Abstract
The processing of personal changes across time and the ability to differentiate between representations of present and past selves are crucial for developing a mature sense of identity. In this study, we explored the neural correlates of self-reflection across time using functional magnetic resonance imaging (fMRI). College undergraduates were asked to reflect on their own psychological characteristics and those of an intimate other, for both the present time period (i.e. at college) and a past time period (i.e. high school years) that involved significant personal changes. Cortical midline structures (CMS) were commonly recruited by the four reflective tasks (reflecting on the present self, past self, present other and past other), relative to a control condition (making valence judgments). More importantly, however, the degree of activity in CMS also varied significantly according to the target of reflection, with the ventral and dorsal medial prefrontal cortex and the posterior cingulate cortex being more recruited when reflecting on the present self than when reflecting on the past self or when reflecting on the other person. These findings suggest that CMS may contribute to differentiate between representations of present and past selves.
Keywords: self, fMRI, medial prefrontal cortex, time
The sense of continuity of self across time is an essential aspect of human consciousness (Damasio, 1999; Gallagher, 2000; Morin, 2006), which when disturbed can lead to severe mental disorders such as schizophrenia (Vogeley and Kupke, 2007). This sense of personal continuity is intimately related to autobiographical memory and emerges, in particular, from the creation of internalized narratives that integrate temporally disparate self-elements into a coherent whole (McAdams, 2001; Conway, 2005). Continuity does not mean total sameness, however. Life circumstances are continually evolving and when significant changes occur (e.g. changing job, getting married, moving to a new country), the self-concept is updated and remodelled to take these alterations into account (Deutsch et al., 1988; Demo, 1992). Thus, besides the creation of a sense of continuity, the processing of personal changes across time and the ability to differentiate between representations of present and past selves are important components of self-processing. In particular, they probably play a critical role in the formation and consolidation of a stable identity during late adolescence and early adulthood (McAdams, 2001).
Recent research in social psychology has started to investigate the process by which people differentiate between representations of present and past selves. There is evidence that when people perceive they have changed, they tend to distance themselves from their past self and regard it as ‘another person’ (Libby and Eibach, 2002; Pronin and Ross, 2006). For example, people frequently adopt a third-person visual perspective when recalling past behaviors that are discrepant with their present self-concept, as if they were looking at someone else (Libby and Eibach, 2002). Furthermore, attributions made about past selves more closely resemble attributions made about others than attributions regarding the present self (e.g. in terms of the tendency to make dispositional attributions; Pronin and Ross, 2006). Therefore, it seems that following personal changes, people process information regarding their past self as they would process information regarding others. This distancing mechanism with regard to past selves may help differentiate representations of present self-attributes from representations of past self-attributes. The purpose of this study was to investigate the brain regions that may contribute to this process.
Functional brain imaging studies have revealed activations in cortical midline structures (CMS) when people reflect on psychological characteristics (Craik et al., 1999; Johnson et al., 2002; Kelley et al., 2002; Kjaer et al., 2002; Fossati et al., 2003; Lieberman et al., 2004; Lou et al., 2004; Schmitz et al., 2004; Mitchell et al., 2005; Ochsner et al., 2005; D’Argembeau et al., 2005, 2007; Heatherton et al., 2006; Moran et al., 2006; Saxe et al., 2006; Pfeifer et al., 2007). CMS are recruited when reflecting on one's own characteristics as well as those of others (Amodio and Frith, 2006; Lieberman, 2007), but research nevertheless suggests that these brain structures are more engaged when referring to the self (see Northoff et al., 2006, for a meta-analysis). For example, assessing whether psychological traits are self-descriptive elicited greater activity in medial prefrontal cortex (MPFC) and medial posterior regions (in the posterior cingulate and/or precuneus) compared to assessing whether traits apply to another person (Kelley et al., 2002; Heatherton et al., 2006; D’Argembeau et al., 2007; Pfeifer et al., 2007). These studies were only interested in the neural correlates of thinking about the present self, however; hence, it is currently unknown whether CMS respond differently to representations of past selves. As the perception of personal changes across time leads people to regard their past selves as other persons (Libby and Eibach, 2002; Pronin and Ross, 2006), we hypothesized that CMS activity should be sensitive to temporal perspectives on the self, and more specifically, that reflecting on a past self should elicit less activity in these structures than reflecting on the present self. In this way, CMS may help differentiate between representations of present and past selves.
To examine this issue, we collected functional magnetic resonance imaging (fMRI) data while participants reflected on their own psychological characteristics and those of an intimate other, for both the present and a past time period. The transition from high school to college entails important changes in the self (Libby and Eibach, 2002), which makes it an ideal period for studying how people differentiate between representations of present and past selves. Accordingly, we asked college undergraduates to reflect on their present self (i.e. at college) and their self 5 years ago (i.e. when they were at high school); hence, judgments they made targeted two clearly distinct lifetime periods (i.e. periods associated with different goals, locations, people, activities and so forth; Conway and Pleydell-Pearce, 2000). The tasks consisted in making judgments on a series of adjectives describing psychological traits. In a first condition, participants assessed whether or not each adjective was descriptive of their present self, whereas in a second condition they assessed whether or not the adjectives described their past self. Two other conditions required participants to judge the adjectives in reference to the present and past traits of an intimate other. Finally, a control task was also included (assessing the valence of each adjective), which involved semantic processing but did not require to reflect on the psychological characteristics of a particular person.
