Abstract
Successful control of affect partly depends on the capacity to modulate negative emotional responses through the use of cognitive strategies (i.e., reappraisal). Recent studies suggest the involvement of frontal cortical regions in the modulation of amygdala reactivity and the mediation of effective emotion regulation. However, within-subject inter-regional connectivity between amygdala and prefrontal cortex in the context of affect regulation is unknown. Here, using psychophysiological interaction analyses of functional magnetic resonance imaging data, we show that activity in specific areas of the frontal cortex (dorsolateral, dorsal medial, anterior cingulate, orbital) covaries with amygdala activity and that this functional connectivity is dependent on the reappraisal task. Moreover, strength of amygdala coupling with orbitofrontal cortex and dorsal medial prefrontal cortex predicts the extent of attenuation of negative affect following reappraisal. These findings highlight the importance of functional connectivity within limbic-frontal circuitry during emotion regulation.
Keywords: emotion, fMRI, functional connectivity, psychophysiological interaction, amygdala, prefrontal, regulation, reappraisal
INTRODUCTION
The ability to self-regulate negative emotion in distress enhances mental and physical well-being and loss of such capacity confers risk towards psychopathology (Gross, 2002; John and Gross, 2004). A fundamental question in cognitive affective neuroscience is which neural circuit is involved in the control of emotion. Recently, functional neuroimaging studies have begun to address this question in humans.
Although multiple strategies for conscious control of emotion exist (Lazarus, 1991; Gross, 1999b), extant neuroimaging research on the neural correlates of affect regulation has concentrated on two empirical approaches—suppression and reappraisal (Ochsner and Gross, 2005; Quirk and Beer, 2006). Functional brain imaging of suppression-based (voluntary inhibition of reaction to emotional stimuli) and reappraisal-based (cognitive re-interpretation of evocative stimuli to reduce negative affect) paradigms have shown that specific frontal brain regions such as the orbitofrontal cortex (OFC), dorsolateral prefrontal cortex (DLPFC), dorsal medial prefrontal cortex (DMPFC), ventrolateral prefrontal cortex (VLPFC) and anterior cingulate cortex (ACC) are engaged (Beauregard et al., 2001; Ochsner et al., 2002; Levesque et al., 2003a; Ochsner et al., 2004b; Phan et al., 2005; Urry et al., 2006). Moreover, the recruitment of these frontal regions occurs when subjects engage in active self-regulation and is associated with modulation of amygdala reactivity (Beauregard et al., 2001; Ochsner et al., 2002; Schaefer et al., 2002; Phan et al., 2005; Urry et al., 2006). The amygdala is a region critical to the generation, expression and experience of negative emotions as demonstrated by both animal and human lesion studies (Aggleton, 1993; Angrilli et al., 1996; Davis and Whalen, 2001; Adolphs, 2002; Amaral et al., 2003; Phelps, 2004), and human imaging studies (Phan et al., 2002; Murphy et al., 2003; Phillips et al., 2003a; Wager et al., 2003; Zald, 2003).
Evidence of frontal involvement in the regulation of emotion is further supported by imaging studies in which similar frontal regions are observed to be important for the control of emotion-related behavior. For example, the ACC, VLPFC and DLPFC have been found to activate to response inhibition during the cognitive-emotion interference tasks (Whalen et al., 1998; Bush et al., 2000; Etkin et al., 2006). The ACC, VLPFC, DMPFC and OFC are engaged when subjects divert their attention from threat and/or painful stimuli (Bantick et al., 2002; Tracey et al., 2002; Bishop et al., 2004). Moreover, cognitive labeling (i.e., appraisal) of negative emotional stimuli similarly engages VLPFC, DLPFC and DMPFC (Hariri et al., 2000, 2003; Taylor et al., 2003). As above, these indirect forms of emotion modulation are also associated with attenuation of limbic-amygdala responses (Hariri et al., 2000; Pessoa et al., 2002; Taylor et al., 2003; Etkin et al., 2006).
Interestingly, studies of psychiatric disease also implicate cortico-limbic dysfunction in disorders of affect dysregulation (Phillips et al., 2003b). For example, the ACC, DMPFC, DLPFC and/or OFC appear to be dysfunctional during cognitive-emotional tasks in patients with depression (Mayberg, 1997; Beauregard et al., 2006), anxiety (Shin et al., 2001; Lanius et al., 2004), impulsive aggression (Coccaro et al., in press) and personality disorders (McCloskey et al., 2005; New et al., 2007); furthermore, these patients also exhibit exaggerated amygdala reactivity to emotionally negative stimuli (Coccaro et al., in press; Rauch et al., 2000; Herpertz et al., 2001; Sheline et al., 2001; Donegan et al., 2003). However, an exact neural mechanism for dysregulated emotion in psychiatric disorders remains elusive.
Taken together, the data from animal and human studies point to a specific amygdala–frontal circuit of emotion generation and regulation (Davidson et al., 2000; Phan et al., 2002; Phillips et al., 2003a; Ochsner and Gross, 2005; Quirk and Beer, 2006). Anatomical tracing studies have demonstrated strong reciprocal connections between the amygdala and the ACC, OFC, VLPFC and DMPFC (Amaral and Price, 1984; Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007). However, few studies have examined the dynamic interactions between regions within the amygdala–frontal circuit during the active control of affect. In prior neuroimaging studies of emotion regulation, it has been posited that conscious down-regulation of emotion appears to have a top–down inhibitory effect of prefrontal brain regions on the amygdala (Beauregard et al., 2001; Ochsner et al., 2002, 2004a; Levesque et al., 2003b, 2004; Phan et al., 2005; Ohira et al., 2006; Urry et al., 2006). To date, only two studies have assessed inter-regional relationships during emotion regulation. First, Ochsner et al. (2002) demonstrated a negative correlation of brain activity between ventrolateral PFC and amygdala during active reappraisal. Second, Urry et al. (2006) recently demonstrated that attenuation of amygdala activation was associated with enhanced activation in ventromedial PFC bilaterally during conscious regulation of emotion. However, these findings show a between-subject effect (i.e., correlations of brain activity across subjects), and it remains unknown whether amygdala–frontal interactions can be demonstrated within-subjects as they engage in active regulation of emotion through reappraisal strategies.
