Abstract
Rationale: High-mobility group box 1 (HMGB1) is a potent inflammatory mediator elevated in sepsis and rheumatoid arthritis, although its role in cystic fibrosis (CF) lung disease is unknown.
Objectives: To determine whether HMGB1 contributes to CF lung inflammation, including neutrophil chemotaxis and lung matrix degradation.
Methods: We used sputum and serum from subjects with CF and a Scnn1b-transgenic (Scnn1b-Tg) mouse model that overexpresses β-epithelial Na+ channel in airways and mimics the CF phenotype, including lung inflammation. Human secretions and murine bronchoalveolar lavage fluid (BALF) was assayed for HMGB1 by Western blot and ELISA. Neutrophil chemotaxis was measured in vitro after incubation with human neutrophils. The collagen fragment proline-glycine-proline (PGP) was measured by tandem mass spectroscopy.
Measurements and Main Results: HMGB1 was detected in CF sputum at higher levels than secretions from normal individuals. Scnn1b-Tg mice had elevated levels of HMGB1 by Western blot and ELISA. We demonstrated that dose-dependent chemotaxis of human neutrophils stimulated by purified HMGB1 was partially dependent on CXC chemokine receptors and that this could be duplicated in CF sputum and BALF from Scnn1b-Tg mice. Neutralization by anti-HMGB1 antibody, in both the sputum and BALF-reduced chemotaxis, which suggested that HMGB1 contributed to the chemotactic properties of these samples. Intratracheal administration of purified HMGB1 induced neutrophil influx into the airways of mice and promoted the release of PGP. PGP was also elevated in Scnn1b-Tg mice and CF serum.
Conclusions: HMGB1 expression contributes to pulmonary inflammation and lung matrix degradation in CF airway disease and deserves further investigation as a biomarker and potential therapeutic target.
Keywords: cystic fibrosis, HMGB1, inflammation, collagen;, fragmentation, proline-glycine-proline
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
High mobility group box 1 (HMGB1) is elevated in cystic fibrosis airways, significantly contributes to neutrophil influx, and contributes to lung matrix degradation.
What This Study Adds to the Field
We show that HMGB1 contributes to pulmonary inflammation and lung matrix degradation in cystic fibrosis, and that it may be a potential biomarker and therapeutic target.
High-mobility group box 1 (HMGB1) is a late mediator of the systemic inflammatory response syndrome (SIRS) and is elevated in bacterial sepsis and hemorrhagic shock (1–3). Under normal conditions, HMGB1 serves as a transcription factor that is expressed in the nucleus of a wide variety of cell types. In inflammatory states, HMGB1 is released into the extracellular environment by stimulated macrophages through a nonclassical pathway (4, 5), activating acute inflammation through Toll-like receptor (TLR)-2, TLR-4, and receptor for advanced glycation end products (RAGE) (5–7). HMGB1 is also released by cells undergoing necrosis (8), a mechanism proposed to explain its effects on perpetuating systemic inflammation in conditions in which cell death is prominent (9) although release during apoptosis has also been reported (10). Extracellular HMGB1 has pleomorphic effects on multiple organs in models of sepsis and shock including activation of nuclear factor (NF)-κB, diffuse endothelial activation, pulmonary inflammation reminiscent of the adult respiratory distress syndrome, hepatocellular injury, and systemic activation of inflammatory cells (7, 9, 11). HMGB1 kinetics in sepsis indicates its role as a late inflammatory mediator, increasing only after tumor necrosis factor (TNF)-α and IL-1β have peaked (9), making it an attractive therapeutic target. Inhibitors of the inflammatory activities of HMGB1 have been efficacious in animal models (12–15), and are currently in preclinical development.
Previous studies defining the role of HMGB1 in the lung have included acute inflammatory states, such as in hemorrhage and septic shock, and an animal model of ventilator-induced lung injury, as well as induction of an adult respiratory distress syndrome–like phenotype after direct instillation (2, 14). HMGB1 has also been implicated in chronic inflammatory conditions such as rheumatoid arthritis (16, 17). Given the importance of HMGB1 with regard to innate immunity, we hypothesized that this protein may also have effects on the prominent neutrophilic inflammatory response that characterizes cystic fibrosis (CF) airway disease (18, 19), and could contribute to lung matrix degradation and lung structure damage that characterize progression of the disease (20).
