Abstract
With the advent of studies showing that amygdala responses are not limited to fear-related or highly unpleasant stimuli, studies began to focus on stimulus valence and stimulus-related arousal as predictors of amygdala activity. Recent studies in the chemosensory domain found amygdala activity to increase with the intensity of negative and positive chemosensory stimuli. This has led to the proposal that amygdala activity might be an indicator of emotional arousal, at least in the chemosensory domain. The present study investigated amygdala activity in response to visual and auditory stimuli. By selecting stimuli based on individual valence and arousal ratings, we were able to dissociate stimulus valence and stimulus-related arousal, both on the verbal and the peripheral physiological level. We found that the amygdala was sensitive to stimulus valence even when arousal was controlled for, and that increased amygdala activity was better explained by valence than by arousal. The proposed difference in the relation between amygdala activity and stimulus-related arousal between the chemosensory and the audiovisual domain is discussed in terms of the amygdala's embedding within these sensory systems and the processes by which emotional meaning is derived.
Keywords: amygdala, valence, arousal, visual, auditory, startle reflex, skin conductance response, emotion dimensions
INTRODUCTION
The amygdala, a structure in the anterior medial temporal lobe comprising subcortical nuclei and cortical grey matter (Swanson and Petrovich, 1998) is widely recognized as a core structure in affective processing. Until recently, the amygdala has been seen primarily as being involved in the detection of fear-related or highly unpleasant stimuli (Davis, 1992; Adolphs et al., 1994; LeDoux, 1995; Morris et al., 1996; Phan et al., 2002). Growing evidence that the amygdala responds to a variety of negative and positive stimuli (Breiter et al., 1996 [facial expressions]; Cunningham et al., 2004 [semantic concepts]; Garavan et al., 2001; Hamann et al., 2002 [pictures]; Hamann and Mao, 2002 [words]; Liberzon et al., 2003; Phan et al., 2004; Sabatinelli et al., 2005 [pictures]; Yang et al., 2002; Williams et al., 2004a, 2004b, 2005 [facial expressions]; for review see Zald, 2003) has led to the suggestion that the amygdala might not be sensitive to the valence of a stimulus, that is how negative or positive it is, but might mediate the emotional arousal associated with highly unpleasant and pleasant stimuli.
For many visual and auditory stimuli, the tight connection between stimulus valence and stimulus-associated arousal makes it difficult to dissociate brain circuits involved in the processing of valence and arousal. Visual stimuli employed to test the relation between amygdala activity and stimulus-related arousal have often been more negative or positive than pictures used as reference stimuli (Phan et al., 2003; Sabatinelli et al., 2005). In the chemosensory domain, stimulus-related arousal is closely associated with the perceived stimulus intensity (Bensafi et al., 2002) and can thus be readily manipulated independently from stimulus valence. Two studies in the chemosensory domain found amygdala activity to increase with the perceived intensity of both positive and negative olfactory and gustatory stimuli (Anderson et al., 2003; Small et al., 2003). These findings have been interpreted as evidence that the amygdala mediates stimulus-related arousal, at least in the chemosensory domain (Anderson and Sobel, 2003; Hamann, 2003). However, a subsequent study by Winston et al. (2005) that additionally incorporated neutral stimuli demonstrated that the amygdala is sensitive to the physical intensity of negative and positive stimuli as suggested by Anderson et al. (2003) and Small et al. (2003), but not to the physical intensity of neutral stimuli. This suggests that the amygdala discriminates negative and positive stimuli from neutral stimuli equated for arousal, at least at sufficient intensities.
Moreover, the relation between amygdala activity and stimulus-related arousal might fundamentally differ between the chemosensory and the audiovisual domain. While the emotional impression of a chemosensory stimulus is closely tied to its physical characteristics (Schiffman, 1974; Ehrlichman and Bastone, 1992), stimulus valence and stimulus-related arousal are often not directly linked to the physical stimulus characteristics in the auditory and particularly in the visual domain (Bradley and Lang, 2000; Taylor et al., 2000; Junghofer et al., 2001). Many human, animal and environmental sounds with a distinct emotional meaning vary greatly in their physical appearance, and whether a visual scene is judged as pleasant or unpleasant, calm or arousing does often not depend on its contrast, luminance or hue. A different relation between amygdala activity and stimulus-related arousal in the chemosensory and audiovisual domain is further in line with the amygdala's different embedding in these sensory systems (McDonald, 1998; Swanson and Petrovich, 1998).
In the present study, we aimed to determine (i) whether the amygdala is sensitive to the valence of pictures and sounds equated for arousal and (ii) whether increasing amygdala activity in response to emotional pictures and sounds is better explained by valence than by arousal. To test these hypotheses, we employed a fully balanced factorial design based on individual ratings of valence and arousal. Additionally, we performed correlation analyses in which valence and arousal were separated by partialling out any common effects. In separate analyses, the two hypotheses were tested for negative and positive versus neutral stimuli, for pictures and sounds, and for the left and right amygdala. Startle eyeblink amplitude and skin conductance response (SCR) were recorded throughout scanning as additional indicators of stimulus valence and arousal.
METHODS
Subjects
Forty right-handed volunteers with no report of neurological or psychiatric disorders participated in the study. One group of subjects (N = 16; 9 women; mean age 27 years; range 21–43 years) was presented with visual stimuli and a second group of subjects (N = 24; 13 women; mean age 27 years; range 20–49 years) was presented with auditory stimuli. Data of subjects in the visual group were also used for a study on the neural correlates of physiological and subjective emotional responses published elsewhere (Anders et al., 2004b). Subjects gave their written informed consent prior to participation and the study was approved by the Ethics Committee of the University of Tübingen Medical School.
Stimuli and experimental design
Visual stimuli included 40 pictures selected from the International Affective Picture System (IAPS, Lang et al., 1997). Pictures depicted one to three humans or animals and were presented on a translucent screen (stimulus size of 17° vertically and 10–28° horizontally) with a duration of 12 s. Auditory stimuli included 40 non-linguistic human, animal and environmental sounds selected from the International Affective Digitized Sounds (IADS, Bradley and Lang, 1999) and were presented through headphones [HD 570, Sennheiser, Germany, modified after Baumgart et al. (1998) for use with fMRI] with a loudness individually adjusted for each subject and a stimulus duration between 4.5 and 6 s. Both visual and auditory stimuli varied largely and independently in published valence and arousal ratings obtained from a large American sample (Lang et al., 1997; Bradley and Lang, 1999). Relatively long stimulus presentation times were chosen to allow recording of peripheral physiological responses (see below). A white fixation cross on a dark background was presented during intertrial intervals in the visual experiment and during the entire auditory experiment. All stimuli were presented in pseudo-randomized order with an intertrial interval of 24 s, 32 s or 40 s and an additional jittering of 0–3 s relative to scan onset.