The present study aimed to extend existing literature on the neurocircuitry of emotion regulation by examining within-subject functional connectivity (i.e., temporal correlations of activity across spatially distributed brain regions) between the amygdala and frontal regions during regulation of negative affect. We conducted psychophysiological interactions (PPI) analyses (Friston et al., 1997) which identify task-related changes in functional brain connectivity on previously published data (Phan et al., 2005) obtained from a functional magnetic resonance imaging (fMRI) study of emotion regulation via cognitive reappraisal. In that paper, we demonstrated that voluntary regulation by reappraisal relative to passive maintenance was associated with activation of the DMPFC, DLPFC, OFC, VLPFC and ACC and attenuation of the left amygdala, consistent with prior reports using similar paradigms (Ochsner et al., 2002, 2004b). Moreover, activation of the DLPFC, ACC and VLPFC was inversely correlated with intensity of negative affect, suggesting that recruitment of these frontal regions resulted in increasing effectiveness of reappraisal. The PPI analysis allows inference as to whether region-to-region coactivation changes significantly as a function of task. Here, we identified inter-regional interactions using the left amygdala as the seed region, and compared these interactions between emotion regulation and non-regulation tasks. We hypothesized that the amygdala would engage distinct frontal networks reflective of emotion regulation inherent to cognitive reappraisal. Moreover, we hypothesized that the strength of functional coupling between amygdala and frontal regions would predict intensity of negative affect.
METHODS
Participants
Fourteen healthy, right-handed volunteers (six men, aged 22–38 years, mean 27.6 ± 4.4 years) participated in the study. Participants had normal or corrected normal vision and were without a history of psychiatric, medical or neurologic illness, as verified by a semistructured clinical interview. All participants provided written informed consent as approved by the local Human Investigation Committee.
Task procedure
Study protocol and task procedure has been described in detail elsewhere (Phan et al., 2005). In brief, the experimental paradigm employed a task involving active, voluntary regulation of negative emotion by cognitive reappraisal (Ochsner et al., 2002, 2004a, b; Ochsner and Gross, 2005; Phan et al., 2005). The stimulus set consisted of 40 highly aversive and arousing pictures based on normative ratings from the International Affective Picture System (IAPS; Lang et al., 1997) and five blank (BL) gray-scale images with a centered fixation cross. The mean (±s.d.) valence and arousal values on a 9-point scale for the pictures were 2.03 ± 0.44 and 6.03 ± 0.72, respectively (1 = most unpleasant/least arousing, 9 = most pleasant/most arousing) based on normative ratings (Lang et al., 1993). The pictures were transformed to gray-scale using Adobe Photoshop 7 (Adobe Systems, San Jose, California, USA). The pictures elicit both evaluation and experience of negative affect (Lang et al., 1993) and generally depict complex scenes of burn victims, funeral scenes, people crying and dead animals. Of note, the pictures selected for the two experimental/task conditions were matched for general content, including faces and figures and were balanced on subjective valence and arousal (t-test, P > 0.5).
The protocol involved two task conditions of interest, ‘Maintain’ and ‘Reappraise,’ which alternated across blocks in a counterbalanced order. During the Maintain task, participants were instructed to attend to, be aware of and experience naturally (without trying to change or alter) the emotional state elicited by the pictures; they were told to maintain the evoked affect for the entire task block. During the Reappraise task, participants were instructed to voluntarily decrease the intensity of their negative affect by using the cognitive strategy of reappraisal (Lazarus, 1991; Gross, 1999a), adapted for fMRI (Ochsner et al., 2002, 2004a, b; Ochsner and Gross, 2005; Phan et al., 2005; Urry et al., 2006); they were told to reinterpret the content of the picture so that it no longer elicited a negative response. Extensive instruction on the cognitive strategy of reappraisal was provided to participants prior to the initiation of the experiment, and understanding of the task was confirmed prior to scanning by reviewing examples of subject-generated strategies (Phan et al., 2005).
The task involved a block-related design in which subjects viewed 20 s blocks of aversive pictures; each picture was presented for 4 s consecutively without an interstimulus interval. Prior to each block of pictures, the instruction to ‘Maintain’ or ‘Suppress’/Reappraise appeared at the center of a gray screen below a fixation cross for a duration of 4 s (Instruction). Immediately following each Maintain and Reappraise block, a blank screen with a rating scale appeared for 4 s asking participants to rate the intensity of their negative affect on a 5-point scale (1 = least negative/neutral, 5 = extremely negative) via button response by pressing the button 1–5 times (Rating). The Maintain/Reappraise blocks were interspersed with 20 s baseline blocks consisting of blank fixation images (4 s each) to minimize carryover effects (‘Baseline’), and to allow the blood oxygen-level dependent (BOLD) signal to return to baseline. During this period, participants were asked to stop maintaining or reappraising their emotional experience and to ‘relax and clear your mind’. The total task duration was 12 min. The order of task and picture epochs was counterbalanced across subjects. We had previously reported that the intensity of negative emotion was higher following the Maintain block than the Suppress/Reappraise block (Mean rating ± s.d.: 4.27 ± 0.33 vs 1.95 ± 0.70, respectively; t (13) = 11.30, P < 0.001), suggesting that the reappraisal task had the intended effect of reducing negative affect as indexed by self-report (Phan et al., 2005).
