Abstract
Approximately 80% of the body vitamin A is stored in liver stellate cells with in the lipid droplets as retinyl esters. In low vitamin A status or after liver injury, stellate cells are depleted of the stored retinyl esters by their hydrolysis to retinol. However, the identity of retinyl ester hydrolase(s) expressed in stellate cells is unknown. The expression of carboxylesterase and lipase genes in purified liver cell-types was investigated by real-time PCR. We found that six carboxylesterase and hepatic lipase genes were expressed in hepatocytes. Adipose triglyceride lipase was expressed in Kupffer cells, stellate cells and endothelial cells. Lipoprotein lipase expression was detected in Kupffer cells and stellate cells. As a function of stellate cell activation, expression of adipose triglyceride lipase decreased by 2-fold and lipoprotein lipase increased by 32-fold suggesting that it may play a role in retinol ester hydrolysis during stellate cell activation.
Keywords: Hepatic stellate cells, carboxylesterases, lipoprotein lipase, adipocytes triglyceride lipase, real-time PCR, retinyl palmitate, vitamin A
Liver plays an important role in the uptake, metabolism and storage of vitamin A (Figure 1). The first step in liver vitamin A metabolism is sequestration of the chylomicrons remnants in the space of Disse [1]. Lipoprotein lipase and hepatic lipase are secreted in the space of Disse and can further hydrolyze chylomicrons remnants. Hepatocytes are responsible for the uptake of retinyl ester containing chylomicrons remnants [2]. Inside the hepatocytes, the retinyl esters are hydrolyzed to retinol [3] which can either be oxidized to retinoic acid or bound to cellular retinol binding protein (CRBP). Retinol secreted into the circulatory system, binds retinol binding protein (RBP) [4] and is transported to the tissues to meet the body’s retinol requirements. The excess RBP-bound retinol will be taken up by the hepatic stellate cells (HSC) and converted by lecithin-retinol acyltransferase or coenzyme A retinol acyltransferase to retinyl esters for long term storage in intracellular lipid droplets [5]. Hepatic stellate cells (HSC) are located in the perisinusoidal space of Disse and store about 80% of the total body vitamin A [6]. After liver injury caused by chronic alcohol consumption or exposure to hepatotoxins, the HSC become “activated” and transform into myofibroblast-like cells. One of the early events during HSC activation is the loss of vitamin A [7] due to hydrolysis of retinyl esters to retinol [8] by retinyl ester hydrolases (REH).
Figure 1.
Chylomicrons remnants containing retinyl esters (CMR-RE) are cleared by hepatocytes after intestinal absorption. The retinyl esters are hydrolyzed by retinyl ester hydrolase (REH) to retinol (ROL). ROL can either be oxidized to retinoic acid (RA) or can bind to cellular retinol binding protein (CRBP). The CRBP bound ROL can be secreted into circulation as a complex with retinol binding protein (RBP) to meet body’s retinol requirements. Excess retinol is than taken up by stellate cells and converted to retinyl esters (RE) by lecithin-retinol acyltransferase (LRAT) or coenzyme A retinol acyltransferase (ARAT) for long term storage in lipid droplets. These RE’s can be converted back to ROL if necessary by REH.
The total REH activity in different liver cell types was studied by Blaner et. al. [9] but their identity was not determined. Several in vitro studies have identified carboxylesterases (ES10, ES2, ES4, ES3, D50580, AY034877 and AB010635) [10–12] and lipases (lipoprotein lipase (LPL) [13] pancreatic triglyceride lipase (PTL) [14], and hormone sensitive lipase (HSL) [15]) with REH activity. Based on their ability to bind lipid droplets in adipocytes the most interesting candidates would be HSL and adipose triglyceride lipase (ATGL) [16; 17]. It is our hypothesis that intervention of vitamin A loss might lead to treatment option for liver fibrosis. However, the target enzyme responsible for increased retinyl ester hydrolysis has not been identified. Accordingly, the expression of six carboxylesterase and six lipase genes was studied to determine the liver cell-specific expression of REH by quantitative real-time PCR.