White noise standard startle probes (50 ms, Berg and Balaban, 1999), adjusted individually to be unpleasant but not painful, were presented through the headphones during each trial (6–9 s after stimulus onset in the visual experiment and immediately after stimulus offset in the auditory experiment), and during each intertrial interval (15 s, 16 s, 21 s or 22 s after stimulus onset). Intervals were chosen such that startle probes always occurred 2 s after scan onset.
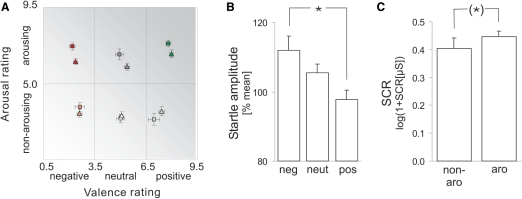
After the scanning procedure, all stimuli were presented in a second pass outside the scanner and subjects were asked to rate how pleasant or unpleasant and how arousing they had experienced each stimulus during scanning on scales ranging from unpleasant (1) to pleasant (9) and non-arousing (1) to arousing (9) on a paper-and-pencil version of the self-assessment manikin (SAM; Lang, 1980; Bradley and Lang, 1994). Based on these ratings, stimuli were rank-ordered, separately for each subject, and stimuli representing six subdivisions of the emotional space (negative non-arousing, negative arousing, neutral non-arousing, neutral arousing, positive non-arousing and positive arousing) were selected. In the visual domain, a stimulus was defined as negative (positive) when it fell into the lower (upper) quartile of valence ratings of a given subject. A stimulus was defined as neutral when it did not fall into the lower or upper quartile of valence ratings. Regardless of its classification with respect to valence, a stimulus was defined as non-arousing (arousing) when it fell into the lower (upper) quartile of arousal ratings of a given subject. In the auditory domain, the same partitioning was used except that non-arousing (arousing) stimuli included stimuli from the lower (upper) half of arousal ratings. This partitioning resulted in emotional categories balanced with respect to individual valence and arousal ratings (Figure 1A).
Fig. 1.
Verbal ratings and peripheral physiological responses. (A) Partitioning resulted in emotional categories well balanced with respect to individual valence and arousal ratings for both pictures and sounds. Axes represent ratings on nine-point scales. Squares and triangles represent pictures and sounds, respectively. Bars reflect s.e.m. Note that not all stimulus categories were represented in all subjects. (B) Mean startle eyeblink amplitude during negative/neutral/positive stimulus presentation. (C) Mean SCR during non-arousing/arousing stimulus presentation. Bars indicate s.e.m. *Significant effect (P < 0.05); (*)marginally significant effect (P < 0.10).
Verbal recordings of experienced valence and arousal after scanning have been shown to reliably represent emotional experiences during scanning (Phan et al., 2004) and avoided biases introduced by monitoring one's own emotion during scanning (Taylor et al., 2003; Phan et al., 2004). To ensure that the delivery of startle probes during the experiment did not lead to an increased level of arousal or a bias toward negative valence, we performed an additional behavioural experiment in which one group of subjects (N = 10) rated all stimuli presented with startle probes, and one group (N = 10) rated all stimuli presented without startle probes. Analyses of variance (ANOVAs) with between-subject factor startle probe and within-subject factor normative stimulus valence or normative stimulus arousal revealed no significant effect of startle probe and no significant interaction between startle probe and stimulus valence or arousal on either valence or arousal ratings [F(1,16) < 1.5, P > 0.20 for all comparisons], while main effects of stimulus valence and arousal on valence and arousal ratings, respectively, were highly significant [F(1,16) > 15, P < 0.0001 for all comparisons].
Data acquisition
Experiments were conducted in a 1.5 Tesla MR Scanner (Siemens, Erlangen, Germany). Functional images were acquired with echoplanar imaging (EPI) of the whole brain (44 coronal slices, slice thickness 3 mm + 1.5 mm gap, 56 × 64 voxel, in-plane resolution 3 × 3 mm2, TE 33 ms, TR 4 s). Scanning was divided into five runs with eight stimulus presentations each. During each run, 64 volumes were acquired, preceded by five scans applied to allow T1 saturation and not included in the analysis. The eyeblink component of the startle response (Vrana et al., 1988) was recorded at 1000 Hz using infrared oculography (Anders et al., 2004c). Skin conductance was recorded at 16 Hz with commercial recording equipment (Vitaport II, Becker Meditec, Karlsruhe, Germany).
Data analysis
Startle eyeblink recordings were smoothed with a 10 ms Gaussian kernel and startle amplitudes were scored as the maximal differential voltage between 21 ms and 150 ms after startle probe onset, relative to the mean of a 20 ms baseline beginning with startle probe onset. All startle amplitudes were scaled to mean per run (Anders et al., 2004c). Skin conductance data were smoothed with a 1 s Gaussian kernel. Skin conductance response (SCR) amplitudes were scored as the largest increase in conductance between 1 and 6 s after picture onset, and between 1 and 5.5 s after sound onset in order to exclude potential effects of the startle probe. SCR amplitudes were log transformed [log(1 + SCR)] for statistical analyses.
Functional imaging data were analysed with SPM99 (Wellcome Department of Imaging Neuroscience, London, UK). Volumes were spatially realigned, corrected for difference in slice acquisition time, normalized into MNI space (Montreal Neurological Institute) and spatially (Gaussian kernel with 15 mm full width at half maximum) and temporally (Gaussian kernel with 4 s full width at half maximum) smoothed. Within-subject design matrices included 40 regressors of interest, one per stimulus, and 45 regressors of no interest (9 per run) modelling responses to startle probes during and between stimulus presentation, estimated head movements (translation and rotation with six degrees of freedom), and run means. Stimulus regressors were derived by convolving box car functions, representing stimulus duration, with the default SPM99 haemodynamic response function. Within these models, individual contrasts were defined for each subject, each representing one subdivision of the emotional space (negative non-arousing, negative arousing, neutral non-arousing, neutral arousing, positive non-arousing and positive arousing).
Parameter estimates for each emotional category were averaged across voxels within the amygdala of each subject [approximated as a 6 mm sphere centred at x = −/+21 mm, y = −3 mm, z = −18 mm (MNI space)]. Averaged parameter estimates of amygdala activity were then entered into eight separate 2 × 2 ANOVAs with within-subject factors valence (negative vs neutral or positive vs neutral) and arousal (non-arousing vs arousing) and subject as random factor (SPSS 12, SPSS Inc, Chicago, IL, USA). Each ANOVA tested two hypotheses: (i) that the amygdala is sensitive to stimulus valence when stimulus-related arousal is balanced (main effect of valence) and (ii) that increased amygdala activity in response to emotional stimuli is better explained by stimulus valence than by stimulus-related arousal (main effect of valence vs main effect of arousal, for comparison see Anderson et al., 2003). As we did not want to preclude the possibility that amygdala responses would differ for negative and positive vs neutral stimuli, for pictures and sounds, and for the left and right amygdala, eight different ANOVAs were run for (i) negative and positive stimuli vs neutral stimuli, (ii) pictures and sounds and (iii) the left and right amygdala. In addition to the two effects testing our two hypotheses, we also report main effects of arousal and interactions of valence and arousal for completeness.