fMRI data acquisition
Functional MRI was performed on a 1.5-T Sonata scanner (Siemens Medical Systems, Erlangen, Germany) to acquire BOLD contrast using single-shot multi-echo T2*-weighted EPI (echoplanar imaging) with six evenly spaced echo times ranging from 18 to 93 ms (repetition time = 2000 ms; 192 mm field of vision, 32 × 32 matrix, 16 slices, 6 mm slice thickness, 0.6 mm slice gap [voxel size = 6mm isotropic with a 0.6 mm skip]; flip angle = 90°). This novel single-shot multi-slice MR imaging technique (TurboPEPSI), which acquires up to six consecutive echoplanar images with multiple echo times and allows acquisition of multiple images that are matched for a wide range of T2* values such that the data are sampled and summed at multiple echo times (i.e., at different relaxation signals), confers enhanced BOLD-contrast sensitivity compared to conventional echoplanar imaging (EPI), as previously described (Posse et al., 1999). Slices were oriented axially or near axially along the AC–PC (anterior commissure–posterior commissure) line. The first four volumes from each run were discarded to allow for T1 equilibration effects. Additionally, a T1-weighted high-resolution structural scan was acquired in each subject for subsequent anatomic localization. Head movement was minimized using a custom-fitted head holder, consisting of two-component polyurethane foam tightly molded around the head and neck.
fMRI data processing
Data processing and analyses for data reported here employed Statistical Parametric Mapping software (SPM2; Wellcome Department of Imaging Neuroscience, London, UK). The functional volumes were spatially realigned to correct for motion and corrected for slice-timing, normalized to a standard template based upon the Montreal Neurologic Institute (MNI) reference brain and smoothed with an 8 mm FWHM Gaussian kernel. For each participant, task-related activity was identified by convolving a vector of the onset times of the experimental blocks (box-car design) with a canonical hemodynamic response. A 100 s high-pass filter was applied to reduce low frequency noise. The statistical analyses relied upon the general linear model to model effects of interest (activation during Maintain and Reappraise) (Friston et al., 1995).
The current analysis carried out an examination of PPI (Friston et al., 1997) which is intended to capture interactions between brain regions in relation to the experimental design. A PPI analysis is used to compare the functional ‘coupling’ of different brain regions (physical component) during different tasks (psychological component); it can capture the modulation of activity in one brain region by activity in another brain region dependent on specific active tasks. In a PPI analysis, a design matrix contains three columns of variables: (i) a ‘psychological’ variable that represents the experimental paradigm; (ii) a time-series ‘physiological’ variable that represents the time course of the source (seed) region; (iii) an interaction variable that represents the interaction of psychological and physiological variables, (i) and (ii) respectively. The regression coefficient for the interaction term provides a measure of PPI; a correlation (or covariance) in activity between the source/seed region and the identified ‘coupled’ region that is significantly different between tasks (Reappraise vs Maintain) yields a significant PPI effect. As such, PPI analysis examines differences in task-dependent (i.e., context-specific) functional connectivity between regions of interest. The PPI analysis examines differences in functional connectivity between regions (i.e., influence of one region on another) as a function of task manipulation. As such, a significant PPI effect would demonstrate that inter-regional coactivation was significantly greater during Reappraise than during Maintain tasks. Of note, a significant PPI does not inform us about the directional nature of the regression slope under each condition individually, it only shows the direction of the change in covariation (increase or decrease) between the tasks (Friston et al., 1997; Friston, 1998).
In the current analysis, we were specifically interested in amygdala–cortical interactions during active emotion regulation. Hence, we chose the amygdala as a potential source region of interest. Based on our experimental design (i.e., involving negatively valenced images) and to perform unbiased contrast analysis, we first performed a conjunction analysis with SPM2 (Price and Friston, 1997; Friston et al., 1999; Nichols et al., 2005) to identify amygdala activation that was present across both Maintain and Reappraise task blocks. The conjunction (i.e., [Maintain > Baseline] AND [Reappraise > Baseline]) analysis identified that the left amygdala cluster (MNI coordinates of peak activation: [−22, 0, −22], 70 voxels, Z = 2.37, P < 0.05, small-volume corrected) was active during both Maintain and Reappraise blocks, consistent with prior reports (Phan et al., 2002, 2005; Wager et al., 2003). We had several reasons for choosing this foci (and the 10 mm spherical ROI that surrounds it) as our seed/source region. First, we wanted to identify active voxels that exhibited enhanced BOLD signal to the aversive/ negatively valenced pictures, rather than examine voxels that were de-activated (negative BOLD values) relative to the passive baseline. Second, this time-series best reflected emotion-related amygdala reactivity to negatively valenced pictures, and this analysis was intended to identify brain regions selectively influenced by general, affect-driven amygdala responses as a function of the Reappraise task. In other words, we chose not to restrict the ‘seed’ region to only those amygdala voxels that were less active in Reappraise than Maintain; choosing only those appraisal-modulated voxels may have biased our results towards finding a task-related (Reappraise > Maintain) connectivity pattern. Third, the current analysis was directed at examining differences in connectivity between the Reappraise and Maintain tasks in relation to amygdala responses to negative pictures, and not necessarily specific to amygdala responses modulated by Reappraisal.
The presence of a significant task-specific (Reappraise vs Maintain) change in coupling between the amygdala and other brain regions can be interpreted either as the influence of the amygdala on the other region during a particular task, or as a change in responsiveness of the amygdala to activity from the other brain region (Friston et al., 1997).