To evaluate the potential role of HMGB1 in CF lung disease, and consequently as a biomarker and potential therapeutic target, we examined expression levels and the biological activity of HMGB1 in samples derived from patients with CF and from a murine model of CF lung disease (Scnn1b-transgenic [Scnn1b-Tg] mouse) characterized by chronic neutrophilic inflammation and airway mucus obstruction (21). We also examined whether HMGB1-related activity led to lung matrix degradation that could be measured through a simple, noninvasive technique. We found increased expression of HMGB1 in human subjects with CF and in Scnn1b-Tg mice, and demonstrated its pathogenic role as a neutrophil chemoattractant partly dependent on a CXCR2-dependent mechanism capable of production of the bioactive collagen fragment proline-glycine-proline (PGP), a lung matrix degradation product that also directly activates neutrophils through CXCR receptors (22). Some of the results of these studies have been previously reported in the form of abstracts (23, 24).
METHODS
Special Reagents
Anti-HMGB1 antibodies were purchased either from Abcam (Abcam, Cambridge, UK) or R&D Systems (Minneapolis, MN). ELISA was performed using commercially available kits (first-generation assay; Shino Test Corp., Tokyo, Japan). Recombinant human HMGB1 was purchased from Sigma-Aldrich Chemical Corporation (St. Louis, MO) at greater than 90% purity and concentration confirmed by ELISA.
Animal Models
Scnn1b-Tg mice, 5–6 weeks of age, and littermate control mice were acquired in collaboration with the University of North Carolina Cystic Fibrosis Center (Chapel Hill, NC), and evaluated at 5–6 weeks of age. Male C3H/HeJ and BALB/c mice, 5–6 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, ME).
Intratracheal Administration of HMGB1
Methoxyfluorane-anesthetized C3H/HeJ and BALB/c mice received 25 or 50 μg HMGB1 intratracheally in 50 μl of sterile phosphate-buffered saline (PBS). Control mice were given 50 μl sterile PBS intratracheally without HMGB1, as previously described (2). After 24 hours of observation with a light incubator, mice were killed and bronchoalveolar lavage (BAL) performed.
Murine BAL
For Scnn1b-Tg and littermate control mice, after avertin (2,2,2 tribromophenol) anesthesia and exsanguination, a tracheal canula was inserted and BAL performed in three or four body weight instillations of PBS (0.035 ml/g) and pooled as previously described (21). BAL in BALB/c and C3H/HeJ mice was performed similarly, except medroxyfluorane was used for anesthesia, and the BAL procedure was performed under direct visualization of lung distension (maximum 1 ml), as previously described (22). Cells were pelleted by centrifugation (500–1,100 × g for 5 min at 4° C) and resuspended in 100–150 μl PBS for total cell count determination. Cytospin preparations were stained with modified Wright-Giemsa staining for differential cell counts. Aliquots of cell-free BAL fluid (BALF) (supernatant) were prepared and stored at −80°C for further assay.
Human Sample Preparation
Freshly collected sputum after cough induction was diluted 1:1 in sterile saline and centrifuged at 1,000 × g for 20 minutes. The resultant supernatants were collected and stored in aliquots at −80°C, as previously reported (25–27).
Western Blot Analysis
Western blotting was used to determine the presence of HMGB1 in the human sputum and murine BALF. Total protein concentration of human samples was measured using Bio-Rad Benchmark Plus Multiplate Spectrophotometer (Bio-Rad, Hercules, CA), and samples were normalized, as previously described (2). Twenty micrograms of total proteins/sample were loaded on a 12% Tris-HCl–sodium dodecyl sulfate–polyacrylamide gel and run for 1 hour at 120 V. Protein was electrotransferred to a nitrocellulose membrane and then blocked with 5% nonfat dry milk and Tris-buffered saline (composition, pH) with 0.1% Tween 20. After being blocked, the membrane was incubated overnight at 4°C with a specific monoclonal mouse primary antibody to HMGB1 (R&D Systems) at a dilution of 1:2,000 followed by anti-mouse horseradish peroxidase–coupled secondary antibody (Bio-Rad) at a dilution of 1:10,000. After three washings, bands were detected using Enhanced Chemiluminescence Plus Western blotting detection reagents (Amersham Pharmacia Biotech, Piscataway, NJ), as previously reported (27). HMGB1 levels were then estimated by comparing with purified HMGB1.
