Abstract
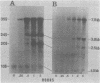
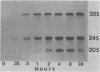
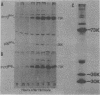
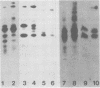
We have studied the kinetics of dexamethasone induction of mouse mammary tumor virus (MMTV) RNAs and proteins in virus-infected rat XC cells and GR mouse mammary tumor cells. A detectable increase in viral RNA in infected XC cells was present within 10 min after hormone addition, and half-maximal induction was achieved in less than 2 h. The increase in viral RNA concentration was apparent first in nuclear RNA and later in the cytoplasm. Within the first 15 min of induction, only genome-sized RNA (35S, 7.8 kilobases) was present in augmented amounts, whereas the major subgenomic RNA (24S, 3.8 kilobases) did not appear until at least 30 to 60 min postinduction. The sequential appearance of these RNAs, the probable mRNA's for the gag and env proteins, paralleled the order of appearance of the gag and env proteins, respectively, after hormone treatment. An additional species of viral RNA (20S, 2.5 kilobases) was detected during these induction experiments, but the role of this RNA is not known. Both subgenomic RNAs contain sequences derived from both the 5′ and 3′ termini of genomic RNA and are presumably spliced. After dexamethasone induction of infected XC cells, we detected two smaller env-related proteins which were not found in full hormone induction. The functional role of these smaller proteins is not known. A previously reported smaller species of RNA (13S, 1.0 kilobase) did not appear to be induced and was shown to be cellular rather than viral in origin. In the fully induced infected XC and GR mammary tumor cells, the only viral RNAs present were the 35S and 24S RNAs. In addition, mammary tumors contained only these two viral RNAs. Thus, tumor cells appear to contain only the viral RNAs which direct the synthesis of the gag, pol, and env proteins of the virion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cordell B., Weiss S. R., Varmus H. E., Bishop J. M. At least 104 nucleotides are transposed from the 5' terminus of the avian sarcoma virus genome to the 5' termini of smaller viral mRNAs. Cell. 1978 Sep;15(1):79–91. doi: 10.1016/0092-8674(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Dahl H. H., Dickson C. Cell-free synthesis of mouse mammary tumor virus Pr77 from virion and intracellular mRNA. J Virol. 1979 Mar;29(3):1131–1141. doi: 10.1128/jvi.29.3.1131-1141.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Composition, arrangement and cleavage of the mouse mammary tumor virus polyprotein precursor Pr77gag and p110gag. Cell. 1979 Aug;17(4):1003–1012. doi: 10.1016/0092-8674(79)90339-8. [DOI] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Polyproteins related to the major core protein of mouse mammary tumor virus. J Virol. 1978 Jun;26(3):660–672. doi: 10.1128/jvi.26.3.660-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Atterwill M. Structure and processing of the mouse mammary tumor virus glycoprotein precursor pr73env. J Virol. 1980 Aug;35(2):349–361. doi: 10.1128/jvi.35.2.349-361.