To perform the PPI analyses, the deconvolved time series from a 5 mm radius sphere around the group peak activation voxel [left amygdala, (−22, 0, −22)] identified in the conjunction analysis (above) was extracted for each subject. The effect of the interaction term was examined using the contrast [1 0 0] in which the first column represents the interaction term, as calculated between the left amygdala time series and a block vector representing the tasks of interest (Reappraise vs Maintain) (Gitelman et al., 2003; Egner and Hirsch, 2005; Etkin et al., 2006); as such the activity within this left amygdala mask was regressed on a voxel-wise basis against the product of this time course and the vector of the psychological variable of interest, with the ‘physiological’ and ‘psychological’ variables serving as regressors of interest. The individual contrast images were then entered into a 2nd-level random effects analysis (df = 13) in which task-dependent effects (Reappraise > Maintain; Maintain > Reappraise) were investigated using one-sample t-tests, in order to identify which, if any, areas of the brain exhibited activity that covaried with that of the left amygdala significantly more during the Reappraise than Maintain task, and vice versa. As such the PPI effect, or task-dependent coupling between regions, is calculated as the difference between regression slopes; a significant effect for PPI (connectivity estimate) indicates that covariance between the seed region (amygdala) and a coupled region during Reappraise is significantly higher than that during Maintain. Significant clusters exhibiting PPI-related amygdala coupling were identified with a threshold of P < 0.001 uncorrected (t > 3.85) with at least 10 contiguous voxels in the cluster (120 mm3); the combination of the height-extent threshold effectively yields equivalent correction for multiple comparisons (Forman et al., 1995).
In addition, based on similar approaches (Ochsner et al., 2002; Phan et al., 2005; Etkin et al., 2006), we were specifically interested in the relationship between within-subject amygdala–cortical coupling with subject-specific behavioral measures. Thus, we examined the relationship between the magnitude of amygdala–cortical coupling and negative affect, a subjective index of successful emotion regulation via reappraisal. Using Spearman's rank correlation analysis on these non-parametric behavioral data, we correlated the extent of amygdala–cortical coupling (represented by the PPI beta estimate) with self-report intensity scores of negative affect (range, 1–5) collected on-line following each Reappraise block.
RESULTS
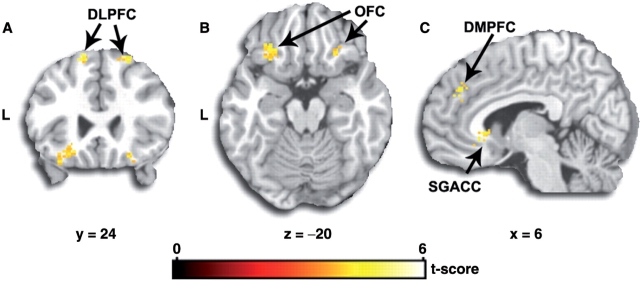
PPI analyses (results shown in Table 1 and Figure 1) showed that activity in the left amygdala was accompanied by task-dependent (Reappraise > Maintain) functional interaction with specific areas: bilateral DLPFC, bilateral OFC, right subgenual/subcallosal ACC (SGACC), right DMPFC, bilateral inferior parietal cortex (IPC). In other words, the covariance in activity between the seed region (amygdala) and the DLPFC, OFC, SGACC, DMPFC and IPC during Reappraise was significantly higher than that during Maintain. The pattern of coupling was observed only in the Reappraise > Maintain contrast (i.e., seed and coupled regions exhibited greater coactivation specifically during Reappraise). The reverse contrast (Maintain > Reappraise) did not reveal a significant PPI effect. An absence of a significant PPI during the reverse contrast broadly suggests that the amygdala activity did not significantly covary with activity in other brain regions during the Maintain task, relative to the Reappraise task, such that the amygdala-related PPI estimate was not greater during the Maintain than during Reappraise. Hence, the null result in this reverse contrast could also reflect an absence of amygdala–frontal coupling in the Reappraise task.
Table 1.
Task-related PPI analysis of left amygdala seed
| Brain region of co-activation | ||||||
|---|---|---|---|---|---|---|
| Reappraise > Maintain | Laterality | (x, | y, | z) | Z-score | Size |
| Dorsolateral prefrontal cortex | L | −12 | 22 | 60 | 4.07 | 50 |
| R | 20 | 24 | 60 | 3.78 | 57 | |
| R | 48 | 28 | 36 | 3.51 | 11 | |
| Orbitofrontal cortex | L | −24 | 28 | −14 | 4.02 | 257 |
| R | 26 | 24 | −22 | 3.69 | 38 | |
| Subgenual anterior cingulate cortex | R | 6 | 24 | −2 | 3.74 | 36 |
| Dorsomedial prefrontal cortex | R | 8 | 44 | 32 | 3.62 | 45 |
| Inferior parietal cortex | L | −44 | −54 | 38 | 3.27 | 15 |
| R | 44 | −62 | 44 | 3.37 | 48 | |
| Maintain > Reappraise | ||||||
| No significant clusters |
Regions showing amygdala coupling with clusters of 10 or more contiguous voxels whose global maxima exceeds a t-statistic of 3.85 (P < 0.001 uncorrected). For each maximal activation foci per cluster, laterality (L, left; R, right), coordinates, Z score and size (number of contiguous voxels within cluster) are provided. Coordinates are defined in Montreal Neurologic Institute stereotactic space in millimeters: x > 0 is right of the midsagittal plane, y > 0 is anterior to the anterior commissure and z > 0 is superior to anterior commissure–posterior commissure plane.
Fig. 1.