ELISA
Immunoreactive HMGB1 was quantified using a commercially available capture ELISA using polyclonal and monoclonal antibody (e.g., Shino Test Corp.), as previously described by our center and others (2, 3). Results were quantified using a relative standard curve method with purified human HMGB1 per the manufacturer's instructions. All BAL samples were run without dilution and in duplicate for verification using the Bio-Rad Benchmark Plus Multiplate Spectrophotometer (Bio-Rad).
Human Neutrophil Chemotaxis
In vitro chemotaxis assays of mouse and human samples were performed using isolated human neutrophils in a 96-well modified Boyden chamber appropriate for the evaluation of leukocyte chemotaxis. Human neutrophils were isolated from peripheral blood by standard methods using Histopaque 1077 and 1119, as previously described (22). Cells were washed twice with Hanks' balanced salt solution containing 1% bovine serum albumin (BSA), counted, and resuspended at 2 × 106 cells/ml in Dulbecco's modified Eagle medium (DMEM) with 5% BSA (all chemicals from Sigma-Aldrich, except where noted). Murine neutrophils were isolated by bone marrow aspiration and centrifugation on 62% Percoll at 1,000 × g for 30 minutes at room temperature. Pelleted cells were collected, subjected to red blood cell lysis with AKC lysis buffer (Biosource International, Camarillo, CA), and resuspended in DMEM with 5% BSA as previously described (28). In vitro assays were then performed in a 96-well polycarbonate filter plate with a 3-μm pore size appropriate for leukocyte chemotaxis (Millipore, Billerica, MA). Cell solution (100 μl) was added to each well in the top filter-plate portion of the assembly, and 150 μl of diluted sample in DMEM was added to the bottom feeder wells. CF sputum was added in 1:10 dilution and incubated with 0.4 μg of neutralizing antibody (antibody [ab] 18256; Abcam, Cambridge, UK) or isotype control at room temperature for 2 hours before chemotaxis assay. After 1 hour incubation at 37°C with 5% CO2, the upper portion was removed, and four photomicrographs (20) per well were digitally acquired with a Nikon Eclipse TE2000-U inverted microscope (Nikon, Melville, NY) interfaced with a Nikon Coolpix 990 digital camera. Polymorphonuclear leukocyte (PMN) counts were made by averaging the four images as previously described (22, 29). All experiments were run in duplicate for verification. Murine BALF experiments were performed similarly using anti-HMGB1 antibody acquired from R&D Systems. For comparison between experiments, data were standardized to a chemotactic index with cell migration to blank medium as a baseline (e.g., chemotactic index = mean cells per field migrating to sample solution per mean cells per field migrating to blank medium). IL-8 was used as a positive control (10–50 ng/ml). Chemokinesis experiments were preformed using varying amounts of HMGB1 in the upper chamber with a fixed concentration of HMGB1 in the lower chamber. Blockade of CXCR1 and CXCR2 neutrophil receptors was performed by preincubating isolated neutrophils with antibody (25 μg/ml, R&D Systems) for 45 minutes at 4°C before assay.
Electron Ionization–Liquid Chromatography Tandem Mass Spectrometry for PGP Detection
PGP was measured in sputum and BALF samples using an MDS Sciex (Applied Biosystems, Foster City, CA) API-4000 spectrometer equipped with a Shimadzu HPLC (Shimadzu Scientific Instruments, Columbia, MD). HPLC was performed using a 2.1 × 150 mm Develosi C30 column with 0.1% formic acid (solution A) and acetonitrile + 0.1% formic acid (solution B). From 0 to 0.6 minutes after sample loading, a gradient was applied containing 20% solution B, and from 0.6 to 5 minutes after sample loading the gradient was increased to 100% solution B. Background was removed by flushing with 100% isopropranol + 0.1% formic acid. Positive electrospray mass transitions were at 270–70 and 270–116 M/z for PGP. Area under the curve was measured, and PGP concentration calculated using a relative standard curve method as previously described (22). Serum samples were processed by filtering through a Millipore 10,000 Molecular Weight cutoff centrifugal filter, then washing with 30 μl of 1 N HCl. Filtrates were evaluated by spectroscopy as noted above.