METHODS
Participants
Data were acquired from couples of close friends (eight women, eight men) or siblings (two women, two men) who knew each other for at least 5 years. All were right-handed, French-speaking college undergraduates (aged between 20 and 23 years; mean age = 21 years). All participants gave their written informed consent to take part in the study, which was approved by the Ethics Committee of the Medical School of the University of Liège. None of them had any history of neurological or psychological disorder.
Task description
Participants made different types of judgments on a series of adjectives describing psychological traits. More specifically, they were asked to assess whether or not the adjectives described their current psychological characteristics (present self), their characteristics 5 years ago (past self), their friend's current characteristics (present other) and their friend's characteristics 5 years ago (past other). All participants were at high school 5 years ago, whereas they currently were college undergraduates. Furthermore, most of them had moved to another city to come to the university. Judgments about the past vs the present thus targeted clearly distinct lifetime periods (Conway and Pleydell-Pearce, 2000). A control condition was also included (judging whether or not the adjectives designated a positive trait), which involved semantic processing but did not require to reflect on the psychological characteristics of a particular person.
The same set of 40 trait adjectives [20 positive and 20 negative adjectives selected from Anderson's (1968) list; e.g. modest, shy] was used in all five conditions. We decided to use the same set of adjectives for all judgment conditions in order to closely follow previous behavioral studies on which this work was based (Pronin and Ross, 2006). In addition, it allowed us to assess perceived changes in personal characteristics across time. The five conditions were presented within a single session, using a block design.1 There were 10 blocks per condition, with each block consisting of four trials. Before the start of each block, an instruction cue appeared on the screen (for a variable duration comprised between 3000 and 3500 ms: random Gaussian distribution centered on a mean duration of 3250 ms) to inform participants about the type of judgment they had to make for the adjectives presented subsequently [present self: At present, I am; past self: Five years ago, I was; present other: At present, X is (where X was replaced by the friend's first name); past other: Five years ago, X was (where X was replaced by the friend's first name); control: Positive trait]. The four trials were then presented sequentially. Each trial consisted of the presentation of a fixation cross first (for a variable duration: random Gaussian distribution centered on a mean duration of 875 ms) and then of an adjective (for 3500 ms, during which participants made a yes/no decision by pressing one of two buttons). After each block, the screen was emptied (for a variable duration: random Gaussian distribution centered on a mean duration of 550 ms) before the instruction pertaining to the next block was presented.
Blocks were presented in pseudo-random order, such that all five conditions were presented before their presentation was repeated and with the restriction that two blocks of the same condition could not be repeated immediately and could not be separated by more than six blocks of a different condition. Furthermore, the 40 adjectives were all presented before their presentation was repeated, thus ensuring that possible repetition effects were not confounded with conditions (i.e. each condition included eight adjectives that were presented for the first time, eight adjectives that were presented for the second time, eight adjectives that were presented for the third time, eight adjectives that were presented for the fourth time and eight adjectives that were presented for the fifth time). Each block consisted of two positive and two negative adjectives presented in random order.
Before the fMRI session, participants were asked to take a few minutes to think about their life 5 years ago (the experimenter helped them to remember this period by asking questions such as how old they were, what school they went to and so forth) and about their present life. Participants were instructed to keep in mind the past or present lifetime period when making the corresponding judgments. Then, they made a series of practice trials (with a different set of adjectives) in order to familiarize them with the five types of judgments. After the fMRI session, participants were asked to rate the overall ease/difficulty with which they made each type of judgments in the scanner (using a 10-point rating scale: 1 = not at all difficult, 10 = very difficult).
MRI acquisition
Data were acquired on a 3Tesla scanner (Siemens, Allegra, Erlangen, Germany) using a T2*-weighted echo-planar imaging (EPI) sequence (TR = 2130 ms, TE = 40 ms, FA 90°, matrix size 64 × 64 × 32, voxel size 3.4 × 3.4 × 3.4 mm3). Thirty-two 3 mm thick transverse slices (FOV 22 × 22 cm2) were acquired, with a distance factor of 30%, covering the whole brain. Around 540 functional volumes were obtained. The first three volumes were discarded to account for T1 saturation. A structural MR scan was obtained at the end of the session [T1-weighted 3D magnetization-prepared rapid acquisition gradient echo (MP-RAGE) sequence, TR = 1960 ms, TE = 4.4 ms, FOV 23 × 23 cm2, matrix size 256 × 256 × 176, voxel size 0.9 × 0.9 × 0.9 mm]. Head movement was minimized by restraining the subject's head using a vacuum cushion. Stimuli were displayed on a screen positioned at the rear of the scanner, which the subject could comfortably see through a mirror mounted on the standard head coil.
fMRI analyses
fMRI data were preprocessed and analyzed using SPM5 (Wellcome Department of Imaging Neuroscience, http://www.fil.ion.ucl.ac.uk/spm) implemented in MATLAB (Mathworks Inc., Sherborn, MA, USA). Functional scans were realigned using iterative rigid body transformations that minimize the residual sum of squares between the first and subsequent images. They were normalized to the Montreal Neurological Institute (MNI) EPI template (voxel size: 2 × 2 × 2 mm) and spatially smoothed with a Gaussian kernel with full width at half maximum (FWHM) of 8 mm.