Because the factorial design did not use all trials of each subject in order to ensure balanced categories, we also performed a correlation analysis based on each subject's individual valence and arousal ratings that included all trials. In this analysis, valence and arousal were separated by partialling out any common effects. In analogy to the factorial analyses, eight different correlation analyses were performed for (i) positive and negative stimuli, (ii) pictures and sounds and (iii) the left and right amygdala. For each subject stimuli with valence ratings ≥5 were included in the correlation analysis for positive stimuli, and stimuli with valence ratings ≤5 were included in the correlation analysis for negative stimuli. Correlation coefficients were Fisher-transformed to ensure normal distribution and statistical significance was assessed at random effect group level. As for the factorial design, effects corresponding to our two hypotheses (i.e. parametric effects of valence and parametric effects of valence vs parametric effects of arousal) are reported, along with parametric effects of arousal.
To assess brain activity associated with valence and arousal outside the amygdala, we also performed a whole-brain analysis. This analysis provided as an additional check whether our stimuli activated brain regions reported by previous studies. The whole brain analysis was based on the factorial design described above. Because the relatively small number of subjects who rated a sufficient number of stimuli as negative non-arousing and positive non-arousing did not allow a within-subject comparison of brain responses to negative vs positive stimuli, we used a masking approach to identify brain regions that showed common and differential responses to negative and positive stimuli. Random effect statistical parametric maps (SPMs) representing the six emotional categories (Figure 2) were first conjoint across pictures and sounds by computing the minimal t-value for each voxel (Nichols et al., 2005). Maps representing responses to negative non-arousing, negative arousing, neutral arousing, positive non-arousing and positive arousing stimuli were then exclusively masked by the map representing responses to neutral non-arousing stimuli to eliminate responses common to emotional and non-emotional stimuli. These maps were then overlaid to identify regions commonly and distinctly activated by negative and positive stimuli. To derive comparable effects for all contrasts despite different degrees of freedom, all SPMs were thresholded at T = 3.5, corresponding to a voxel-wise probability of error of P < 0.005 for the contrast with the least degrees of freedom (negative non-arousing pictures, N = 8). This relatively lenient threshold was chosen, in order to reduce the increased probability of false rejections associated with conjunction analyses (Friston et al., 2005). Anatomical regions were defined using automated anatomical labelling (Tzourio-Mazoyer et al., 2002).
Fig. 2.
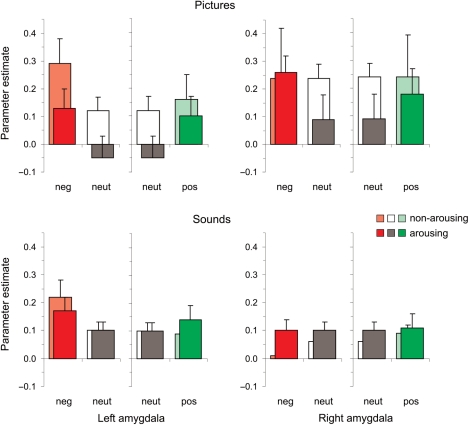
Amygdala activity during visual and auditory stimulation. Mean parameter estimates of amygdala activity elicited by stimuli representing the six emotional categories. Bars indicate s.e.m.
RESULTS
Behavioural data
Stimulus selection based on individual valence and arousal ratings of each subject resulted in balanced valence and arousal categories for both pictures and sounds that did not differ in content or complexity (typical stimuli of each category are given in Table 1). In both modalities, valence categories accounted for >60% of the variance in valence ratings, and for <3% of the variance in arousal ratings. Similarly, arousal categories accounted for >60% of the variance in arousal ratings and for <3% of the variance of valence ratings (visual stimuli; negative vs neutral; valence R2 = 0.62, arousal R2 = 0.01; positive vs neutral; valence R2 = 0.68, arousal R2 = 0.01; auditory stimuli; negative vs neutral; valence R2 = 0.76, arousal R2 = 0.03; positive vs neutral; valence R2 = 0.81, arousal R2 = 0.00). Formal testing confirmed that variance in valence ratings was significantly better explained by valence categories than by arousal categories, and variance in arousal ratings was significantly better explained by arousal categories than by valence categories (P < 0.001 for all comparisons, Figure 1A). Interestingly, subjects showed a higher agreement on valence ratings for a given stimulus than on arousal ratings (visual stimuli; n = 40 stimuli; average s.d. across stimuli, valence = 1.3, arousal = 1.5, Wilcoxon Z = −3.0, P < 0.005; auditory stimuli, n = 40 stimuli, average s.d. across stimuli, valence = 1.4, arousal = 1.7, Wilcoxon Z = −4.7, P < 0.001).
Table 1.
Typical stimuli of the six emotional categories
| Pictures |
| Negative non-arousing |
| Wretched people |
| Unsavory animal predators |
| Negative arousing |
| A severely wounded woman |
| An aggressive dog |
| Neutral non-arousing |
| Three elderly men dressed in tuxedos |
| A cow |
| Neutral arousing |
| A veiled Arabic woman |
| A pack of seemingly wounded seals |
| Positive non-arousing |
| A family |
| Puppies |
| Positive arousing |
| A young man kissing a young woman |
| Laughing chimpanzees |
| Sounds |
| Negative non-arousing |
| Sound of a nose blow |
| A yawn |
| Negative arousing |
| Sounds of a fight |
| A growling dog |
| Neutral non-arousing |
| The soundscape of a restaurant |
| Cackle of chickens |
| Neutral arousing |
| Someone walking slowly |
| Buzzing insects |
| Positive non-arousing |
| Sounds of children playing in a park |
| The song of a cardinal |
| Positive arousing |
| Laughter |
| Erotic utterances |
Analysis of peripheral physiological responses indicated that valence and arousal varied independently across stimulus categories not only on the verbal, but also on the peripheral physiological level. Because peripheral physiological data were not obtained from all subjects (startle; pictures, N = 12, sounds, N = 22; SCR; pictures, N = 16; sounds, N = 21) and did not show statistically significant effects of modality, these data were pooled across the sensory modalities. Startle amplitude was stronger for negative than for positive stimuli (Figure 1B), and did not differ between arousal categories [negative vs positive stimuli, t(37) = 2.5, P < 0.01; arousing vs. non-arousing stimuli, t(32) = 0.4, n.s.]. SCR amplitudes showed a trend to be larger for arousing than for non-arousing stimuli (Figure 1C), and did not differ between valence categories [negative and positive vs neutral stimuli, t(33) = 0.4, NS; arousing vs non-arousing stimuli, t(34) = 1.5, P < 0.10]. Importantly, neither startle amplitudes nor SCR amplitudes showed an interaction of valence and arousal [arousing negative and positive minus neutral vs non-arousing negative and positive minus neutral stimuli; startle, t(12) = 1.4, n.s.; SCR t(26) = 1.3, n.s.] indicating that the two factors were separated in both response systems.