Study Population
All subjects with CF were diagnosed using accepted diagnostic criteria, including a minimum of two clinical features consistent with the diagnosis and either two sweat Cl− values greater than 60 mEq or two disease-causing CF transmembrane conductance regulator (CFTR) mutations (30). Individuals with CF hospitalized for acute pulmonary exacerbation (APE) had at least four of the following criteria: 10% or greater decrease in baseline FEV1, increased cough or sputum production, change in sputum character, dyspnea, tachypnea, fever, weight loss, 5% or greater decrease in O2 saturation, new or worsening crackles on lung auscultation, or findings on chest X-ray consistent with pneumonia (31). Secretions from normal subjects were collected from nonsmoking individuals without known lung disease.
Statistical Analysis
For Western blot densitometry, ELISA, and neutrophil chemotaxis measures, descriptive statistics (mean, SD, and SEM) and paired and unpaired t tests or analysis of variance were performed, as appropriate, using SPSS software (SPSS, Inc., Chicago, IL) and Microsoft Excel (Microsoft Corp., Seattle, WA). Correlation coefficients were determined using simple linear regression. All statistical tests were two-sided and were performed at a 5% significance level (i.e., α = 0.05).
Assurances
All human samples were obtained from subjects with CF who granted written informed consent through protocols approved by the University of Alabama at Birmingham Institutional Review Board and the General Clinical Research Center. All animals were maintained using approved protocols of the University of Alabama at Birmingham or the University of North Carolina at Chapel Hill.
RESULTS
HMGB1 Is Elevated in CF Sputum
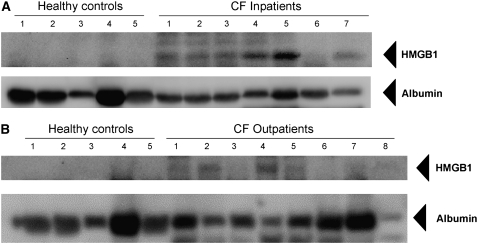
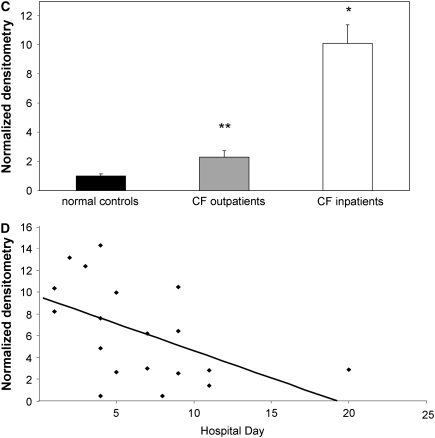
To test whether HMGB1 is elevated in human CF lung disease, we compared HMGB1 expression in sputum from subjects with CF suffering from APE (a population known to have intense neutrophilic inflammation in the airways [18, 20]) with stable CF patients presenting for routine outpatient evaluation and normal controls (Table 1). Western blot analysis showed high levels of HMGB1 in subjects with CF and with APE, whereas HMGB1 was essentially undetectable in healthy control subjects (Figure 1A). Evaluation of sputum from outpatients with stable CF demonstrated intermediate results (Figure 1B). Densitometry estimated an approximate 10-fold increase in relative abundance in patients with CF with APE and a twofold greater expression in outpatients with CF (Figure 1C). Relative concentrations of HMGB1 negatively correlated with the duration of systemic antibiotic therapy in inpatients with CF and with APE (Figure 1D), suggesting that the abundance of HMGB1 decreases as airway inflammation abates. Although HMGB1 levels in inpatients with CF were not correlated with lung function measurements (FEV1% predicted or FVC% predicted) upon admission, when both inpatient and outpatient subjects with CF were included, a significant negative correlation was observed (r = −0.44, P < 0.05).
TABLE 1.
DEMOGRAPHIC CHARACTERISTICS OF SUBJECTS WITH CYSTIC FIBROSIS AND ACUTE PULMONARY EXACERBATION
| Patients with CF and APE (n = 23) | Outpatients with CF (n = 9) | |
|---|---|---|
| Age, yr | 27.1 ± 6.6 | 25.8 ± 7.0 |
| Females, n (%) | 6 (31.6) | 7 (64.0) |
| FEV1, % predicted | 33.5 ± 13.4 | 60.3 ± 15.1 |
| FVC, % predicted | 45.1 ± 14.1 | 73.9 ± 23.1 |
| Colonized with Pseudomonas aeruginosa, n (%) | 17 (89.5) | 9 (81.8) |
| Genotype (alleles), n (%) | ||
| ΔF508 | 21 (55.3) | 14 (63.6) |
| Unknown/not identified | 13 (34.2) | 1 (4.5) |
| Other | 4 (10.5) | 8 (31.9) |
Definition of abbreviations: APE = acute pulmonary exacerbation; CF = cystic fibrosis.