SPM t-map showing areas that exhibit significant task-dependent (Reappraise > Maintatin) coupling with the left amygdala: (A) DLPFC; (B) OFC; (C) DMPFC and SGACC. Maps are overlaid on coronal slice of a canonical brain rendering constructed using MRIcro software (http://www.sph.sc.edu/comd/rorden/mricro.html), shown on cororonal (A), axial (B) and sagital (C) canonical brain slices, with location of slices in coordinates as defined in Montreal Neurologic Institute stereotactic space. Activations are thresholded at P < 0.001 (uncorrected) showing only significant clusters with more than 10 contiguous voxels. L, Left.
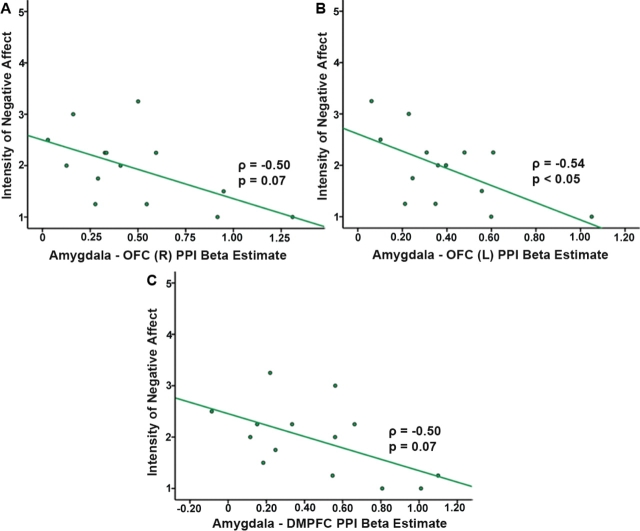
As noted above, the PPI beta estimate (a measure of task-dependent functional connectivity) of the extent of coupling between the left amygdala seed region and each ‘coupled’ region (identified in Table 1) was extracted and correlated with intensity of negative affect following the Reappraise task. Of the amygdala-coupled brain regions (shown in Table 1) that exhibited task-dependent (Reappraise > Maintain) coactivation with the amygdala during voluntary emotion regulation, only two regions exhibited a significant correlation with the self-report intensity scores of negative affect: (i) bilateral OFC; (ii) right DMPFC. Those individuals whose amygdala predicted greater activation (in the psychophysiological interaction) of the OFC and DMPFC during Reappraise showed less negative affect: the pattern was significant in the left OFC (Spearman r = −0.536, P = 0.048), and the pattern showed trend-level significance in the right OFC (Spearman r = −0.498, P = 0.070) and DMPFC (Spearman r = −0.498, P = 0.070) (Figure 2).
Fig. 2.
Spearman rank correlation graphs showing the relationship of negative affect (self-report intensity scores) and PPI beta estimate of amygdala coupling with right orbitofrontal cortex (A), left orbitofrontal cortex (B) and dorsal medial prefrontal cortex (C). OFC, DMPFC; L, Left, R, Right. Greater amygdala-predicted activation of OFC and DMPFC is associated with lower intensity of negative affect.
DISCUSSION
Using PPI analyses (Friston et al., 1997), we demonstrate amygdala–frontal coupling specifically during emotion regulation. In particular, we observed that left amygdala activity covaries with activity in the DLPFC, DMPFC, OFC, SGACC and IPC to a greater extent during reappraisal-based control of negative affect compared to maintenance of that affect. Moreover, we show that the strength of coupling between the amygdala and the OFC/DMPFC predicted successful emotion regulation, as indexed by reduction in self-reported negative affect. These findings specifically point to amygdala–frontal interactions as being an important neural mechanism that underlies the control of emotion.
The reappraisal-modulated coactivation of specific frontal regions (DLPFC, DMPFC, OFC, ACC) with amygdala reactivity to negative affect is well supported by prior neuroimaging studies that implicate these same areas as being engaged during active, voluntary emotion regulation (Ochsner et al., 2002, 2004b; Levesque et al., 2003b; Phan et al., 2005; Ohira et al., 2006; Urry et al., 2006). Two recent critical meta-analyses of neuroimaging data show that these frontal regions exert greater activity when subjects engage cognitive strategies (i.e., reappraisal, detachment) to modulate negative affect and less activity when subjects passively maintain affect (i.e., negative emotion unaltered) (Ochsner and Gross, 2005; Quirk and Beer, 2006). This is broadly consistent with the role of these regions in cognitive inhibition/control and executive function (Cabeza and Nyberg, 2000; Miller and Cohen, 2001), processes that are relevant to reappraisal-based affect regulation.
These findings of amygdala–frontal interactions are further supported by known reciprocal anatomical connections between the amygdala and the frontal circuit (ACC, OFC, DMPFC) (Amaral and Price, 1984; Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007), specifically identified as an emotion generation–regulation circuit (Ghashghaei et al., 2007). In both animal and human studies, lesions to these areas result in emotion dysregulation (Williams and Mateer, 1992; Damasio, 1994; Izquierdo and Murray, 2005; Sanchez-Navarro et al., 2005). It has been posited that the prefrontal cortex has a top–down inhibitory effect on the amygdala (Pears et al., 2003; Rosenkranz et al., 2003; Izquierdo and Murray, 2005; Quirk and Beer, 2006). The prefrontal projections to the amygdala originate mostly in those layers of the cortex involved in feed-forward mechanisms, and terminate on inhibitory interneurons in the amygdala (Carmichael and Price, 1995; McDonald et al., 1996) providing some evidence for a top–down role for the prefrontal cortex on limbic regions (Carmichael and Price, 1995; McDonald et al., 1996). In support, based on between-subjects correlational analyses, two studies have observed increased activation of prefrontal brain regions, including OFC and rostral ACC, as associated with attenuated amygdala reactivity during emotion regulation (Ochsner et al., 2002; Urry et al., 2006), consistent with the notion that frontal cortex exerts a top–down inhibitory influence on the amygdala.