Material and Methods
Isolation of rat liver cells
The following protocol was approved by the Institutional Animal Care and Use committee at Indiana University Purdue University at Indianapolis. The isolation of rat liver cells was adapted from published methods [18–20]. Male Wistar rats (Charles Rivers Labs, Wilmington, MA) weighing 500–600g were used. In situ liver perfusion was performed via the portal vein, sequentially with 150 ml MEM without Ca2+ and Mg2+, 100 ml of 0.4 % Pronase (Roche, Indianapolis, IN) and 150 ml 0.0125% type IV collagenase (Sigma, St. Louis, MO) in DMEM/Ham’s F12 medium. After perfusion the liver was incubated in 100 ml 0.035% pronase containing 10 µg /ml of DNase (Roche Diagnostics, Indianapolis, IN) at 37°C for 30 min. The cell suspension was centrifuged at 60 × g for 2 min and the supernatant was used for isolation of non-parenchymal cells.
Hepatic stellate cells (HSC)
The supernatant (above) was centrifuged at 800 × g for 10 min. The pellet was re-suspended in 14 ml of DMEM/Ham’s F12 and 6.4 ml of 28.7 % Histodenz (Sigma, St. Louis, MO). This mixture was distributed in two tubes and layered with DMEM/Ham’s F12 media and centrifuged at 1,400 × g for 17 min. Enriched HSC at the junction of medium and Histodenz were collected. Purified non-activated HSC were obtained by Fluorescence Activated Cell Sorting (as described below) of enriched HSC. To obtain activated HSC, the enriched HSC were cultured overnight in RPMI 1640 (Cambrex BioScience, Walkersville, MA) with 25 mM Hepes, 10% FBS and penicillin-streptomycin, followed by sorting (as described below) and 2 additional days of culturing. Fluorescence Activated Cell Sorting was done using FACSVantage™ SE (Becton Dickinson, Sunnyvale, CA) instrument equipped with a 488nm laser for side scatter and forward scatter analysis and a 325nm UV laser coupled with a 440/40 nm band pass filter for vitamin A autofluorescence analysis.
Kupffer cells
The cell pellet from the Histodenz centrifugation (above) was re-suspended in 7 ml of DMEM/Ham’s F12 and layered onto a discontinuous stractan gradient [19] of 1.084, 1.058, 1.044 and 1.035 densities. The stractan gradient was centrifuged at 21,400 rpm in a SW41 rotor (Beckman Coulter, Inc.) for 40 min without the brake. The cells at interphase of 1.058/1.084 density were collected, washed and plated in RPMI 1640 containing 10% FBS. The following day, the cells were washed with PBS, trypsinized and aliquots were snap frozen in liquid nitrogen and stored in −80°C freezer. The purity of the Kupffer cells was determined to be >90% using 2 µm fluoresbrite-YG beads (Polysciences Inc., Warrington, PA) in a phagocytosis assay coupled with fluorescence imaging using Axiovert 200 (Zeiss) microscope equipped with GFP filter set.
Sinusoidal endothelial cells
Cells were provided by the Non-Parenchymal Liver Cell Core at the Research Center for Alcoholic Liver and Pancreatic Diseases at the University of Southern California.
Hepatocytes
Rat liver was perfused in situ via portal vein with 150 ml minimal essential medium without Ca2+ and Mg2+, and 250 ml of 0.03% type IV collagenase. The liver was re-suspended and the hepatocytes were pelleted at 60 × g and washed five times in DMEM/Ham’s F12. The hepatocytes with >90% purity were aliquoted, snap frozen and stored in −80°C freezer.
Fluorescence microscopy
Images were acquired on a Zeiss Axiovert 200 epifluorescent microscope equipped with DAPI, GFP, and Texas red filter sets (Chroma Technology, Rockingham, VT) with an Axiocam HR camera using Axiovision 4.01 software, and analyzed using MetaMorph 5.0 software (Universal Imaging, Downingtown, PA).