For each participant, BOLD responses were modeled at each voxel, using a general linear model with epoch regressors. All five conditions (present self, past self, present other, past other, control) were included in the model. For each condition, each epoch ranged from the onset of the first adjective on the screen until the last adjective disappeared from the screen. Boxcar functions representative of these epoch regressors were convolved with the canonical hemodynamic response. The design matrix also included the realignment parameters to account for any residual movement-related effect. A high pass filter was implemented using a cut-off period of 128 s in order to remove the low-frequency drifts from the time series. Serial autocorrelations were estimated with a restricted maximum likelihood algorithm with an autoregressive model of order 1 (+white noise). Four linear contrasts were performed, looking at the effect of each reflective task relative to the control task (present self–control; past self–control; present other–control; past other–control). The corresponding contrast images were smoothed (6-mm FWHM Gaussian kernel) in order to reduce remaining noise due to inter-subject differences in anatomical variability in the individual contrast images. They were then entered in a second-level analysis, corresponding to a random-effects model.
To examine brain regions that were commonly engaged by the four reflective tasks relative to the control task, a conjunction analysis (conjunction null; Friston et al., 2005) was performed with the four contrast images. Next, in order to identify brain regions that showed differential activity across reflective tasks, the four contrast images were entered in a one-way whole-brain voxel-wise ANOVA (using a single task factor with four levels) [1 −1 0 0; 0 1 −1 0; 0 0 1 −1]. Correction for nonsphericity was used to account for possible differences in error variance across conditions and any nonindependent error terms for the repeated-measures ANOVA. The resulting set of voxel values was thresholded at P < 0.001 (uncorrected). Statistical inferences were corrected for multiple comparisons using Gaussian random field theory at the voxel level in a small spherical volume [radius 10 mm; small volume correction, (SVC)] around a priori locations of structure of interest taken from the literature on self-referential processing. We selected coordinates of brain regions that have been associated with reflecting on the present self using tasks similar to the one employed in this study (i.e. judging trait information). These regions concerned areas in the ventral MPFC (−8, 50, −2; −4, 58, −12; Lieberman et al., 2004; D’Argembeau et al. 2007), the dorsal MPFC (−6, 52, 28; Pfeifer et al., 2007) (in this study, we refer to ventral MPFC for z coordinate ≤10 mm and to dorsal MPFC for z coordinate >10 mm) and medial posterior areas (posterior cingulate cortex, PCC: −2, −62, 32; Johnson et al., 2002).
Finally, region of interest (ROI) analyses were conducted to further explore patterns of activity in ventral MPFC, dorsal MPFC and PCC across conditions. Functional ROIs were defined using 6 mm radius spheres around the local maxima of the MPFC and PCC activation clusters yielded by the whole-brain voxel-wise ANOVA. Parameter estimates from these regions were extracted for each participant and each contrast (present self–control; past self–control; present other–control; past other–control) and were submitted to a series of one-way repeated-measures ANOVAs. Significant differences between conditions were then assessed using paired t-tests. These analyses were performed with STATISTICA 7.1 (StatSoft Inc., Tulsa, OK, USA).
RESULTS
Behavioral data
Response times differed significantly across conditions, as revealed by a one-way repeated-measures ANOVA, F(4, 76) = 5.57, P = 0.001. Follow-up comparisons showed that response times were faster for the control task (M = 1570 ms, s.d. = 267) compared to each reflective task (present self: M = 1674 ms, s.d. = 250; past self: M = 1735 ms, s.d. = 258; present other: M = 1696 ms, s.d. = 277; past other: M = 1732 ms, s.d. = 250; all Ps < 0.05). However, the four reflective tasks did not differ from each other (all Ps > 0.13). To estimate perceived changes in personal characteristics across time, we computed the percentages of adjectives for which participants gave a different answer between the present and the past. There was a substantial amount of perceived changes for both self (M = 29%, s.d. = 10%) and other (M = 25%, s.d. = 12%),2 with no significant difference between self and other, t(19) = 1.10, P = 0.28.
Ratings for difficulty in making judgments differed significantly across conditions, F(4, 76) = 15.99, P < 0.001. Follow-up comparisons showed that the control task was rated as being easier (M = 2.25, s.d. = 1.68) than each reflective task (past self: M = 4.20, s.d. = 1.82; present other: M = 3.55, s.d. = 1.76; past other: M = 5.15, s.d. = 2.46; all Ps < 0.05), except the present self (M = 2.60, s.d. = 1.57). Furthermore, the present self was rated as being easier than past self and past other, and the present other was rated as being easier than past other (all Ps < 0.05); all other comparisons were not significant (all Ps > 0.16).
fMRI data
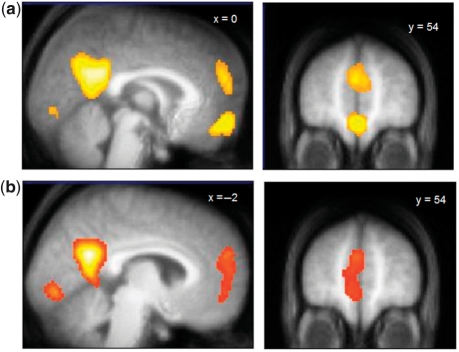
We first investigated the brain regions that were commonly engaged by the four reflective tasks (judgments regarding the present self, past self, present other and past other) relative to the control task (valence judgments), using a conjunction analysis (see Methods section). As shown in Figure 1a, this analysis revealed activations in the ventral MPFC (MNI coordinates of peak voxel: 0, 54, −12; Z-score = 4.52, PSVC < 0.001), the dorsal MPFC (MNI coordinates of peak voxel: −2, 54, 24; Z-score = 4.00, PSVC = 0.002), and the PCC (MNI coordinates of peak voxel: 6, −54, 28; Z-score = 5.85, PSVC < 0.001).
