 000682).
References
- (1) (a).Lakowicz JR. Anal. Biochem. 2005;337:171. doi: 10.1016/j.ab.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lakowicz JR. Plasmonics. 2006;1:5. doi: 10.1007/s11468-005-9002-3. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Malicka J, Gryczynski I, Kusba J, Lakowicz JR. Biopolymers. 2003;70:595. doi: 10.1002/bip.10507. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lakowicz JR, Shen Y, Gryczynski Z, D’Auria S, Gryczynski I. Biochem. Biophys. Res. Commun. 2001;286:875. doi: 10.1006/bbrc.2001.5445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2) (a).Aslan K, Gryczynski I, Malicka J, Matveeva E, Lakowicz JR, Geddes CD. Curr. Opin. Biotechnol. 2005;16:55. doi: 10.1016/j.copbio.2005.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Geddes CD, Lakowicz JR, editors. Topics in Fluorescence Spectroscopy. Kluwer Academic/Plenum Publishers; New York: 2005. [Google Scholar]
- (3) (a).Moerner WE. J. Phys. Chem. B. 2002;106:910. [Google Scholar]; (b) Michalet X, Pinaud F, Lacoste TD, Dahan M, Bruchez MP, Alivisatos AP, Weiss S. Single Molecules. 2001;2:261. [Google Scholar]
- (4).Michalet X, Pinaud FF, Bentolila LA, Tsay JM, Doose S, Li JJ, Sundaresan G, Wu AM, Gambhir SS, Weiss S. Science. 2005;307:538. doi: 10.1126/science.1104274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5) (a).Nirmal M, Dabbousi BO, Bawendi MB, Macklin JJ, Trautman JK, Harris TD, Brus LE. Nature. 1996;383:802. [Google Scholar]; (b) Tittel J, Gohde W, Koberling F, Basche T, Kornowski A, Weller H, Eychmuller A. J. Phys. Chem. B. 1997;101:3013. [Google Scholar]; (c) Shimizu KT, Neuhauser RG, Leatherdale CA, Empedocles SA, Woo WK, Bawendi MB. Phys. Rev. B. 2001;63:205316. [Google Scholar]; (d) English DA, Pell LE, Yu Z, Barbara PF, Korgel BA. Nano Lett. 2002;2:681. [Google Scholar]; (e) Kuno M, Fromm DP, Gallagher A, Nesbitt DJ, Micic OI, Nozik AJ. Nano Lett. 2001;1:557. [Google Scholar]
- (6) (a).Song J-H, Atay T, Shi S, Urabe H, Nurmikko AV. Nano Lett. 2005;5:1557. doi: 10.1021/nl050813r. [DOI] [PubMed] [Google Scholar]; (b) Lee J, Govorov AO, Dulka J, Kotov NA. Nano Lett. 2004;4:2323. [Google Scholar]; (c) Lee J, Govorov AO, Kotov NA. Angew. Chem. Int. Ed. 2005;44:7439. doi: 10.1002/anie.200501264. [DOI] [PubMed] [Google Scholar]
- (7).Wang Y, Li M, Jia H, Song W, Han X, Zhang J, Yang B, Xu W, Zhao B. Spectrochim. Acta, A. 2006;64:101. doi: 10.1016/j.saa.2005.07.003. [DOI] [PubMed] [Google Scholar]
- (8).Gaponik N, Talapin DV, Rogach AL, Hoppe K, Shevchenko EV, Kornowski A, Eychmüller A, Weller H. J. Phys. Chem. B. 2002;106:7177. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