RNA isolation and reverse transcription
Hepatocyte RNA was extracted with TRIzol® (Invitrogen, Carlsbad, CA) using manufacturer’s protocol. RNeasy mini kit (Qiagen, Valencia, CA) with DNase treatment was used to isolate RNA from non-activated and activated HSC, Kupffer cells, endothelial cells and to purify 100 µg of hepatocyte RNA. The quantity and the quality of the RNA were assessed using a ND-1000 Spectrophotometer (Nanodrop, Wilmington, DE) by determining the absorbance at 260 and monitoring the 260/280 and 260/230 ratios. One µg of total RNA from each cell sample was reverse transcribed using the GeneAmp Gold RNA PCR kit (Applied Biosystems, Foster City, CA) in a 50-µl reaction. The reaction conditions were previously described [21].
Real-time PCR
The primers used for each gene are reported in Table 1, their specificity was determined in a control experiment by sequencing their respective PCR products. SYBR Green kit (Applied Biosystems, Foster City, CA) was used and each 25 µl PCR reaction contained 3 mM Mg+2, 0.3–0.4 µM of each primer, 0.2 mM of deoxynucleotide triphosphates and cDNA equivalent to 20 ng of RNA. Each cDNA sample was evaluated in triplicate using PCR cycling conditions of 50°C for 2 min, 95°C for 10 min and 40 cycles of 95°C for 15 s, 65°C for 15 s, and 72°C for 1 min on Applied Biosystems 7300 system (Applied Biosystems, Foster City, CA). The presence of single PCR product was determined by dissociation curve analysis. A standard curve for each gene was generated using clones constructed in our laboratory. The copy numbers of each gene was determined by comparison to their respective standard curve.
Table 1.
Real-time PCR primers: The primer sequences are reported in 5’→3’ orientation.
| Gene (Accession Number)* | Forward Primer | Reverse Primer |
|---|---|---|
| ES10 (X51974) | ATTACAACAGATAGCAAGCCCATT | AGAGATTTTCAGTGTTGGGTAGG |
| D50580 (D50580) | TGTGGATTCAGAGGCCTTGATGAG | TGTTCTTCAGAACAGCCCGCATG |
| ES3 (X81395) | CCCTCACTGCAGGCCTGGTCAA | GAGTCTCCGTGCAAATCCAGCG |
| ES4 (X81825) | CCAGAGGATATTATTCCAGTTGCC | TCTCCTACTACATGCTTGGGTCTT |
| AB010635 (AB010635) | GAGCTATCCAGAGAGAACCTGCA | ATTCTTGAAATAGCTGGGTGCATGT |
| AY034877 (AY034877) | GAAGACTGCCTGTATCTCAACAT | CTTGTAGACCACCTCAGATTTATC |
| LPL (NM_012598) | CTTAAGTGGAAGAACGACTCCTACT | GTCATGGCATTTCACAAACACTGCA |
| ATGL NM_001108509.2) | TAGCATCTGCCAGTATCTGGTGAT | AGGCCACATTGGTGCAGAAGAGA |
| CEL (NM_016997) | ACCTTTGACATCTACACTGAGTCCT | ACGTACTGGAGGTCATCAGCGTG |
| HSL (NM_012859) | TCCTCTTCTACCACTGAGCGTAC | ACTCCTGCGCATAGACTCCGTAA |
| Hepatic Lipase (NM_012597) | GAACACAGTGCAGACCATAATGCT | TTCAGGTCACATTTCACGAAGACTT |
| PTL (NM_013161) | ACTTCGCACGTTGGCGGTACCA | AATCTCCAACATCCATATCAGAGTC |
The accession number used for designing primers for each gene is indicated in the first column in parenthesis.
RESULTS
Isolation of liver cells
Disruption of the liver basement membrane with collagenase generates a mixture of hepatocytes and non-parenchymal cells. Hepatocytes are larger in size and spin down at 50 × g. Repeated washing of the hepatocyte pellet removes most of the smaller non-parenchymal cells. The typical yield of hepatocytes was 1.7 × 108 cells per liver. For isolation of non-parenchymal cells, which account for approximately 25~35% of liver cells, the liver was perfused with 0.4 % pronase to digest most of the hepatocytes. HSC were enriched using Histodenz centrifugation to >60% purity as determined by light microscopy. In order to achieve >95% purity the HSC were subjected to cell sorting using their vitamin A autofluorescence (Figure 2A) and side scatter (Figure 2B). The R1 gate (Figure 2A) separates the vitamin A containing stellate cells from other cells and debris. These vitamin A containing stellate cells were further sorted by forward and side scatter to separate the single cell population of HSC (R2 gate) from cell clumps (R3 gate; Figure 2B). The R2 cell population had >95% purity by microscopic analysis for both activated and non-activated HSC. The cultured and sorted HSC acquire a myofibroblast-like or “activated” phenotype (Figure 3A). A few non-activated cells are marked by arrowheads in Figure 3. The perinuclear localization of vitamin A autofluorescence in activated HSC (marked by arrows) is shown in Figure 3B.