Fig. 1.
Brain activity associated with reflecting on self and other in the present and past. (a) The ventral MPFC, dorsal MPFC and PCC were commonly activated by the four reflective tasks (present self, past self, present other and past other) relative to the control task, as revealed by a conjunction analysis. (b) Nevertheless, activity in the ventral MPFC, dorsal MPFC and PCC varied across reflective conditions, as revealed by a whole-brain voxel-wise ANOVA. Displayed at P < 0.001 (uncorrected) on the mean structural MRI of all participants.
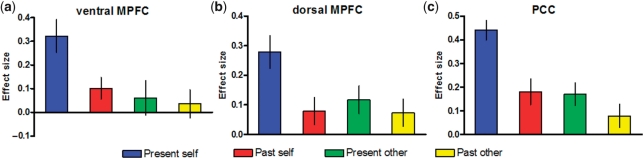
Next we examined the brain regions that showed differential activity across the four reflective tasks by conducting a whole-brain voxel-wise ANOVA (see Methods section). As shown in Figure 1b, differential activity occurred in the PCC (MNI coordinates of peak voxel: −2, −58, 26; Z-score = 6.10, PSVC < 0.001) and in a large portion of MPFC that included both the ventral MPFC (MNI coordinates of peak voxel: −6, 54, −2; Z-score = 3.48, PSVC = 0.015) and the dorsal MPFC (MNI coordinates of peak voxel: −2, 56, 26; Z-score = 3.88, PSVC = 0.004). We then conducted ROI analyses to further specify patterns of activity in these regions (Figure 2). One-way repeated-measures ANOVAs showed significant differences between tasks in the three ROIs [ventral MPFC: F(3, 57) = 5.82, P = 0.002; dorsal MPFC: F(3, 57) = 3.81, P = 0.01; PCC: F(3, 57) = 10.67, P < 0.001] and paired t-tests further revealed that each of these regions was more recruited during reflection about the present self than when reflecting on the past self or when reflecting on the other person in the present or past [all t(19) ≥ 2.02, P ≤ 0.05]; the past self, present other and past other did not differ from each other [all t(19) < 1.38, P > 0.18].
Fig. 2.
Activity in the ventral MPFC, dorsal MPFC and PCC. Functional ROIs were defined using 6 mm radius spheres around the local maxima of (a) ventral MPFC (MNI coordinates of peak voxel: −6, 54, −2), (b) dorsal MPFC (MNI coordinates of peak voxel: −2, 56, 26) and (c) PCC (MNI coordinates of peak voxel: −2, −58, 26) activation clusters yielded by the whole-brain voxel-wise ANOVA. Effect sizes correspond to average parameter estimates for each reflective condition (present self, past self, present other, past other) relative to the control condition (valence judgments). Error bars represent the standard error of the mean.
Additional analyses
The main purpose of this study was to explore whether CMS activity varies as a function of the type of reflective task. Considering that CMS might show a graded response across tasks (e.g. highest activity for the present self condition, lowest activity for the present and past other conditions, with the past self condition falling in between), we analyzed data using a one-way ANOVA (see Methods section) because this analysis would better capture such patterns of graded responses compared to the fully factorial (target × time) ANOVA. However, for the sake of completeness, we also report the results of the 2 (target: self vs other) × 2 (time: present vs past) ANOVA. For each participant, four contrast images were computed (present self, past self, present other, past other) and were then entered in a two-way whole-brain voxel-wise ANOVA (using two factors with two levels each) to examine the main effect of target, the main effect of time and their interaction. We report brain regions that were significantly activated at P < 0.001 (uncorrected) with an extent threshold of 10 voxels (see Table 1 for MNI coordinates and F values).
Table 1.