Correlation analyses of each subject's verbal ratings and peripheral physiological responses also indicated a good agreement of verbal and physiological responses on the two factors. In the visual modality, startle amplitude correlated significantly stronger with valence ratings than with arousal ratings, and SCR amplitude correlated marginally stronger with arousal ratings than with valence ratings [mean correlation, startle–valence −0.18, startle–arousal 0.02, SCR–arousal 0.19, SCR–valence −0.10; absolute startle–valence vs absolute startle–arousal, t(11) = 3.2, P < 0.05; absolute SCR–arousal vs absolute SCR–valence, t(15) = 1.5, P < 0.10]. In the auditory modality, startle amplitude correlated significantly with valence ratings and not with arousal ratings, and SCR amplitude correlated significantly with arousal ratings and not with valence ratings, but these differences did not reach statistical significance [mean correlation, startle–valence −0.07, t(21) = −2.3, P < 0.05, startle–arousal 0.02, t(21) = 0.5, SCR–arousal 0.07, t(22) = 1.8, P < 0.05, SCR–valence −0.01, t = −0.3]. This pattern remained the same when common effects of valence and arousal were partialled out.
fMRI data
We first used a factorial design to test the two hypotheses that (i) the amygdala is sensitive to valence of emotional stimuli when arousal is equated (main effect of valence) and (ii) that increased amygdala activity in response to emotional stimuli is better explained by valence than by arousal (main effect of valence vs main effect of arousal) (first and second column of Table 2). Evidence for our first hypothesis was found in the left amygdala for negative pictures and sounds. Evidence for our second hypothesis was also found in the left amygdala. Here, the significant main effect of valence for negative pictures was also significantly stronger than the main effect of arousal, providing evidence for the second hypothesis. Additionally, the main effect of valence was significantly stronger than the main effect of arousal for positive pictures in the left amygdala and for negative pictures in the right amygdala, but these effects were partly due to the (unexpected) negative main effect of arousal.
Table 2.
Amygdala activity during visual and auditory stimulation: factorial design
| Main effect of valance | Main effect of valence vs main effect of arousal | Main effect of arousal | Interaction valence × arousal | |
|---|---|---|---|---|
| Left amygdala | ||||
| Negative vs neutral pictures | T(14) = 2.1; P <.05* | T(7) = 2.6; P <.05* | T(16) = −2.8; NS | T(7) = 0.1; NS |
| Positive vs neutral pictures | T(15) = 1.0; NS | T(8) = 2.1; P <.05* | T(15) = −1.3; NS | T(8) = 0.0; NS |
| Negative vs neutral sounds | T(25) = 1.6; P <.05* | T(16) = 1.4; P <.10 | T(26) = 0.0; NS | T(16) = 0.1; NS |
| Positive vs neutral sounds | T(23) = 0.5; NS | T(22) = 0.1; NS | T(23) = 0.3; NS | T(20) = 0.4; NS |
| Right amygdala | ||||
| Negative vs neutral pictures | T(15) = 0.3; NS | T(7) = 1.9; P <.05* | T(15) = −0.4; NS | T(7) = 1.7; P <.10 |
| Positive vs neutral pictures | T(15) = 1.0; NS | T(8) = 1.7; P <.10 | T(15) = −1.3; NS | T(8) = 2.1; P <.05 |
| Negative vs neutral sounds | T(25) = 0.9; NS | T(16) = −1.6; NS | T(26) = 2.7; P <.05 | T(16) = 1.1; NS |
| Positive vs neutral sounds | T(23) = 0.5; NS | T(22) = 0.2; NS | T(23) = 0.4; NS | T(20) = −0.6; NS |
| Left vs right amygdala | ||||
| Negative vs neutral pictures | F(1,16) = 2.1, P <.10 | F(1,16) = 1.4; NS | ||
| Negative vs neutral sounds | F(1,26) = 7.2; P <.05 | F(1,28) = 7.1; P <.05 |
*Significant effects for which we had an a priori hypothesis. T-values are given in cases where effects were expected to be larger than zero, F-values otherwise.
To obtain a statistical measure for the observed tendency for negative pictures and sounds for stronger valence effects in the left amygdala, we tested for possible interactions between hemisphere and valence and arousal in post hoc 2 × 2 × 2 ANOVAs (Table 2). For pictures, the observed difference did not reach statistical significance. For sounds, the left amygdala showed a stronger valence effect and the right amygdala showed a stronger arousal effect. However, because we had no a priori hypothesis these differences will not be discussed further. Finally, we obtained a statistical measure for the observed tendency of stronger valence effects for negative than positive stimuli in the left amygdala. Direct comparison of negative and positive stimuli in post hoc 2 × 2 ANOVAs revealed no statistically significant differences between negative and positive stimuli in the left amygdala [pictures; F(1,16) = 1.7; sounds; F(1,22) = 0.5].
The same two hypotheses were then tested with correlation analyses based on each subject's individual valence and arousal ratings (first and second column of Table 3). As in the factorial design, evidence for both hypotheses was found in the left amygdala for negative pictures. Additionally, the correlation analyses provided evidence for both hypotheses in both amygdalae for positive pictures.
Table 3.
Amygdala activity during visual and auditory stimulation: Correlation analyses
| Correlation with valence | Correlation with valence vs correlation with arousal | Correlation with arousal | |
|---|---|---|---|
| Left amygdala | |||
| Negative–neutral pictures | T = 1.8; P< 0.05* | T = 2.0; P< 0.05* | T = −2.0; n.s. |
| Positive–neutral pictures | T = 2.3; P< 0.05* | T = 2.3; P< 0.05* | T = −1.6; n.s. |
| Negative–neutral sounds | T = 0.8; n.s. | T = 0.7; n.s. | T = −0.5; n.s. |
| Positive–neutral sounds | T = 0.2; n.s. | T = 0.1; n.s. | T = 0.1; n.s. |
| Right amygdala | |||
| Negative–neutral pictures | T = 0.3; n.s. | T = 0.6; n.s. | T = −0.8; n.s. |
| Positive–neutral pictures | T = 2.5; P < 0.05* | T = 2.5; P < 0.05* | T = −1.7; n.s. |
| Negative–neutral sounds | T = −0.9; n.s. | T = −1.1; n.s. | T = 1.2; n.s. |
| Positive–neutral sounds | T = 0.6; n.s. | T = 0.4; n.s. | T = 0.1; n.s. |
| Left vs right amygdala | |||
| Negative–neutral pictures | F(1,15) = 1.7; n.s. | F(1,15) = 0.25; n.s. | |
| Positive–neutral pictures | F(1,15) = 0.36; n.s. | F(1,15) = 0.01; n.s. |
*Significant effects for which we had an a priori hypothesis. T-values are given in cases where effects were expected to be larger than zero, F-values otherwise. Df = 15 for pictures; df = 23 for sounds.