Figure 2.
Vitamin A fluorescence-directed cell sorting of HSC. Panel A: Autofluorescence of enriched HSC using a 325 UV laser shows a clear separation between vitamin A containing cells (R1) and contaminants cells (R4). Panel B: The vitamin A positive cells in R1 gate (Panel A) were analyzed for side and forward scatter. Two distinct populations R2 and R3 are observed. R2 cells were highly purified single-cell HSC population. R3 cells represented aggregates of HSC themselves or other cell-types and were associated with higher forward scatter values.
Figure 3.
Images of purified cultured HSC. Images were collected at 10x magnification on an Axiovert 200 microscope equipped with Dapi filter set. Panel A shows the phase contrast image of activated HSC purified by flow sorting and cultured on plastic dish. Activated (marked by arrows) and non-activated HSC (marked by arrowheads) were both seen. Panel B shows an inverted vitamin A autofluorescence image collected with Dapi filter set of the cells in panel A. Typical perinuclear autofluorescence was observed in activating HSC (marked by arrows).
Normalization of real-time PCR data
We studied two house keeping genes (β-actin and glyceraldehyde-6-phosphate dehydrogenase) by real-time PCR in our samples. In agreement with the literature [22] we found high variation in expression of both genes among the different cell-types. Expression of β-actin was lower by 8-fold in hepatocytes in comparison to non-parenchymal cells. Expression of glyceraldehyde-6-phosphate dehydrogenase was 8-fold higher in activated and 20-fold higher in Kupffer cells in comparison to hepatocytes. Therefore, we examined expression of each gene in identical RNA aliquot.
Real-time PCR
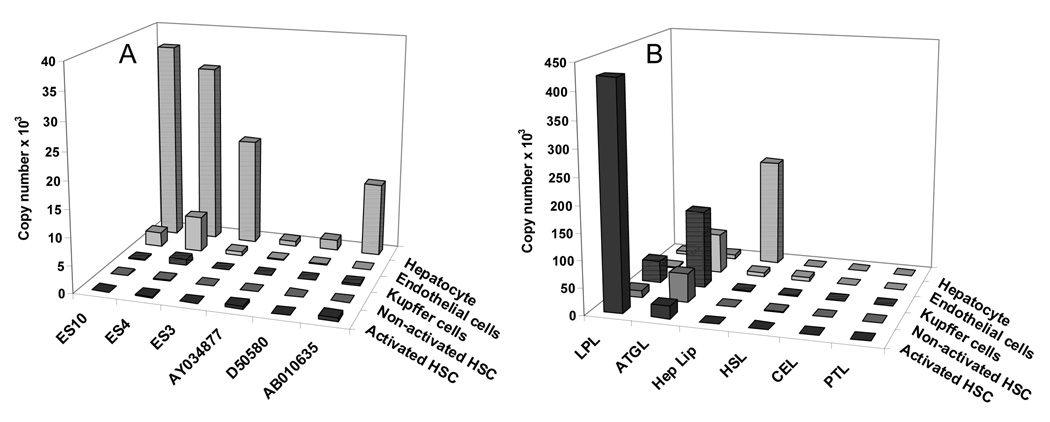
The expression of carboxylesterase in specific liver cells was investigated by real-time PCR. Six major rat liver carboxylesterase genes were analyzed and all of them were highly expressed in hepatocytes (Figure 4A). Expression of carboxylesterases was not detected in non-parenchymal cell-types with the exception of sinusoidal endothelial cells (Figure 4A). In endothelial cells, ES4 was expressed at a slightly higher level than ES10 but the expression of both carboxylesterases was 5–10-fold lower than that in the hepatocytes (Figure 4A). Expression of six major lipases ATGL, LPL, hepatic lipase, HSL, PTL and cholesterol ester lipase (CEL, lysophospholipase), was investigated in liver cell-types by real-time PCR (Figure 4B). Hepatic lipase was expressed only in the hepatocytes with no detectable expression in any of the non-parenchymal cells (Figure 4B). The only lipase expressed in the endothelial cells was ATGL. Kupffer cells express ATGL and LPL. Non-activated stellate cells express ATGL and small amount of LPL. Activated HSC showed a 2-fold decrease in the expression of ATGL and remarkable 32-fold increase in the expression of LPL (Figure 4B). Expression of HSL, PTL and CEL was either very low or not detected in any liver cell-types.