fMRI results of the fully factorial (target x time) ANOVA
| MNI coordinates |
F | Voxels | |||
|---|---|---|---|---|---|
| x | y | z | |||
| Main effect of target | |||||
| Ventral MPFC | −6 | 46 | −10 | 12.62 | 26 |
| Dorsal MPFC | −12 | 38 | 40 | 14.01 | 16 |
| Posterior cingulate | −2 | −60 | 24 | 22.69 | 1183 |
| Lingual gyrus | −16 | −86 | 2 | 23.90 | 210 |
| 12 | −80 | −4 | 15.25 | 76 | |
| R caudate nucleus | 8 | 12 | 18 | 15.54 | 28 |
| R inferior frontal gyrus | 60 | 14 | 10 | 21.55 | 198 |
| L inferior parietal lobule | −58 | −42 | 54 | 14.67 | 195 |
| Main effect of time | |||||
| R inferior temporal gyrus | 64 | −52 | −8 | 27.63 | 898 |
| L inferior temporal gyrus | −56 | −62 | −12 | 21.87 | 482 |
| L middle temporal gyrus | −60 | −14 | −22 | 16.13 | 67 |
| R superior temporal gyrus | 62 | −34 | 14 | 14.57 | 95 |
| L postcentral gyrus/superior temporal gyrus | −58 | −34 | 34 | 12.72 | 222 |
| R inferior/middle frontal gyrus | 46 | 46 | 6 | 25.38 | 749 |
| L inferior/middle frontal gyrus | −46 | 42 | 14 | 13.64 | 26 |
| R middle frontal gyrus | 40 | 36 | 40 | 14.31 | 26 |
| Precuneus | 6 | −50 | 72 | 17.49 | 121 |
| −14 | −64 | 58 | 14.01 | 72 | |
| Posterior cingulate | 12 | −48 | 24 | 16.34 | 48 |
| −8 | −54 | 26 | 14.23 | 127 | |
| R occipital cortex | 46 | −80 | 30 | 36.17 | 180 |
| L occipital cortex | 22 | −72 | 58 | 17.71 | 402 |
| Interaction | |||||
| Dorsal MPFC | 4 | 46 | 44 | 14.11 | 122 |
| 6 | 62 | 16 | 14.02 | 72 | |
| Posterior cingulate | 2 | −58 | 24 | 12.58 | 16 |
| L inferior frontal gyrus | −34 | 26 | −2 | 13.95 | 98 |
Reported brain regions were significantly activated at P < 0.001 (uncorrected) with an extent threshold of 10 voxels. L, left hemisphere; R, right hemisphere. MPFC, medial prefrontal cortex. Ventral MPFC refers to z coordinate ≤10 mm and dorsal MPFC to z coordinate >10 mm.
The brain regions that were associated with the main effect of target are shown in Table 1. Follow-up t-tests were computed to examine the direction of effects, which showed that the ventral and dorsal MPFC, the PCC, the lingual gyrus and the right caudate nucleus were more activated for judgments targeting the self than for judgments targeting the other person. These findings are broadly consistent with previous studies of self-referential processing (Heatherton et al., 2006; Moran et al., 2006; D’Argembeau et al., 2007). The right inferior frontal gyrus and the left inferior parietal lobule showed greater activation for other than for self.
With regard to the main effect of time, the results showed that the lateral temporal cortex (the inferior and superior temporal gyrus bilaterally), the lateral prefrontal cortex (the inferior/middle frontal gyrus bilaterally), the precuneus and the occipital cortex were more activated for judgments concerning the past than for judgments concerning the present. These regions have been associated with autobiographical memory (Svoboda et al., 2006; Cabeza and St Jacques, 2007) and may thus reflect the retrieval of semantic and sensory information regarding the past period participants had to refer to when making their judgements. The PCC and the left middle temporal gyrus were recruited to a greater extent for the present than for the past.
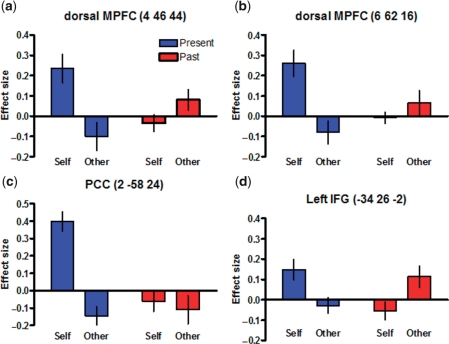
Finally, there was a significant interaction between target and time in the dorsal MPFC, the PCC and the left inferior frontal gyrus. Examination of average parameter estimates (Figure 3) revealed that activity in the dorsal MPFC and PCC was higher for the present self than for the other three conditions, which is consistent with results of the one-way ANOVA (see above). Activity in the left inferior frontal gyrus is more difficult to interpret because it was higher for both the present self and past other conditions compared to the past self and present other conditions.
Fig. 3.
Activity in brain regions that showed a significant interaction between target and time. Functional ROIs were defined using 6 mm radius spheres around the local maxima of (a) dorsal MPFC (MNI coordinates of peak voxel: 4, 46, 44), (b) dorsal MPFC (MNI coordinates of peak voxel: 6, 62, 16), (c) PCC (MNI coordinates of peak voxel: 2, −58, 24) and (d) left inferior frontal gyrus (MNI coordinates of peak voxel: −34, 26, 2). Effect sizes correspond to average parameter estimates for each reflective condition (present self, past self, present other, past other) relative to baseline. Error bars represent the standard error of the mean.
DISCUSSION
The main purpose of this study was to investigate whether CMS activity is sensitive to temporal perspectives on the self. To examine this issue, we collected fMRI data while participants reflected on their own psychological characteristics and those of an intimate other, for both the present and a past time period. The finding that CMS were recruited when reflecting on one's own traits as well as those of another person (relative to valence judgments) confirms the general role of these brain structures in reflecting on mental states and psychological characteristics, be they one's own or those of others (Amodio and Frith, 2006; Lieberman, 2007). More importantly, however, the degree of activity in CMS also varied significantly according to the target of reflection. More specifically, reflecting on the present self elicited greater activity in the ventral and dorsal MPFC and PCC compared to reflecting on the past self or reflecting on an intimate other (in the present or past). Interestingly, the past self and other person conditions did not differ from each other, which fits well with recent findings that people perceive their past self as an ‘other’ following significant personal changes (Libby and Eibach, 2002; Pronin and Ross, 2006). Thus, this study demonstrates that the level of activity in CMS is indeed sensitive to temporal perspectives on the self, suggesting that these brain structures may help differentiate between representations of present and past selves.