As in the factorial design, we tested whether the observed tendency of stronger valence effects for negative pictures in the left amygdala and for positive pictures in the right amygdala would have reached statistical significance had there been an a priori hypothesis. This was not the case. Finally, we also directly compared valence effects for negative and positive pictures. Neither of the differences reached statistical significance [left amygdala; F(1,16) = 0.02, right amygdala; F(1,16) = 3.2].
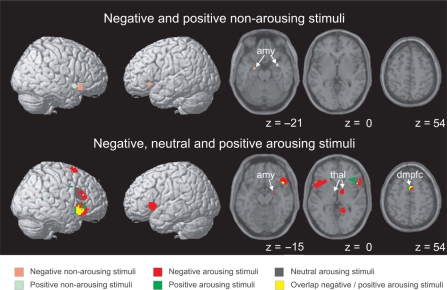
The whole-brain analysis confirmed the results of the amygdala analyses; the amygdala responded to both negative and positive stimuli, and this response did not increase with arousal. In contrast, thalamic and cortical activity increased with arousal. Negative and positive stimuli activated both common and distinct cortical regions. While the right caudolateral orbitofrontal cortex responded to emotional stimuli of either valence, responses in the left caudolateral orbitofrontal region were confined to negative stimuli. Activity in response to negative and positive arousing stimuli overlapped in the orbital part of the inferior frontal gyrus in the right hemisphere, and in a region in the dorsomedial prefrontal cortex corresponding to the supplementary motor area (SMA) (Figure 3 and Table 4). These results are in line with previous studies (Royet et al., 2000; Dolcos et al., 2004).
Fig. 3.
Brain activity during visual and auditory stimulation. SPMs are shown as surface projections, and superimposed on horizontal sections of a T1-weighted image of a standard brain. Maps are exclusively masked by voxels activated by neutral non-arousing stimuli to eliminate responses common to emotional and non-emotional stimuli. Z-values and coordinates of local maxima are listed in Table 4. amy, amygdala; thal, thalamus; dmpfc, dorsomedial prefrontal cortex.
Table 4.
Overlap of brain activity during visual and auditory stimulation
| MNI coordinates | Z | k | |
|---|---|---|---|
| Negative non-arousing | |||
| L Inferior frontal gyrus/Insula* | −33 21 −9 | 2.6 | 13 |
| R Inferior frontal gyrus, orbital part*/Insula/Superior temporal pole | 48 21 –9 | 2.5 | 27 |
| L Amygdala*/Hippocampus | −27 −6 −18 | 2.4 | 5 |
| L Pallidum*/Putamen | −18 3 −6 | 2.3 | 2 |
| Positive non-arousing | |||
| R Superior temporal pole*/Insula | 57 9 –9 | 2.7 | 30 |
| R Superior parietal gyrus | 9 −81 51 | 2.3 | 3 |
| R Amygdala | 33 −3 −21 | 2.1 | 2† |
| Neutral arousing | |||
| R Inferior frontal gyrus triangular* and opercular part/middle frontal gyrus | 60 18 21 | 2.8 | 129 |
| L Thalamus | −6 −9 −3 | 2.4 | 6 |
| R Insula*/Inferior frontal gyrus triangular and orbital part | 36 30 −3 | 2.4 | 3 |
| R Middle temporal gyrus | 51 −51 9 | 2.4 | 4 |
| Negative arousing | |||
| R Inferior frontal gyrus*/Superior temporal pole/Insula | 51 24 −9 | 2.9 | 151 |
| L Insula/Inferior frontal gyrus*/Superior temporal pole | −45 18 0 | 2.8 | 87 |
| R/L SMA*/Cingulum/Superior medial frontal gyrus | 9 9 66 | 2.7 | 134 |
| R Inferior frontal gyrus, triangular* and opercular part | 54 30 18 | 2.7 | 24 |
| R Thalamus*/Pallidum | 12 −12 6 | 2.5 | 43 |
| R Lingual gyrus*/Calcarine sulcus/Precuneus | 15 −45 0 | 2.4 | 29 |
| R Amygdala | 24 −3 −15 | 2.4 | 2 |
| Positive arousing | |||
| R Insula*/Inferior frontal gyrus/Superior temporal pole | 48 15 −9 | 2.8 | 99 |
| R/L SMA | 0 6 57 | 2.5 | 12 |
| R Middle temporal gyrus | 54 −48 12 | 2.3 | 3 |
| R Precentral gyrus | 51 3 36 | 2.3 | 2 |
Anatomical descriptions are based on automated anatomical labeling (Tzourio-Mazoyer et al. 2002). Regions are ordered with respect to fractions of clusters they contain. Coordinates and Z-scores correspond to local maxima derived from SPMs thresholded at T = 3.5, corresponding to a voxel-wise probability of error of P < 0.005 of the contrast with the least degrees of freedom. Only clusters that contain at least two voxels are listed.
*The region with the highest activated voxel; k, number of activated voxels; L, left hemisphere; R, right hemisphere.
†Amygdala activity in response to positive non-arousing stimuli became only evident with a voxel-wise probability of p < 0.005, based on the degrees of freedom of this contrast.
DISCUSSION
This study investigated amygdala activity in response to emotional pictures and sounds. Specifically, we tested the hypothesis that (i) the amygdala is sensitive to valence of emotional stimuli equated for arousal and (ii) that increased amygdala activity in response to emotional stimuli is better explained by valence than by arousal. Both hypotheses were tested separately for eight different cases: (i) negative and positive vs neutral stimuli, (ii) pictures and sounds and (iii) the left and right amygdala. We found evidence that the left amygdala is sensitive to valence of pictures and negative sounds, and that the right amygdala is sensitive to valence of positive pictures. Moreover, increasing amygdala activity in response to emotional pictures was better explained by valence than by arousal. The factorial analysis was more sensitive to valence effects of negative pictures and sounds, while the correlation analysis was more sensitive to valence effects of positive pictures. This might be partly due to the fact that valence and arousal were less strongly correlated for positive stimuli and thus partialling out common effects might have reduced sensitivity less severely in the case of positive stimuli. Positive effects of arousal were observed only in the factorial analysis and only in one case, namely for positive pictures in the right amygdala. Our findings are in agreement with studies in the chemosensory domain (Anderson et al., 2003; Small et al., 2003, Winston et al., 2005) in so far that we found the amygdala to respond to both negative and positive emotional stimuli. Our findings do not match with those studies in that amygdala activity did not consistently increase with increasing stimulus-related arousal. In fact, left amygdala activity was decreased in response to arousing compared to non-arousing pictures.