Figure 4.
Expression analysis of carboxylesterases and lipase genes in liver cell-types by real-time PCR. Panel A shows the expression of six carboxylesterase genes ES10, ES4, ES3, D50580, AY034877, and AB010635 as copy numbers. Panel B shows the expression of six lipase genes ATGL (Adipose triglyceride lipase), LPL (Lipoprotein lipase), HSL (hormone sensitive lipase), CEL (Cholesterol ester lipase), Hep L (Hepatic lipase), and PTL (Pancreatic triglyceride lipase).
DISCUSSION
The primary goal of this study was to identify the liver cell-specific expression of carboxylesterases and lipases that could be candidates for REH. Among different liver cell-types, hepatic lipase is exclusively expressed in the hepatocytes and is the only lipase expressed in these cells (Figure 4B). Hepatic lipase can be secreted in the space of Disse by the hepatocytes where it can play a role in hydrolysis of chylomicrons remnants (Figure 1) and their uptake [1; 23]. In this study, we find that all carboxylesterases were almost exclusively expressed in the hepatocytes with relative abundance of, ES10>ES4>ES3>AB010635>>D50580 > AY034877. In our previous study, we had isolated five glycosylated carboxylesterase proteins from rat liver extracts with relative abundance of ES10>ES4>AY034877>ES3>>D50580 and they could all hydrolyze retinyl palmitate [12]. ES10 and ES4 together accounted for 85% of glycosylated carboxylesterase. ES4 was the most efficient REH with specific activities reported between 3 – 7.5 nmole min−1 mg−1 [10; 12]. In agreement with our previous report, our current study finds that ES10 and ES4 account for 66% of the total carboxylesterase transcript in hepatocytes (Figure 4A). In addition to hepatic lipase, ES10 and ES4 would be the most likely candidates for REH in the hepatocytes (Figure 1) converting retinyl esters in the chylomicrons remnants to retinol.
Two bile salt dependent lipases, CEL and PTL, exhibit in vitro retinyl palmitate hydrolase activity [14; 24]. However in comparison to wild-type mice, CEL knockout mice did not exhibit any change in absorption or tissue and serum levels of retinoid [25]. The retinoid absorption was much less efficient in PTL−/− (45%) mice and CEL−/− and PTL−/− double knockout mice (65%) [26]. This suggests that CEL does not, while PTL does play a role in uptake of retinyl esters from the intestine (Figure 1). Lack of CEL and PTL expression in liver cells suggests that they are not involved in liver retinoid metabolism.
In BFC-1β adipocytes an activator of HSL stimulated hydrolysis of stored retinyl esters [15]. To determine if HSL played a similar role in liver adipocytes or stellate cells, we investigated its expression in activated and non-activated HSC. We determined that, HSL was expressed in HSC (Figure 4B) but its expression was significantly lower than ATGL or LPL, 43 and 11-fold respectively, in non-activated stellate cells and 250 and 4200-fold, respectively, in activated stellate cells. Due to its low expression, we conclude that HSL does not play a significant role of REH in HSC.