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Peters G. Protein-coding potential of mouse mammary tumor virus genome RNA as examined by in vitro translation. J Virol. 1981 Jan;37(1):36–47. doi: 10.1128/jvi.37.1.36-47.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson C., Puma J. P., Nandi S. Identification of a precursor protein to the major glycoproteins of mouse mammary tumor virus. J Virol. 1975 Jan;17(1):275–282. doi: 10.1128/jvi.17.1.275-282.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donehower L. A., Huang A. L., Hager G. L. Regulatory and coding potential of the mouse mammary tumor virus long terminal redundancy. J Virol. 1981 Jan;37(1):226–238. doi: 10.1128/jvi.37.1.226-238.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Verma I. M. Size analysis and relationship of murine leukemia virus-specific mRNA's: evidence for transposition of sequences during synthesis and processing of subgenomic mRNA. J Virol. 1978 May;26(2):468–478. doi: 10.1128/jvi.26.2.468-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Salden M. H., Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Hynes N. E., Diggelmann H. Identification of mouse mammary tumor virus-specific mRNA. J Virol. 1979 Apr;30(1):417–420. doi: 10.1128/jvi.30.1.417-420.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett P. B., Varmus H. E., Bishop J. M. The genesis of Rous sarcoma virus messenger RNAs. Virology. 1981 Jul 30;112(2):714–728. doi: 10.1016/0042-6822(81)90316-0. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lasfargues E. Y., Lasfargues J. C., Dion A. S., Greene A. E., Moore D. H. Experimental infection of a cat kidney cell line with the mouse mammary tumor virus. Cancer Res. 1976 Jan;36(1):67–72. [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Nusse R., Asselbergs F. A., Salden M. H., Michalides R. J., Bloemendal H. Translation of mouse mammary tumor virus RNA: precursor polypeptides are phosphorylated during processing. Virology. 1978 Nov;91(1):106–115. doi: 10.1016/0042-6822(78)90359-8. [DOI] [PubMed] [Google Scholar]
- Oppermann H., Bishop J. M., Varmus H. E., Levintow L. A joint produce of the genes gag and pol of avian sarcoma virus: a possible precursor of reverse transcriptase. Cell. 1977 Dec;12(4):993–1005. doi: 10.1016/0092-8674(77)90164-7. [DOI] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Phosphorylation of murine mammary tumor virus precursor polypeptides. J Virol. 1979 Apr;30(1):241–247. doi: 10.1128/jvi.30.1.241-247.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racevskis J., Sarkar N. H. Synthesis and processing of precursor polypeptides to murine mammary tumor virus structural proteins. J Virol. 1978 Jan;25(1):374–383. doi: 10.1128/jvi.25.1.374-383.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M. Glucocorticoid regulation of mouse mammary tumor virus gene expression. Biochim Biophys Acta. 1979 Dec 19;560(4):487–508. doi: 10.1016/0304-419x(79)90014-3. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Varmus H. E., Ring J., Yamamoto K. R. Integration and transcription of mouse mammary tumor virus DNA in rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):665–669. doi: 10.1073/pnas.76.