Valence and arousal in the chemosensory and audiovisual domain
The concept of a dimensional organization of emotion dates back to Wilhelm Wundt (1913), who proposed three fundamental components of emotional experience: Unlust/Lust (listlessness/lust), Erregung (excitation) and Spannung (tension). Since then, the dimensional organization of emotion has thoroughly been explored empirically with the consistent finding that most of the variance in self-reported feelings and verbal judgements of emotional stimuli is explained by two factors, now most commonly termed valence and arousal, whereby valence refers to a bipolar continuum of stimulus characteristics or emotional experience ranging from negative to neutral to positive, and arousal refers to the magnitude of bodily arousal and/or the subjective experience thereof (Russel, 1980; Feldman et al., 1999; Yik et al., 1999; Fontaine et al., 2007). However, these terms are not used consistently in the neuroimaging literature. For example, researchers have used the term ‘valence’ to refer to the sign of valence (i.e. whether something is negative or positive) and ‘intensity’ to refer to the magnitude of positive or negative valence (i.e. distance to neutral irrespective of the sign). Thus, the notion that the amygdala is not sensitive to valence sometimes means that the amygdala does not discriminate between stimuli that have a similar distance to neutral, but into opposite directions (i.e. the same emotional ‘intensity’). Moreover, because emotional ‘intensity’, tends to be closely associated with arousal, ‘intensity’ and ‘arousal’ are sometimes used interchangeably. However, this is not in line with psychological research that has shown that emotional space is at least two-dimensional.
In the current study, we have shown that the amygdala is sensitive to both positive and negative valence even when arousal is equated. Our design regards psychological models of emotion, and supplements previous neuroimaging studies that found amygdala activity in response to valenced pictures but did not fully control for both factors (Mourao-Miranda et al., 2003; Phan et al., 2004). It further extends previous studies in the chemosensory domain by showing that results on amygdala function cannot easily be transferred from one sensory domain to another.
In the chemosensory domain, valence and arousal are closely associated with physical stimulus characteristics. The valence of an odour is determined by its chemical compounds (Schiffman, 1974; Ehrlichman and Bastone, 1992), and rated arousal of odours is directly linked to stimulus intensity (Bensafi et al., 2002). Given the close anatomical connections between the amygdala and chemosensory cortices (McDonald, 1998; Swanson and Petrovich, 1998), the positive relation between physical stimulus intensity and amygdala activity in the chemosensory domain (Anderson et al., 2003; Small et al., 2003, Winston et al., 2005) is well in line with other studies demonstrating increased neural activity in response to physically intense stimuli in primary and secondary sensory areas (Jancke et al., 1998 [auditory stimuli]; Mohamed et al., 2002 [visual stimuli]).
In the visual and auditory domain, valence and arousal are very often, and particularly for the stimuli used here, not determined by the physical appearance of the stimuli, or the sensory percept they elicit (Bradley and Lang, 2000; Junghofer et al., 2001), but by their semantic content. There is evidence that semantic valence decoding might require less resources than the processes that determine stimulus-related arousal. In a review of pre-attentive emotional processing, Robinson (1998) concludes that the valence of a stimulus, but not the features that lead to stimulus-related arousal, is detected by pre-attentive processes that direct attention and resources to the stimulus. This ‘quick-and-dirty’ processing has often been associated with the amygdala (LeDoux, 1996). A key function of the amygdala in signalling stimulus valence is further supported by studies in patients with partial destruction of primary visual cortex. In the absence of a cortical stimulus representation, differential amygdala activity as well as graded peripheral physiological and verbal responses along the valence dimension are observed (Morris et al., 2001; Hamm et al., 2003; Anders et al., 2004a), while arousal-related responses are lacking (Hamm et al., 2003; Anders et al., 2004a).
Interestingly, in the present study valence ratings for both visual and auditory stimuli varied significantly less across subjects than arousal ratings. A similar trend was found in published ratings of the complete IAPS and IADS sets (IAPS, n = 603, valence s.d. = 1.6, arousal s.d. = 2.2, Wilcoxon Z = −25.3, P < 0.001, Lang et al., 1997; IADS, n = 110, valence s.d. = 1.7, arousal s.d. = 1.9, Wilcoxon Z = 6.1, P < 0.001, Bradley and Lang, 1999). This suggests that valence ratings are more directly and invariantly related to the stimulus than arousal ratings. Given that the amygdala has been implicated in the processing of inherent stimulus meaning (Phan et al., 2004) a role of the amygdala in signalling stimulus valence rather than arousal is well in line with the low variability of valence reports. Arousal reports, on the other hand, might depend more strongly upon cognitive processing and cortically represented personal experiences and attitudes. In line with this, cortical activity increased with reported arousal. Such cortical processing might lead to a reduction of amygdala activity (Hariri et al., 2000, 2003), particularly with the long stimulus presentation times used here.
This reasoning is also in line with results from a recent study on the amygdala's function in emotional memory. Kensinger and Corkin (2004) found that amygdala activity was generally stronger during the processing of negative than neutral words irrespective of their arousal. Moreover, negative words were remembered better when they had elicited strong amygdala responses, but this effect was larger for arousing than for non-arousing words, possibly as a result of cortico–amygdalar interactions. Thus, impaired arousal judgements in patients with longstanding amygdalar lesions (Adolphs et al., 1999; Glascher and Adolphs, 2003) may be due to impaired encoding rather than retrieval of arousal-related information.
In contrast to our findings, one study that used emotional words as stimuli (Lewis et al., 2007) did find amygdala activity to increase with arousal but not with valence when both factors were controlled. However, this study included only stimuli from the extreme ends of the valence scale (i.e. very negative and very positive words) and used published valence and arousal ratings rather than the subjects’ individual ratings for regression analysis. The limited range of valence and the averaging of ratings over a large number of subjects might have led to a reduced sensitive to valence effects which might have biased the results in this study. Alternatively, the processing of words whose semantic content is highly overlearned might be more similar to the processing of chemical signals than the processing of pictures and sounds. Clearly, more studies that control both valence and arousal are needed to draw a more comprehensive picture.
While our findings suggest that amygdala activity might not necessarily increase with increasing stimulus-related arousal when emotional meaning is conveyed by pictures and sounds, they do not preclude the possibility that amygdala activity does increase with stimulus-related arousal when it is associated with physical stimulus characteristics of visual or auditory stimuli. Although the amygdala does not receive direct input from primary or secondary visual and auditory cortices (Amaral, 1992; McDonald, 1998), it does receive subcortical input from tectal visual and auditory nuclei, which might give rise to such effects.