Until recently, it was believed that HSL was a key lipolytic enzyme in adipose tissue. However, HSL (−/−) mice were found to hydrolyze triglycerides (TAG) and accumulate diglycerides [27]. This led to the discovery of ATGL [28–30]. ATGL is a cytosolic protein that is recruited to the lipid droplet by CGI-58 resulting in lipolysis [31]. We find that ATGL is expressed in both non-activated and activated HSC and that its expression decreases with HSC activation. This decrease may reflect the loss of adipogenic transcriptional regulation such as PPARγ activity which is reduced in activated HSC [32] and positively regulates ATGL expression in adipocytes [33]. Zimmermann et. al. reported that ATGL did not possess REH activity [28] however, in their later report they found that efficient lypolytic activity of ATGL required activation by CGI-58 [31], which was not included in their earlier report. It is possible that the recruitment of cytosolic ATGL to the lipid droplets by CGI-58 is necessary for REH activity in HSC.
Lipoprotein lipase is expressed in the livers of newborn animals but not in adult livers [34]. In agreement with this, we found only limited expression of LPL in adult rat hepatocytes, endothelial cells, and non-activated stellate cells (Figure 4B). However, a 32-fold induction of LPL was observed in activated HSC. It is possible that this induction is due to high level of lipids present in the medium. LPL has two distinct functions, a catalytic function responsible for hydrolysis of triglycerides and a non-catalytic bridging function that is responsible for cellular uptake of lipoproteins and fat soluble vitamins [35]. Cellular uptake of lipoproteins and fat-soluble vitamin A in BFC-1β adipocytes increased in the presence of LPL [36]. Intracellular localization of LPL in primary adipocytes showed that, unlike most constitutively secreted proteins, LPL accumulated in the ER [37]. It is therefore possible that, in addition to the extra-cellular function of secreted LPL, it may have an intracellular function. Given the wide range of mediators controlling the expression of LPL, it may have a unique role in activated stellate cells as retinyl ester hydrolase in lipid droplets.
In conclusion, we determined liver cell-specific expression of carboxylesterase and lipase genes. Carboxylesterases and hepatic lipase are almost exclusively expressed in hepatocytes. ATGL and LPL are the two lipases expressed in activated stellate cells. The increase in the expression of LPL with stellate cell activation suggests that it may play a role in retinyl ester hydrolysis in HSC. The roles of ATGL and LPL as a retinyl ester hydrolase in hepatic stellate cells need to be examined at the protein and activity level.
Acknowledgement
This work was supported by NIH grant R01DK063141. We dedicate this work in fond memory of Dr. Judy White who had initiated the cell imaging work on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References List
- 1.Harrison EH. Lipases and carboxylesterases: possible roles in the hepatic utilization of vitamin A. J.Nutr. 2000;130:340S–344S. doi: 10.1093/jn/130.2.340S. [DOI] [PubMed] [Google Scholar]
- 2.Blomhoff R, Helgerud P, Rasmussen M, Berg T, Norum KR. In vivo uptake of chylomicron [3H]retinyl ester by rat liver: evidence for retinol transfer from parenchymal to nonparenchymal cells. Proc.Natl.Acad.Sci U.S.A. 1982;79:7326–7330. doi: 10.1073/pnas.79.23.7326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blaner WS, Dixon JL, Moriwaki H, Martino RA, Stein O, Stein Y, Goodman DS. Studies on the in vivo transfer of retinoids from parenchymal to stellate cells in rat liver. Eur.J.Biochem. 1987;164:301–307. doi: 10.1111/j.1432-1033.1987.tb11058.x. [DOI] [PubMed] [Google Scholar]