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Bishop J. M., Varmus H. E. Glucocorticoid-stimulated accumulation of mouse mammary tumor virus RNA: increased rate of synthesis of viral RNA. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2879–2883. doi: 10.1073/pnas.74.7.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Yamamoto K. R., Tomkins G. M., Bishop M., Varmus H. E. Dexamethasone-mediated induction of mouse mammary tumor virus RNA: a system for studying glucocorticoid action. Cell. 1975 Nov;6(3):299–305. doi: 10.1016/0092-8674(75)90181-6. [DOI] [PubMed] [Google Scholar]
- Ringold G., Lasfargues E. Y., Bishop J. M., Varmus H. E. Production of mouse mammary tumor virus by cultured cells in the absence and presence of hormones: assay by molecular hybridization. Virology. 1975 May;65(1):135–147. doi: 10.1016/0042-6822(75)90014-8. [DOI] [PubMed] [Google Scholar]
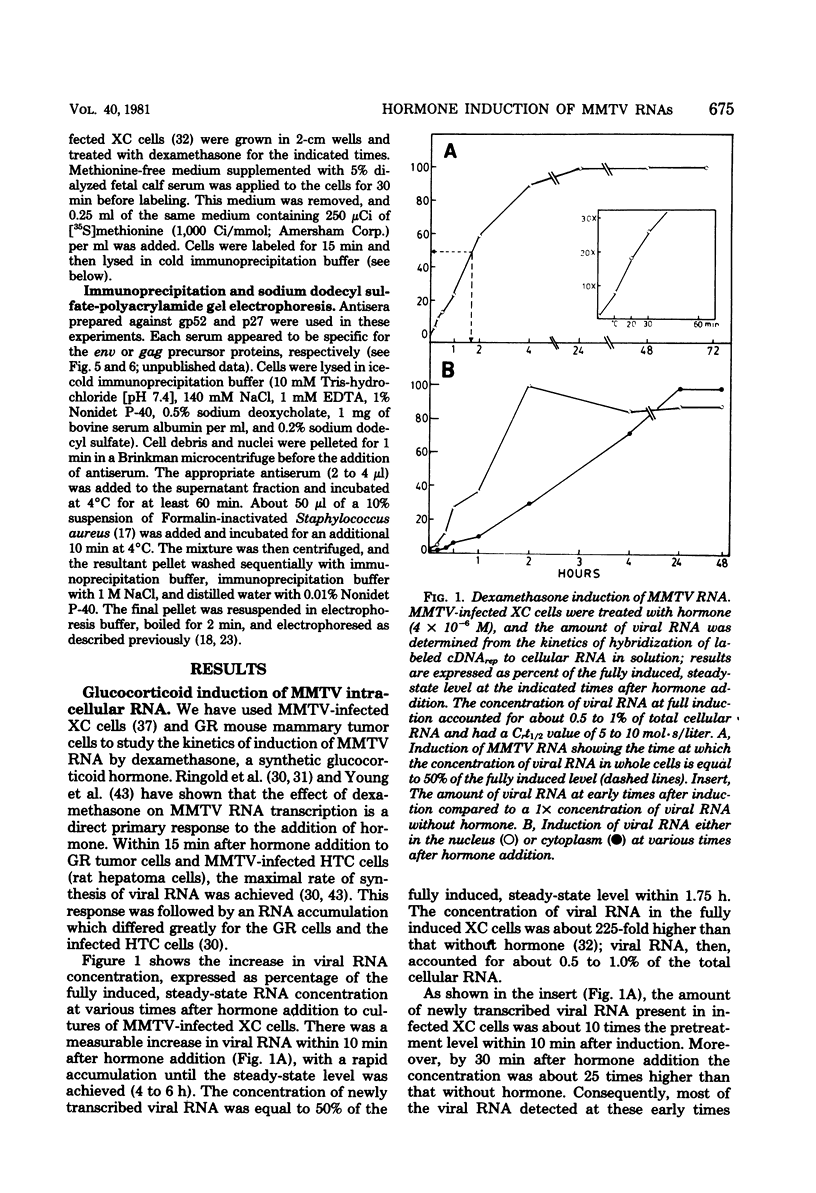
- Robertson D. L., Varmus H. E. Structural analysis of the intracellular RNAs of murine mammary tumor virus. J Virol. 1979 May;30(2):576–589. doi: 10.1128/jvi.30.2.576-589.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- SVOBODA J. Presence of chicken tumour virus in the sarcoma of the adult rat inoculated after birth with Rous sarcoma tissue. Nature. 1960 Jun 18;186:980–981. doi: 10.1038/186980b0. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Smith S. W., Marcus S. L., Sarkar N. H. Identification of the messenger RNAs coding for the gag and env gene products of the murine mammary tumor virus. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1736–1740. doi: 10.1073/pnas.76.4.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacey D. W., Hanafusa H. Nuclear conversion of microinjected avian leukosis virion RNA into an envelope-glycoprotein messenger. Nature. 1978 Jun 29;273(5665):779–782. doi: 10.1038/273779a0. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Shine J., Chirgwin J., Pictet R., Tischer E., Rutter W. J., Goodman H. M. Rat insulin genes: construction of plasmids containing the coding sequences. Science. 1977 Jun 17;196(4296):1313–1319. doi: 10.1126/science.325648. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Heubel G., Lasfargues J. C., Moore D. H. Murine mammary tumor virus: characterization of infection of nonmurine cells. J Virol. 1976 Jun;18(3):911–917. doi: 10.1128/jvi.18.3.911-917.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E., Ringold G., Yamamoto K. R. Regulation of mouse mammary tumor virus gene expression by glucocorticoid hormones. Monogr Endocrinol. 1979;12:253–278. doi: 10.1007/978-3-642-81265-1_14. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Varmus H. E., Bishop J. M. The size and genetic composition of virus-specific RNAs in the cytoplasm of cells producing avian sarcoma-leukosis viruses. Cell. 1977 Dec;12(4):983–992. doi: 10.1016/0092-8674(77)90163-5. [DOI] [PubMed] [Google Scholar]
- Young H. A., Shih T. Y., Scolnick E. M., Parks W. P. Steroid induction of mouse mammary tumor virus: effect upon synthesis and degradation of viral RNA. J Virol. 1977 Jan;21(1):139–146. doi: 10.1128/jvi.21.1.139-146.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]