Negative and positive valence
Like previous studies (Anderson et al., 2003; Small et al., 2003; Cunningham et al., 2004; Phan et al., 2004; Winston et al., 2005), we found that the amygdala responds to both negative and positive stimuli with an increase of activity. At the moment, evidence is lacking whether these responses are subserved by the same or different subsystems within the amygdala. Advanced imaging techniques like fMR-adaptation paradigms (Grill-Spector and Malach, 2001) might further our understanding of human amygdala function in this regard.
CONCLUSION
In conclusion, we provide evidence that at least in some cases the amygdala is sensitive to negative and positive valence when arousal is equated, and that increased amygdala activity in response to emotional pictures and sounds is better explained by stimulus valence than arousal. Although we note that our results need replication to allow firm conclusions on the amygdala's role in audiovisual emotional processing as they rest on a relatively small number of trials per subject, the present study clearly shows that results on amygdala function can not easily be transferred from one sensory domain to another.
Acknowledgments
This study was supported by the Junior Science Program of the Heidelberger Academy of Sciences and Humanities. We thank Thomas Ethofer for comments on earlier drafts of the article.
REFERENCES
- Adolphs R, Russell JA, Tranel D. A role for the human amygdala in recognizing emotional arousal from unpleasant stimuli. Psychological Science. 1999;10:167–71. [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372(6507):669–72. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton J, editor. The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York: Wiley; 1992. pp. 1–66. [Google Scholar]
- Anders S, Birbaumer N, Sadowski B, Erb M, Mader I, Grodd W, Lotze M. Parietal somatosensory association cortex mediates affective blindsight. Nature Neuroscience. 2004a;7(4):339–40. doi: 10.1038/nn1213. [DOI] [PubMed] [Google Scholar]
- Anders S, Lotze M, Erb M, Grodd W, Birbaumer N. Brain activity underlying emotional valence and arousal: a response-related fMRI study. Human Brain Mapping. 2004b;23(4):200–9. doi: 10.1002/hbm.20048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anders S, Weiskopf N, Lule D, Birbaumer N. Infrared oculography-validation of a new method to monitor startle eyeblink amplitudes during fMRI. Neuroimage. 2004c;22(2):767–70. doi: 10.1016/j.neuroimage.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Christoff K, Stappen I, et al. Dissociated neural representations of intensity and valence in human olfaction. Nature Neuroscience. 2003;6(2):196–202. doi: 10.1038/nn1001. [DOI] [PubMed] [Google Scholar]
- Anderson AK, Sobel N. Dissociating intensity from valence as sensory inputs to emotion. Neuron. 2003;39(4):581–3. doi: 10.1016/s0896-6273(03)00504-x. [DOI] [PubMed] [Google Scholar]
- Baumgart F, Kaulisch T, Tempelmann C, et al. Electrodynamic headphones and woofers for application in magnetic resonance imaging scanners. Medicine and Physics. 1998;25(10):2068–70. doi: 10.1118/1.598368. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Rouby C, Farget V, Bertrand B, Vigouroux M, Holley A. Psychophysiological correlates of affects in human olfaction. Neurophysiologie Clinique. 2002;32(5):326–32. doi: 10.1016/s0987-7053(02)00339-8. [DOI] [PubMed] [Google Scholar]
- Berg WK, Balaban MT. Startle elicitation: stimulus parameters, recording techniques, and quantification. In: Dawson ME, Schell AE, Boehmelt AH, editors. Startle Modification: Implications for Neuroscience, Cognitive Science, and Clinical Science. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Bradley MM, Lang PJ. International Affective Digitized Sounds (IADS): Stimuli, Instruction Manual and Affective Ratings. The Center for Research in Psychopysiology: Technical Report B-2. FL: University of Florida; 1999. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the self-assessment manikin and the semantic differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25(1):49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Affective reactions to acoustic stimuli. Psychophysiology. 2000;37(2):204–15. [PubMed] [Google Scholar]
- Breiter HC, Etcoff NL, Whalen PJ, et al. Response and habituation of the human amygdala during visual processing of facial expression. Neuron. 1996;17(5):875–87. doi: 10.1016/s0896-6273(00)80219-6. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Bernston G. Relationship between attitudes and evaluative space: a critical review with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–23. [Google Scholar]
- Cunningham WA, Raye CL, Johnson MK. Implicit and explicit evaluation: FMRI correlates of valence, emotional intensity, and control in the processing of attitudes. Journal of Cognitive Neuroscience. 2004;16(10):1717–29. doi: 10.1162/0898929042947919. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–75. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage. 2004;23(1):64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Ehrlichman H, Bastone L. Olfaction and emotion. In: Serby M, Chobor KL, editors. Science of Olfaction. New york: springer; 1992. pp. 410–38. [Google Scholar]
- Feldman Barrett L, Russell JA. The structure of current affect: controversies and emerging consensus. Current Directions in Psychological Science. 1999;8:10–14. [Google Scholar]
- Fontaine JR, Scherer KR, Roesch EB, Ellsworth PC. The world of emotions is not two-dimensional. Psychological Science. 2007;18(12):1050–7. doi: 10.1111/j.1467-9280.2007.02024.x. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25(3):661–7. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Garavan H, Pendergrass JC, Ross TJ, Stein EA, Risinger RC. Amygdala response to both positively and negatively valenced stimuli. Neuroreport. 2001;12(12):2779–83. doi: 10.1097/00001756-200108280-00036. [DOI] [PubMed] [Google Scholar]
- Glascher J, Adolphs R. Processing of the arousal of subliminal and supraliminal emotional stimuli by the human amygdala. Journal of Neuroscience. 2003;23(32):10274–82. doi: 10.1523/JNEUROSCI.23-32-10274.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grill-Spector K, Malach R. fMR-adaptation: a tool for studying the functional properties of human cortical neurons. Acta Psychologica. 2001;107(1–3):293–321. doi: 10.1016/s0001-6918(01)00019-1. [DOI] [PubMed] [Google Scholar]
- Hamann S. Nosing in on the emotional brain. Nature Neurosci. 2003;6(2):106–8. doi: 10.1038/nn0203-106. [DOI] [PubMed] [Google Scholar]
- Hamann S, Mao H. Positive and negative emotional verbal stimuli elicit activity in the left amygdala. Neuroreport. 2002;13(1):15–9. doi: 10.1097/00001756-200201210-00008. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Hoffman JM, Kilts CD. Ecstasy and agony: activation of the human amygdala in positive and negative emotion. Psychological Science. 2002;13(2):135–41. doi: 10.1111/1467-9280.00425. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI, Schupp HT, Treig T, Dressel A, Kessler C. Affective blindsight: intact fear conditioning to a visual cue in a cortically blind patient. Brain. 2003;126(Pt 2):267–75. doi: 10.1093/brain/awg037. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Bookheimer SY, Mazziotta JC. Modulating emotional responses: effects of a neocortical network on the limbic system. Neuroreport. 2000;11(1):43–8. doi: 10.1097/00001756-200001170-00009. [DOI] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biological Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Jancke L, Shah NJ, Posse S, Grosse-Ryuken M, Muller-Gartner HW. Intensity coding of auditory stimuli: an fMRI study. Neuropsychologia. 1998;36(9):875–83. doi: 10.1016/s0028-3932(98)00019-0. [DOI] [PubMed] [Google Scholar]