- 4.Blomhoff R, Berg T, Norum KR. Transfer of retinol from parenchymal to stellate cells in liver is mediated by retinol-binding protein. Proc.Natl.Acad.Sci.U.S.A. 1988;85:3455–3458. doi: 10.1073/pnas.85.10.3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaner WS. In: The Liver:Biology and Pathobiology. Arias IM, Boyer J, Fausto N, Jakoby WB, Schachter DA, Shafritz DA, editors. New York: Raven Press, Ltd.; 1994. pp. 529–541. [Google Scholar]
- 6.Blomhoff R, Rasmussen M, Nilsson A, Norum KR, Berg T, Blaner WS, Kato M, Mertz JR, Goodman DS, Eriksson U. Hepatic retinol metabolism. Distribution of retinoids, enzymes, and binding proteins in isolated rat liver cells. J.Biol.Chem. 1985;260:13560–13565. [PubMed] [Google Scholar]
- 7.Friedman SL. Molecular mechanisms of hepatic fibrosis and principles of therapy. J.Gastroenterol. 1997;32:424–430. doi: 10.1007/BF02934504. [DOI] [PubMed] [Google Scholar]
- 8.Reeves HL, Friedman SL. Activation of hepatic stellate cells--a key issue in liver fibrosis. Front Biosci. 2002;7:d808–d826. doi: 10.2741/reeves. [DOI] [PubMed] [Google Scholar]
- 9.Blaner WS, Hendriks HF, Brouwer A, de Leeuw AM, Knook DL, Goodman DS. Retinoids, retinoid-binding proteins, and retinyl palmitate hydrolase distributions in different types of rat liver cells. J.Lipid Res. 1985;26:1241–1251. [PubMed] [Google Scholar]
- 10.Mentlein R, Heymann E. Hydrolysis of retinyl esters by non-specific carboxylesterases from rat liver endoplasmic reticulum. Biochem.J. 1987;245:863–867. doi: 10.1042/bj2450863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun G, Alexson SE, Harrison EH. Purification and characterization of a neutral, bile salt-independent retinyl ester hydrolase from rat liver microsomes. Relationship To rat carboxylesterase ES-2. J.Biol.Chem. 1997;272:24488–24493. doi: 10.1074/jbc.272.39.24488. [DOI] [PubMed] [Google Scholar]
- 12.Sanghani SP, Davis WI, Dumaual NG, Mahrenholz A, Bosron WF. Identification of microsomal rat liver carboxylesterases and their activity with retinyl palmitate. Eur.J.Biochem. 2002;269:4387–4398. doi: 10.1046/j.1432-1033.2002.03121.x. [DOI] [PubMed] [Google Scholar]
- 13.van Bennekum AM, Kako Y, Weinstock PH, Harrison EH, Deckelbaum RJ, Goldberg IJ, Blaner WS. Lipoprotein lipase expression level influences tissue clearance of chylomicron retinyl ester. J.Lipid Res. 1999;40:565–574. [PubMed] [Google Scholar]
- 14.van Bennekum AM, Fisher EA, Blaner WS, Harrison EH. Hydrolysis of retinyl esters by pancreatic triglyceride lipase. Biochemistry. 2000;39:4900–4906. doi: 10.1021/bi9927235. [DOI] [PubMed] [Google Scholar]
- 15.Wei S, Lai K, Patel S, Piantedosi R, Shen H, Colantuoni V, Kraemer FB, Blaner WS. Retinyl ester hydrolysis and retinol efflux from BFC-1beta adipocytes. J.Biol.Chem. 1997;272:14159–14165. doi: 10.1074/jbc.272.22.14159. [DOI] [PubMed] [Google Scholar]
- 16.Smirnova E, Goldberg EB, Makarova KS, Lin L, Brown WJ, Jackson CL. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006;7:106–113. doi: 10.1038/sj.embor.7400559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brasaemle DL, Levin DM, dler-Wailes DC, Londos C. The lipolytic stimulation of 3T3-L1 adipocytes promotes the translocation of hormone-sensitive lipase to the surfaces of lipid storage droplets. Biochim.Biophys.Acta. 2000;1483:251–262. doi: 10.1016/s1388-1981(99)00179-1. [DOI] [PubMed] [Google Scholar]
- 18.Troen G, Nilsson A, Norum KR, Blomhoff R. Characterization of liver stellate cell retinyl ester storage. Biochem.J. 1994;300:793–798. doi: 10.1042/bj3000793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman SL, Roll FJ. Isolation and culture of hepatic lipocytes, Kupffer cells, and sinusoidal endothelial cells by density gradient centrifugation with Stractan. Anal.Biochem. 1987;161:207–218. doi: 10.1016/0003-2697(87)90673-7. [DOI] [PubMed] [Google Scholar]
- 20.Hendriks HF, Brouwer A, Knook DL. Isolation, purification, and characterization of liver cell types. Methods Enzymol. 1990;190:49–58. doi: 10.1016/0076-6879(90)90008-o. [DOI] [PubMed] [Google Scholar]