However, a number of recent studies on emotion and self-relevance processing using within-subjects functional connectivity analyses have shown positive temporal correlations in activation signal between or coactivation of amygdala and frontal cortex, specifically the OFC/ventral MPFC (Kim et al., 2004; Heinz et al., 2005; Schmitz and Johnson, 2006), dorsal MPFC (Williams et al., 2006) and ACC (Das et al., 2005; Pezawas et al., 2005; Williams et al., 2006). This is further supported by the recent observation that metabolic rates in the amygdala are positively correlated with those in the OFC, DLPFC and ACC (New et al., 2007). Moreover, recent studies using similar PPI-based analyses have shown increased coactivation between amygdala and MPFC (Erk et al., 2006; Schmitz and Johnson, 2006; Williams et al., 2006) and between amygdala and DLPFC (Siegle et al., 2007) during cognitive-emotional tasks. These findings support our own data that stronger coupling exists between amygdala and these areas of the frontal cortex during emotion regulation. However, it remains unclear the directional nature (i.e., reciprocity) of these observed interactions within task (Reappraise or Maintain) given that the PPI analysis only tests if differences in inter-regional coupling exist as function of task (higher or lower covariation between Reappraise and Maintain). Therefore, it is possible that enhanced amygdala–frontal coupling during Reappraise could reflect cognitive consequences or enhanced cognitive effort due to failing to down-regulate amygdala activity. We note that the behavioral results, which show that subjective negative affect did decrease following Reappraise compared to Maintain blocks, would suggest that the intent to suppress was successful. However, the temporal correlation of these amygdala–frontal associations between extended time-series data and the bidirectional nature of connections between these regions do not imply causal inference. Future studies are needed to disambiguate when and how the amygdala and frontal cortex exert their effects on one another during emotion regulation.
We observed that the extent of coupling between amygdala and specifically OFC and DMPFC was related to effective emotion regulation, such that greater functional connectivity between amygdala and OFC/DMPFC during Reappraise was associated with less intensity of negative affect. Urry et al. (2006) have recently observed that individual variation in OFC/ventral MPFC is related to successful regulation of negative affect, such that individuals with higher activity in this region during the reappraisal task also exhibited lower amygdala signal and more normative diurnal decline of the stress-related hormone cortisol. Moreover, Beauregard et al. (2006) showed that activation of the OFC and MPFC was related to the emotional demands or subjectively perceived difficulty while suppressing sadness. It has been posited that OFC serves as an important relay or coordination region for amygdala and MPFC interactions (Kim et al., 2004; Quirk and Beer, 2006; Urry et al., 2006). The MPFC is consistently implicated in studies of emotional processing, including emotional arousal and personal salience (Phan et al., 2003, 2004) and has been linked to the integration of emotion–cognition interactions (Phan et al., 2002) and to processes that underlie cognitive reappraisal (Ochsner and Gross, 2005). Consistent with its role in emotional appraisal and response, the MPFC has been shown to be positively correlated with activity in the amygdala during anticipation of negative emotion (Erk et al., 2006), and associated with monitoring and/or appraising one's own emotional state (Taylor et al., 2003; Ochsner et al., 2004a) and making self-referential judgments regarding emotionally salient stimuli (Gusnard et al., 2001). Some have posited a functional distinction between ventral and dorsal MPFC, such that ventral MPFC is involved in appraisal for emotional salience of stimuli and dorsal MPFC is involved in regulation of appropriate behaviors following appraisal (Phillips et al., 2003a). Schmitz and Johnson (2006) recently demonstrated task-dependent (self-appraisal vs non-referential affective appraisal) connectivity between ventral MPFC and amygdala. Future studies are needed to parse out the direct and indirect relationships among prefrontal (dorsal vs ventral) brain regions during emotion regulation.
In addition to frontal regions, we observed amygdala coactivation of IPC during emotion regulation. This region was not observed to be activated in the Reappraise task in our initial analyses (Phan et al., 2005). Ochsner et al. (2002, 2004b) did show this region (‘supramarginal gyrus’, ‘inferior parietal lobule’) to be activated during cognitive reappraisal, relative to attend. The parietal cortex is strongly anatomically interconnected with the prefrontal cortex (Petrides and Pandya, 1984), and functional connectivity has also been indicated between these regions (Chafee and Goldman-Rakic, 2000). However, the exact nature of non-frontal involvement in affect regulation remains unclear. Modulation by inferior parietal cortices during reappraisal could reflect attentional selection, working memory and/or resolving cognitive interference (Cabeza and Nyberg, 2000). Future studies are needed to clarify the role of brain regions outside of the amygdala–frontal circuit in emotion regulation.
Certain limitations of this study are noteworthy. First, we did not include positive emotional stimuli (i.e., pleasant pictures), thus these findings are not generalizable to other forms of emotional experience. Second, our task specifically involved cognitive appraisal, and thus the findings do not extend to other strategies of emotion regulation (i.e., suppression, detachment). Third, the study employed a small sample of subjects, and replication in a larger sample is needed. Fourth, this study relied on self-report measures to infer effective emotion regulation, and did not employ corroborative objective measures (i.e., skin conductance, performance) to index regulatory success. Fifth, the present investigation contributes further evidence for functional connectivity between amygdala and frontal regions during emotion regulation; future studies driven by these findings and anatomical studies would develop an a priori mechanistic neural network model of how prefrontal cortex influences amygdala, and vice versa and pursue effective connectivity analyses in order to infer causal relationships (Friston et al., 2003).