- Junghofer M, Bradley MM, Elbert TR, Lang PJ. Fleeting images: a new look at early emotion discrimination. Psychophysiology. 2001;38(2):175–8. [PubMed] [Google Scholar]
- Kensinger EA, Corkin S. Two routes to emotional memory: distinct neural processes for valence and arousal. Proceedings of the National Academy of Science of the United States of America. 2004;101(9):3310–5. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnsons JH, Wlliams TA, editors. Technology in Mental Health Care Delivery Systems. New Jersey: Alex Publishing; 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. NIMH Center for the Study of Emotion and Attention; 1997. International Affective Picture System (IAPS): Technical Manual and Affective Ratings. [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annual Review of Psychology. 1995;46:209–35. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. The Emotional Brain. New York: Simon & Schuster; 1996. [Google Scholar]
- Lewis PA, Critchley HD, Rotshtein P, Dolan RJ. Neural correlates of processing valence and arousal in affective words. Cerebral Cortex. 2007;17:742–8. doi: 10.1093/cercor/bhk024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Phan KL, Decker LR, Taylor SF. Extended amygdala and emotional salience: a PET activation study of positive and negative affect. Neuropsychopharmacology. 2003;28(4):726–33. doi: 10.1038/sj.npp.1300113. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55(3):257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- Mohamed FB, Pinus AB, Faro SH, Patel D, Tracy JI. BOLD fMRI of the visual cortex: quantitative responses measured with a graded stimulus at 1.5 Tesla. Journal of Magnetic Resonance Imaging. 2002;16(2):128–36. doi: 10.1002/jmri.10155. [DOI] [PubMed] [Google Scholar]
- Morris JS, DeGelder B, Weiskrantz L, Dolan RJ. Differential extrageniculostriate and amygdala responses to presentation of emotional faces in a cortically blind field. Brain. 2001;124(Pt 6):1241–52. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- Morris JS, Frith CD, Perrett DI, et al. A differential neural response in the human amygdala to fearful and happy facial expressions. Nature. 1996;383(6603):812–5. doi: 10.1038/383812a0. [DOI] [PubMed] [Google Scholar]
- Mourao-Miranda J, Volchan E, Moll J, et al. Contributions of stimulus valence and arousal to visual activation during emotional perception. Neuroimage. 2003;20(4):1955–63. doi: 10.1016/j.neuroimage.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–60. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, et al. Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biological Psychiatry. 2003;53(3):211–5. doi: 10.1016/s0006-3223(02)01485-3. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–80. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I. Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage. 2002;16(2):331–48. doi: 10.1006/nimg.2002.1087. [DOI] [PubMed] [Google Scholar]
- Robinson MD. Running from William James’ bear: a review of preattentive mechanisms and their contributions to emotional experience. Cognition and Emotion. 1998;12(5):667–96. [Google Scholar]
- Royet JP, Zald D, Versace R, et al. Emotional responses to pleasant and unpleasant olfactory, visual, and auditory stimuli: a positron emission tomography study. Journal of Neuroscience. 2000;20(20):7752–9. doi: 10.1523/JNEUROSCI.20-20-07752.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell JA. A circumplex model of affect. Journal of Personality and Social Psychology. 1980;39(6):1161–78. [Google Scholar]
- Sabatinelli D, Bradley MM, Fitzsimmons JR, Lang PJ. Parallel amygdala and inferotemporal activation reflect emotional intensity and fear relevance. Neuroimage. 2005;24(4):1265–70. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Schiffman SS. Physicochemical correlates of olfactory quality. Science. 1974;185(146):112–7. doi: 10.1126/science.185.4146.112. [DOI] [PubMed] [Google Scholar]
- Small DM, Gregory MD, Mak YE, Gitelman D, Mesulam MM, Parrish T. Dissociation of neural representation of intensity and affective valuation in human gustation. Neuron. 2003;39(4):701–11. doi: 10.1016/s0896-6273(03)00467-7. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD. What is the amygdala? Trends in Neurosciences. 1998;21(8):323–31. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Liberzon I, Koeppe RA. The effect of graded aversive stimuli on limbic and visual activation. Neuropsychologia. 2000;38(10):1415–25. doi: 10.1016/s0028-3932(00)00032-4. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Phan KL, Decker LR, Liberzon I. Subjective rating of emotionally salient stimuli modulates neural activity. Neuroimage. 2003;18(3):650–9. doi: 10.1016/s1053-8119(02)00051-4. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Vrana SR, Spence EL, Lang PJ. The startle probe response: a new measure of emotion? Journal of Abnormal Psychology. 1988;97(4):487–91. doi: 10.1037//0021-843x.97.4.487. [DOI] [PubMed] [Google Scholar]
- Williams LM, Liddell BJ, Rathjen J, et al. Mapping the time course of nonconscious and conscious perception of fear: an integration of central and peripheral measures. Human Brain Mapping. 2004a;21(2):64–74. doi: 10.1002/hbm.10154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams MA, Mattingley JB. Unconscious perception of non-threatening facial emotion in parietal extinction. Experimental Brain Research. 2004b;154(4):403–6. doi: 10.1007/s00221-003-1740-x. [DOI] [PubMed] [Google Scholar]
- Williams MA, McGlone F, Abbott DF, Mattingley JB. Differential amygdala responses to happy and fearful facial expressions depend on selective attention. Neuroimage. 2005;24(2):417–25. doi: 10.1016/j.neuroimage.2004.08.017. [DOI] [PubMed] [Google Scholar]
- Winston JS, Gottfried JA, Kilner JM, Dolan RJ. Integrated neural representations of odor intensity and affective valence in human amygdala. Journal of Neuroscience. 2005;25(39):8903–7. doi: 10.1523/JNEUROSCI.1569-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wundt W. Grundriss der Psychologie. Leipzig: Alfred Kröner; 1913. [Google Scholar]
- Yang TT, Menon V, Eliez S, et al. Amygdalar activation associated with positive and negative facial expressions. Neuroreport. 2002;13(14):1737–41. doi: 10.1097/00001756-200210070-00009. [DOI] [PubMed] [Google Scholar]
- Yik MSM, Russell JA. Structure of self-reported current affect: integration and beyond. Journal of Personality and Social Psychology. 1999;77:600–9. [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Research. Brain Research Reviews. 2003;41(1):88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]