- 21.Sanghani SP, Quinney SK, Fredenburg TB, Sun Z, Davis WI, Murry DJ, Cummings OW, Seitz DE, Bosron WF. Carboxylesterases expressed in human colon tumor tissue and their role in CPT-11 hydrolysis. Clin.Cancer Res. 2003;9:4983–4991. [PubMed] [Google Scholar]
- 22.Lee PD, Sladek R, Greenwood CM, Hudson TJ. Control genes and variability: absence of ubiquitous reference transcripts in diverse mammalian expression studies. Genome Res. 2002;12:292–297. doi: 10.1101/gr.217802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ji ZS, Lauer SJ, Fazio S, Bensadoun A, Taylor JM, Mahley RW. Enhanced binding and uptake of remnant lipoproteins by hepatic lipase-secreting hepatoma cells in culture. J.Biol.Chem. 1994;269:13429–13436. [PubMed] [Google Scholar]
- 24.Lombardo D, Guy O. Studies on the substrate specificity of a carboxyl ester hydrolase from human pancreatic juice. II. Action on cholesterol esters and lipid-soluble vitamin esters. Biochim.Biophys.Acta. 1980;611:147–155. doi: 10.1016/0005-2744(80)90050-9. [DOI] [PubMed] [Google Scholar]
- 25.van Bennekum AM, Li L, Piantedosi R, Shamir R, Vogel S, Fisher EA, Blaner WS, Harrison EH. Carboxyl ester lipase overexpression in rat hepatoma cells and CEL deficiency in mice have no impact on hepatic uptake or metabolism of chylomicron-retinyl ester. Biochemistry. 1999;38:4150–4156. doi: 10.1021/bi981680+. [DOI] [PubMed] [Google Scholar]
- 26.Gilham D, Labonte ED, Rojas JC, Jandacek RJ, Howles PN, Hui DY. Carboxyl ester lipase deficiency exacerbates dietary lipid absorption abnormalities and resistance to diet-induced obesity in pancreatic triglyceride lipase knockout mice. J.Biol.Chem. 2007;282:24642–24649. doi: 10.1074/jbc.M702530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haemmerle G, Zimmermann R, Hayn M, Theussl C, Waeg G, Wagner E, Sattler W, Magin TM, Wagner EF, Zechner R. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J.Biol.Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 28.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J.Biol.Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 30.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J.Biol.Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 31.Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 32.Hazra S, Xiong S, Wang J, Rippe RA, Krishna V, Chatterjee K, Tsukamoto H. Peroxisome proliferator-activated receptor gamma induces a phenotypic switch from activated to quiescent hepatic stellate cells. J.Biol.Chem. 2004;279:11392–11401. doi: 10.1074/jbc.M310284200. [DOI] [PubMed] [Google Scholar]
- 33.Kershaw EE, Schupp M, Guan HP, Gardner NP, Lazar MA, Flier JS. PPARgamma regulates adipose triglyceride lipase in adipocytes in vitro and in vivo. Am.J.Physiol Endocrinol.Metab. 2007;293:E1736–E1745. doi: 10.1152/ajpendo.00122.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenkovich CF, Chen SH, Wims M, Luo CC, Li WH, Chan L. Lipoprotein lipase and hepatic lipase mRNA tissue specific expression, developmental regulation, and evolution. J.Lipid Res. 1989;30:423–431. [PubMed] [Google Scholar]
- 35.Mead JR, Irvine SA, Ramji DP. Lipoprotein lipase: structure, function, regulation, and role in disease. J.Mol.Med. 2002;80:753–769. doi: 10.1007/s00109-002-0384-9. [DOI] [PubMed] [Google Scholar]
- 36.Blaner WS, Obunike JC, Kurlandsky SB, al Haideri M, Piantedosi R, Deckelbaum RJ, Goldberg IJ. Lipoprotein lipase hydrolysis of retinyl ester. Possible implications for retinoid uptake by cells. J.Biol.Chem. 1994;269:16559–16565. [PubMed] [Google Scholar]
- 37.Roh C, Roduit R, Thorens B, Fried S, Kandror KV. Lipoprotein lipase and leptin are accumulated in different secretory compartments in rat adipocytes. J.Biol.Chem. 2001;276:35990–35994. doi: 10.1074/jbc.M102791200. [DOI] [PubMed] [Google Scholar]