Along with others (Pfeifer et al., 2007), we do not believe that CMS are a storage site for self-knowledge, but rather that these structures support component processes that are recruited when reflecting on oneself. Northoff et al. (2006) recently argued that CMS mediate the process of relating stimuli to one's own person (see also Schmitz and Johnson, 2007). The primary function of these structures would be to locate information on a continuum of self-relatedness or self-relevance: the more activity a particular stimulus or mental content elicits in CMS, the more strongly it will be related to the self (Northoff and Bermpohl, 2004; Northoff et al., 2006; Schmitz and Johnson, 2007). In agreement with this view, it has been shown that CMS activity increased in a linear fashion with increasing self-relevance (Moran et al., 2006). When reflecting on their own psychological characteristics, people can generate different mental models of themselves for different time periods (by retrieving relevant knowledge from semantic and/or episodic memory; Klein et al., 2002; Conway, 2005; Sakaki, 2007), and CMS might index the degree to which a particular mental model refers to the present self. In other words, CMS might sustain the process of identifying oneself with the current mental model of the self (which is therefore regarded as ‘me’) vs distancing oneself from representations of past selves (which are therefore considered as ‘not-me’ anymore; see James, 1890, for an early account of the process of ‘appropriating’ vs ‘rejecting’ particular mental contents as part of oneself). In this way, differences in levels of activity within CMS may contribute to differentiate between representations of present and past selves.
It is likely that distinct regions within CMS support different subprocesses (Northoff and Bermpohl, 2004; Amodio and Frith, 2006; Northoff et al., 2006; Lieberman, 2007; Schmitz and Johnson, 2007). In a recent meta-analysis of neuroimaging studies of self-referential processing, Northoff et al. (2006) identified three different regional clusters within CMS, corresponding to the ventral MPFC, dorsal MPFC and PCC. Northoff et al. (2006) suggested that the ventral and dorsal MPFC are involved in coding and appraising the self-relatedness of information and that the PCC sustains the integration of self-related information in the individual's autobiographical context. In this study, these three subregions were more activated when reflecting on the present self than when reflecting on the past self, suggesting that all three processes may contribute to differentiate between representations of present and past selves. Further studies are needed, however, to clarify the specific contribution of each subregion to the processing of self across time.
The question of whether CMS (or some subregions within CMS) play some specific roles in self-referential processing or whether their activation during self-referential tasks can be entirely explained in terms of more general (nonspecific) processes (e.g. metacognitive evaluation) is highly debated (see e.g. Amodio and Frith, 2006; Rameson and Lieberman, 2007, for further discussion of this issue). Thus, an alternative interpretation of the present findings would be that differences in activity within CMS across conditions simply reflect the differential recruitment of evaluative and reasoning processes (Ruby and Legrand, 2007). As people tend to consider situational variability more often when reflecting on their present self than when reflecting on their past selves or when reflecting on others (Pronin and Ross, 2006), it could be argued that participants had to reason more before they could ascribe traits to the present self. Although the argument cannot be completely refuted, participants’ response times and ratings for difficulty do not fit well with this interpretation. The four reflective conditions did not differ in terms of response times, and judgments regarding the present self were actually rated as being easier than judgments regarding the past self and did not differ from judgments regarding the present other; hence, data concerning response times and ratings for difficulty cannot account for the specific pattern of activity observed in CMS across the four reflective tasks. Therefore, although the four tasks undoubtedly shared some common processes (e.g. metacognitive evaluation), we are inclined to believe that the increased activity in CMS when reflecting on the present self cannot be entirely explained in terms of differences in amount of evaluative and reasoning processes.
In the current study, we contrasted present and past time periods that were 5 years apart. It should be noted, however, that the critical factor is probably the perception of personal changes between the present and past, rather than the actual time interval. We chose a 5-year interval because it included the transition from high school to college, which entails significant personal changes in young adults (Libby and Eibach, 2002). Young adults are still in a phase of formation and consolidation of identity during the college years, and the ability to differentiate between present and past selves probably plays an important role in the creation of a stable identity (e.g. by enabling the reflection on personal changes and the construction of life narratives that make sense of how one has become the person one currently is; McAdams, 2001). After the self-concept has been stabilized, however, people may perceive less differences between their present self and their self 5 years ago (unless they have encountered important changes in their life circumstances), such that the processing of present and past selves may be more similar to each other.