In conclusion, this study provides further evidence for the importance of specific frontal brain regions, namely the DLPFC, OFC, ACC, in the regulation of negative affect. These regions covaried to a greater extent with activation of amygdala reactivity specifically during active attempts at emotion suppression via reappraisal, and the greater the task-dependent amygdala–OFC/DMPFC coupling, the more effective the reappraisal strategy, as noted by attenuated intensity of negative affect. Though directionality of these influences is unknown, these findings highlight the role of amygdala–frontal interactions during emotion regulation. Given that a number of psychiatric disorders are associated with affective instability and emotion dysregulation (Koenigsberg et al., 2002; Phillips et al., 2003b), paradigms and analyses such as the one presented here should be extended to studies of patients with anxiety, mood and personality disorders.
Acknowledgments
We would like to thank Dan Fitzgerald for his assistance in data collection, and Dan Fitzgerald, Greg Moore, Tom Uhde and Manny Tancer for their assistance in the experimental and fMRI design of the initial study from which the current data analysis is based. This work was partly supported by the Joe F. Young Sr. Psychiatric Research and Training Program and National Institute of Mental Health Grant MH076198.
Footnotes
Conflict of Interest
None declared.
REFERENCES
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–77. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Aggleton JP. The contribution of the amygdala to normal and abnormal emotional states. Trends in Neuroscience. 1993;16:328–33. doi: 10.1016/0166-2236(93)90110-8. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Bauman MD, Capitanio JP, et al. The amygdala: is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:517–22. doi: 10.1016/s0028-3932(02)00310-x. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) Journal of Comparative Neurology. 1984;230:465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Angrilli A, Mauri A, Palomba D, et al. Startle reflex and emotion modulation impairment after a right amygdala lesion. Brain. 1996;119:1991–2000. doi: 10.1093/brain/119.6.1991. [DOI] [PubMed] [Google Scholar]
- Bantick SJ, Wise RG, Ploghaus A, Clare S, Smith SM, Tracey I. Imaging how attention modulates pain in humans using functional MRI. Brain. 2002;125:310–9. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Levesque J, Bourgouin P. Neural correlates of conscious self-regulation of emotion. Journal of Neuroscience. 2001;21:RC165. doi: 10.1523/JNEUROSCI.21-18-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–6. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–8. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–41. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. Journal of Neurophysiology. 2000;83:1550–66. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, McCloskey M, Fitzgerald DA, Phan KL. Amygdala and orbitofrontal reactivity to social threat in individuals with impulsive aggression. Biological Psychiatry. 62:168–78. doi: 10.1016/j.biopsych.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ Error: Emotion, reason and the human brain. New York: Avon Books, Inc; 1994. [Google Scholar]
- Das P, Kemp AH, Liddell BJ. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26:141–8. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation – a possible prelude to violence. Science. 2000;289:591–4. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Donegan NH, Sanislow CA, Blumberg HP, et al. Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation. Biological Psychiatry. 2003;54:1284–93. doi: 10.1016/s0006-3223(03)00636-x. [DOI] [PubMed] [Google Scholar]
- Egner T, Hirsch J. Cognitive control mechanisms resolve conflict through cortical amplification of task-relevant information. Nature Neuroscience. 2005;8:1784–90. doi: 10.1038/nn1594. [DOI] [PubMed] [Google Scholar]
- Erk S, Abler B, Walter H. Cognitive modulation of emotion anticipation. European Journal of Neuroscience. 2006;24:1227–36. doi: 10.1111/j.1460-9568.2006.04976.x. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Peraza DM, Kandel ER, Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51:871–82. doi: 10.1016/j.neuron.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33:636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Friston KJ. Imaging neuroscience: principles or maps?. Proceedings of the National Academy of Science USA; 1998. pp. 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Harrison L, Penny W. Dynamic causal modelling. Neuroimage. 2003;19:1273–302. doi: 10.1016/s1053-8119(03)00202-7. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Price CJ, Buchel C, Worsley KJ. Multisubject fMRI studies and conjunction analyses. Neuroimage. 1999;10:385–96. doi: 10.1006/nimg.1999.0484. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RS. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–79. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: affective, cognitive, and social consequences. Psychophysiology. 39:281–91. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 1993;85:348–62. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: past, present and future. Cognition and Emotion. 1999a;13:551–73. [Google Scholar]
- Gross JJ. Emotion regulation: past, present, future. Cognition and Emotion. 1999b;13:551–73. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proceedings of the National Academy of Science USA; 2001. pp. 4259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11:43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53:494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Heinz A, Braus DF, Smolka MN, et al. Amygdala-prefrontal coupling depends on a genetic variation of the serotonin transporter. Nature Neuroscience. 2005;8:20–1. doi: 10.1038/nn1366. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Dietrich TM, Wenning B, et al. Evidence of abnormal amygdala functioning in borderline personality disorder: a functional MRI study. Biological Psychiatry. 2001;50:292–8. doi: 10.1016/s0006-3223(01)01075-7. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. European Journal of Neuroscience. 2005;22:2341–6. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, et al. Contextual modulation of amygdala responsivity to surprised faces. Journal of Cognitive Neuroscience. 2004;16:1730–45. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Koenigsberg HW, Harvey PD, Mitropoulou V, et al. Characterizing affective instability in borderline personality disorder. American Journal of Psychiatry. 2002;159:784–8. doi: 10.1176/appi.ajp.159.5.784. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–73. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. Gainesville, FL: NIMH Center for the Study of Emotion and Attention, University of Florida; 1997. [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, et al. The nature of traumatic memories: a 4-T FMRI functional connectivity analysis. American Journal of Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Emotion and Adaptation. New York: Oxford University Press; 1991. [Google Scholar]
- Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003a;53:502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levesque J, Eugene F, Joanette Y, et al. Neural circuitry underlying voluntary suppression of sadness. Biological Psychiatry. 2003b;53:502–10. doi: 10.1016/s0006-3223(02)01817-6. [DOI] [PubMed] [Google Scholar]
- Levesque J, Joanette Y, Mensour B, et al. Neural basis of emotional self-regulation in childhood. Neuroscience. 2004;129:361–9. doi: 10.1016/j.neuroscience.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Mayberg HS. Limbic-cortical dysregulation: a proposed model of depression. Journal of Neuropsychiatry and Clinical Neuroscience. 1997;9:471–81. doi: 10.1176/jnp.9.3.471. [DOI] [PubMed] [Google Scholar]
- McCloskey MS, Phan KL, Coccaro EF. Neuroimaging and personality disorders. Current Psychiatry Reports. 2005;7:65–72. doi: 10.1007/s11920-005-0027-2. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: a meta-analysis. Cognitive Affective and Behavioural Neuroscience. 2003;3:207–33. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- New AS, Hazlett EA, Buchsbaum MS, et al. Amygdala-prefrontal disconnection in borderline personality disorder. Neuropsychopharmacology. 2007;32:1629–40. doi: 10.1038/sj.npp.1301283. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25:653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Jounal of Cognitive Neuroscience. 2002;14:1215–29. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience. 2004a;16:1746–72. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004b;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Ohira H, Nomura M, Ichikawa N, et al. Association of neural and physiological responses during voluntary emotion suppression. Neuroimage. 2006;29:721–33. doi: 10.1016/j.neuroimage.2005.08.047. [DOI] [PubMed] [Google Scholar]
- Pears A, Parkinson JA, Hopewell L, Everitt BJ, Roberts AC. Lesions of the orbitofrontal but not medial prefrontal cortex disrupt conditioned reinforcement in primates. Journal of Neuroscience. 2003;23:11189–201. doi: 10.1523/JNEUROSCI.23-35-11189.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessoa L, Kastner S, Ungerleider LG. Attentional control of the processing of neural and emotional stimuli. Brain Research Cognitive Brain Research. 2002;15:31–45. doi: 10.1016/s0926-6410(02)00214-8. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. Journal of Comparative Neurology. 1984;228:105–16. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, et al. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nature Neuroscience. 2005;8:828–34. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Decker LR, Noll DC, Nichols TE, et al. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a functional magnetic resonance imaging study. Biological Psychiatry. 2003;53:211–15. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21:768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16:331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Human emotion and memory: interactions of the amygdala and hippocampal complex. Current Opinion Neurobiology. 2004;14:198–202. doi: 10.1016/j.conb.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003a;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biological Psychiatry. 2003b;54:515–28. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Posse S, Wiese S, Gembris D, Mathiak K, Kessler C, Grosse-Ruyken ML, et al. Enhancement of BOLD-contrast sensitivity by single-shot multi-echo functional MR imaging. Magnetic Resonance in Medicine. 1999;42:87–97. doi: 10.1002/(sici)1522-2594(199907)42:1<87::aid-mrm13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Cognitive conjunction: a new approach to brain activation experiments. Neuroimage. 1997;5:261–70. doi: 10.1006/nimg.1997.0269. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–76. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rosenkranz JA, Moore H, Grace AA. The prefrontal cortex regulates lateral amygdala neuronal plasticity and responses to previously conditioned stimuli. Journal of Neuroscience. 2003;23:11054–64. doi: 10.1523/JNEUROSCI.23-35-11054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Navarro JP, Martinez-Selva JM, Roman F. Emotional response in patients with frontal brain damage: effects of affective valence and information content. Behavioral Neuroscience. 2005;119:87–97. doi: 10.1037/0735-7044.119.1.87. [DOI] [PubMed] [Google Scholar]
- Schaefer SM, Jackson DC, Davidson RJ, Aguirre GK, Kimberg DY, Thompson-Schill SL. Modulation of amygdalar activity by the conscious regulation of negative emotion. Journal of Cognitive Neuroscience. 2002;14:913–21. doi: 10.1162/089892902760191135. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Self-appraisal decisions evoke dissociated dorsal-ventral aMPFC networks. Neuroimage. 2006;30:1050–8. doi: 10.1016/j.neuroimage.2005.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biological Psychiatry. 2001;50:651–8. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Shin LM, Whalen PJ, Pitman RK, Bush G, Macklin ML, Lasko NB, et al. An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biological Psychiatry. 2001;50:932–42. doi: 10.1016/s0006-3223(01)01215-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biological Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18:650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tracey I, Ploghaus A, Gati JS, Clare S, Smith S, Menon RS, et al. Imaging attentional modulation of pain in the periaqueductal gray in humans. Jounal of Neuroscience. 2002;22:2748–52. doi: 10.1523/JNEUROSCI.22-07-02748.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, Schaefer HS, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. Neuroimage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biological Psychiatry. 1998;44:1219–28. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- Williams D, Mateer CA. Developmental impact of frontal lobe injury in middle childhood. Brain and Cognition. 1992;20:196–204. doi: 10.1016/0278-2626(92)90069-x. [DOI] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell BJ, Kemp AH, Rennie CJ, Gordon E. Mode of functional connectivity in amygdala pathways dissociates level of awareness for signals of fear. Jounal of Neuroscience. 2006;26:9264–71. doi: 10.1523/JNEUROSCI.1016-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research Brain Research Reviews. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]