To avoid any confusion, it should also be noted that our use of the term ‘present self ’ does not refer to the self as experienced in the immediate moment, that is, ‘the consciousness of oneself as an immediate subject of experience, unextended in time’ (Gallagher, 2000, p. 15). In this study, the notion of present self refers to the mental representation of one's own current psychological characteristics (e.g. one's own traits, abilities and preferences), which is constructed from knowledge stored in memory structures (semantic and/or episodic) and actually involves some temporal extension. The mental model of the present self contains knowledge of who we are in the current ‘lifetime period’, which boundaries are probably delimited by the individual's current life circumstances (e.g. the period delimited by one's current job or intimate relationships; Conway and Pleydell-Pearce, 2000; Conway, 2005). Strictly speaking, then, what we call ‘present’ actually refers to an extended period of time that encompasses the recent past and expected near future. Interestingly, a recent study suggests that self-awareness in the immediate moment, as induced by mindfulness training (i.e. being aware of one's sensory experience in the present moment), actually reduces memory-based forms of self-reference (such as reflecting on one's own present traits) and is associated with decreased activity in CMS (Farb et al., 2007). Other studies have demonstrated that CMS also show decreased activity when people are engaged in demanding cognitive tasks as opposed to so-called resting states (Shulman et al., 1997; Gusnard and Raichle, 2001; Wicker et al., 2003; Mason et al., 2007) and there is evidence that the level of activity in CMS during rest is positively related to the amount of experienced self-referential thoughts (D’Argembeau et al., 2005). These data provide additional evidence for the role of CMS in memory-based forms of self-reference, and further suggest that this type of self-referential processing tends to occur spontaneously in the absence of external demands for attention.
More generally, this study is also in keeping with the idea that the self is not a fixed and completely isolated entity; it is a fluctuating, context-dependent process (James, 1890; Northoff et al., 2006). According to this view, strength of self-relatedness is distributed along a continuum of identifying oneself with (vs distancing oneself from) particular mental contents, and what is perceived as self-related may vary as a function of contextual factors. As the current study illustrates, we sometimes treat ourselves as an ‘other’ when making self-judgments (Libby and Eibach, 2002; Pronin and Ross, 2006), which is reflected in differential activity within CMS. Conversely, we often use information about ourselves when we reflect on others, especially when considering people who are perceived to be like ourselves (Van Boven and Loewenstein, 2005). Thus, reflecting on similar others engage the ventral MPFC more than reflecting on dissimilar others (Mitchell et al., 2006). The relative malleability of the boundary between self and other is also illustrated by a recent study of cultural differences in the neural correlates of self-processing. Zhu et al. (2007) observed that the MPFC was more activated when thinking about the self vs a close other for Western participants but not for Chinese participants, whose self-concept overlaps more with intimate others. Thus, what is considered as the ‘self’ might depend on which information one identifies with a particular occasion (i.e. what one includes in the current self-concept), which in turn depends on cultural influences, temporal perspectives and very likely numerous other contextual factors.
Acknowledgments
This research was supported by a grant from the French speaking community of Belgium (ARC, Convention 06/11-340), the FRS-FNRS, an Interuniversity Attraction Poles Programme (P6/29)–Belgian State – Belgian Science Policy, and a grant from the University of Liège (Fonds Spéciaux pour la Recherche).
Footnotes
1 We used a block design in this study because pilot testing revealed that some participants experienced difficulty in switching between the present and past time periods on each trial. It has been argued that employing a blocked-design approach may result in weaker differences in MPFC activity between conditions, especially when inter-trial intervals are long (see Heatherton et al., 2006, for further discussion of this issue). Therefore, short inter-trial intervals were used in this study (mean duration of 875 ms). Using a similar block design in an earlier study, we were previously able to observe significant differences in CMS activity for judgments targeting the self vs an intimate other (D’Argembeau et al., 2007).
2 In this study, we used a yes/no response format to simplify motor responses. Estimates of perceived changes might have been even higher if participants had the opportunity to make more nuanced responses (e.g. by using a Likert-type scale). Furthermore, there is evidence that people make dispositional attributions more often when reflecting on their past self than when reflecting on their present self, a processing difference that could not be captured by the yes/no response format (Pronin and Ross, 2006). Thus, it is likely that the present indexes actually underestimated perceived changes across time.
References
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Anderson NH. Likableness ratings of 555 personality-trait words. Journal of Personality and Social Psychology. 1968;9:272–9. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11:219–27. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Conway MA. Memory and the self. Journal of Memory and Language. 2005;53:594–628. [Google Scholar]
- Conway MA, Pleydell-Pearce CW. The construction of autobiographical memories in the self-memory system. Psychological Review. 2000;107:261–88. doi: 10.1037/0033-295x.107.2.261. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Moroz TM, Moscovitch M, et al. In search of the self: a positron emission tomography study. Psychological Science. 1999;10:26–34. [Google Scholar]
- D’Argembeau A, Collette F, Van der Linden M, et al. Self-referential reflective activity and its relationship with rest: a PET study. NeuroImage. 2005;25:616–24. doi: 10.1016/j.neuroimage.2004.11.048. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Ruby P, Collette F, et al. Distinct regions of the medial prefrontal cortex are associated with self-referential processing and perspective taking. Journal of Cognitive Neuroscience. 2007;19:935–44. doi: 10.1162/jocn.2007.19.6.935. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of what Happens: Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. [Google Scholar]
- Demo DH. The self-concept over time: research issues and directions. Annual Review of Sociology. 1992;18:303–26. [Google Scholar]
- Deutsch FM, Ruble DN, Fleming A, Brooks-Gunn J, Stangor C. Information-seeking and maternal self-definition during the transition to motherhood. Journal of Personality and Social Psychology. 1988;55:420–31. doi: 10.1037//0022-3514.55.3.420. [DOI] [PubMed] [Google Scholar]
- Farb NAS, Segal ZV, Mayberg H, et al. Attending to the present: mindfulness meditation reveals distinct neural modes of self-reference. Social Cognitive and Affective Neuroscience. 2007;2:313–22. doi: 10.1093/scan/nsm030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, et al. In search of the emotional self: an fMRI study using positive and negative emotional words. American Journal of Psychiatry. 2003;160:1938–45. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Penny WD, Glaser DE. Conjunction revisited. NeuroImage. 2005;25:661–7. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conceptions of the self: implications for cognitive sciences. Trends in Cognitive Sciences. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nature Reviews Neuroscience. 2001;2:685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Wyland CL, Macrae CN, Demos KE, Denny BT, Kelley WM. Medial prefrontal activity differentiates self from close others. Social Cognitive and Affective Neuroscience. 2006;1:18–25. doi: 10.1093/scan/nsl001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. New York: Holt; 1890. [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125:1808–14. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–94. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17:1080–6. [PubMed] [Google Scholar]
- Klein SB, Rozendal K, Cosmides L. A social-cognitive neuroscience analysis of the self. Social Cognition. 2002;20:105–35. [Google Scholar]
- Libby LK, Eibach RP. Looking back in time: self-concept change affects visual perspective in autobiographical memory. Journal of Personality and Social Psychology. 2002;82:167–79. [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience: a review of core processes. Annual Review of Psychology. 2007;58:259–89. doi: 10.1146/annurev.psych.58.110405.085654. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Jarcho JM, Satpute AB. Evidence-based and intuition-based self-knowledge: an FMRI study. Journal of Personality and Social Psychology. 2004;87:421–35. doi: 10.1037/0022-3514.87.4.421. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental Self. Proceedings of the National Academy of Sciences of the USA. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–5. doi: 10.1126/science.1131295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams DP. The psychology of life stories. Review of General Psychology. 2001;5:100–22. [Google Scholar]
- Mitchell JP, Banaji MR, Macrae CN. General and specific contributions of the medial prefrontal cortex to knowledge about mental states. NeuroImage. 2005;28:757–62. doi: 10.1016/j.neuroimage.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heatherton TF, Wyland CL, Kelley WM. Neuroanatomical evidence for distinct cognitive and affective components of self. Journal of Cognitive Neuroscience. 2006;18:1586–94. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Morin A. Levels of consciousness and self-awareness: a comparison and integration of various neurocognitive views. Consciousness and Cognition. 2006;15:358–71. doi: 10.1016/j.concog.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends in Cognitive Sciences. 2004;8:102–7. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Northoff G, Heinzel A, de GM, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain-a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–57. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Beer JS, Robertson ER, et al. The neural correlates of direct and reflected self-knowledge. NeuroImage. 2005;28:797–814. doi: 10.1016/j.neuroimage.2005.06.069. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. ‘I know you are but what am I?!’: neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19:1323–37. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pronin E, Ross L. Temporal differences in trait self-ascription: when the self is seen as an other. Journal of Personality and Social Psychology. 2006;90:197–209. doi: 10.1037/0022-3514.90.2.197. [DOI] [PubMed] [Google Scholar]
- Rameson L, Lieberman MD. Thinking about the self from a social cognitive neuroscience perspective. Psychological Inquiry. 2007;18:117–22. [Google Scholar]
- Ruby P, Legrand D. Neuroimaging the self ? In: Haggard P, Rossetti Y, Kawato M, editors. Sensorimotor Foundations of Higher Cognition (Attention and Performance XXII). New York: Oxford University Press; 2007. pp. 293–318. [Google Scholar]
- Sakaki M. Semantic self-knowledge and episodic self-knowledge: independent or interrelated representations? Memory. 2007;15:1–16. doi: 10.1080/09658210601055750. [DOI] [PubMed] [Google Scholar]
- Saxe R, Moran JM, Scholz J, Gabrieli J. Overlapping and non-overlapping brain regions for theory of mind and self relfection in individual subjects. Social Cognitive and Affective Neuroscience. 2006;1:229–34. doi: 10.1093/scan/nsl034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Johnson SC. Relevance to self: a brief review and framework of neural systems underlying appraisal. Neuroscience and Biobehavioral Reviews. 2007;31:585–96. doi: 10.1016/j.neubiorev.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. NeuroImage. 2004;22:941–7. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Fiez JA, Corbetta M, et al. Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. Journal of Cognitive Neuroscience. 1997;9:648–63. doi: 10.1162/jocn.1997.9.5.648. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Boven L, Loewenstein G. Cross-situational predictions. In: Alicke MD, Dunning DA, Krueger JI, editors. The Self in Social Judgment. New York: Psychology Press; 2005. pp. 43–64. [Google Scholar]
- Vogeley K, Kupke C. Disturbances of time consciousness from a phenomenological and an neuroscientific perspective. Schizophrenia Bulletin. 2007;33:157–65. doi: 10.1093/schbul/sbl056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicker B, Ruby P, Royet JP, Fonlupt P. A relation between rest and the self in the brain? Brain Research Reviews. 2003;43:224–30. doi: 10.1016/j.brainresrev.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Zhang L, Fan J, Han S. Neural basis of cultural influence on self-representation. NeuroImage. 2007;34:1310–6. doi: 10.1016/j.neuroimage.2006.08.047. [DOI] [PubMed] [Google Scholar]