Values are mean ± SD or n (%).
Figure 1.
High-mobility group box 1 (HMGB1) levels are elevated in sputum from subjects with cystic fibrosis (CF) and inversely correlated with the duration of intravenous antimicrobial therapy. HMGB1 detected by Western blot in spontaneously expectorated sputum from (A) subjects with CF hospitalized for CF exacerbation and (B) outpatients with CF was significantly greater than airway secretions from normal subjects. HMGB1 has a calculated molecular weight of 24.9 kD and migrates as a 29-kD band under sodium dodecyl sulfate–polyacrylamide gel electrophoresis conditions. Albumin loading control is shown for comparison. (C) Summary data of densitometry results showing that HMGB1 levels were significantly elevated in sputa from patients with CF than in secretions from healthy control subjects. All specimens were standardized for total protein concentration and compared by relative densitometry; *P < 0.001, **P < 0.05; n = 5 controls, 23 inpatients with CF, and 9 outpatients with CF, ±SEM. (D) HMGB1 levels in sputum from patients with CF quantified by Western blot densitometry were inversely correlated with the number of days subject was hospitalized for intravenous antimicrobial therapy, reflecting reduced levels of HMGB1 after systemic antibiotic therapy; r = −0.51, P < 0.05.
Elevated HMGB1 in a Model of CF Airway Disease
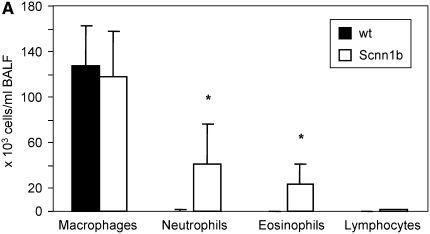
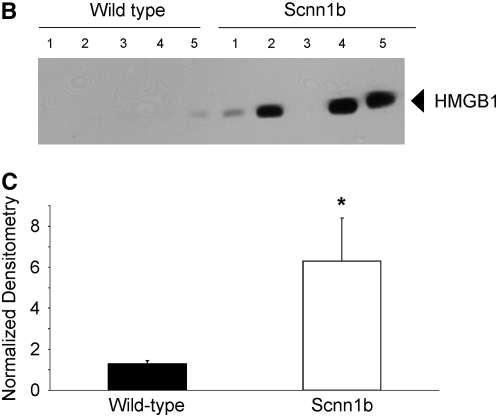
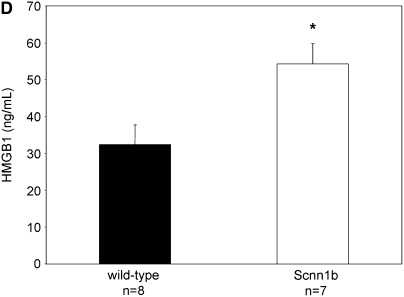
To explore the relevance of HMGB1-mediated inflammation in an in vivo system that would allow mechanistic studies, we investigated a murine model of chronic bronchitis or CF lung disease. Because ΔF508 and CFTR knockout mice exhibit predominantly gastrointestinal pathology and minimal airway disease (32), we used a mouse model in which airway-targeted overexpression of the β subunit of the epithelial Na+ channel causes hyperabsorption of Na+, dehydration of the airway surface liquid, impaired mucociliary clearance, and an airway obstructive phenotype closely resembling the human CF phenotype, except with no evidence of bacterial colonization with Pseudomonas aeruginosa (21). As assayed by BAL, Scnn1b-Tg mice had 33% higher total leukocyte count, and greater neutrophil and eosinophil counts than wild-type (WT) littermates (Figure 2A). HMGB1 levels were significantly elevated in BALF of Scnn1b-Tg mice, as assayed by Western blot (Figures 2B and 2C). Use of an ELISA protocol designed for detection of HMGB1 in serum and plasma confirmed significantly elevated levels of HMGB1 in BALF obtained from Scnn1b-Tg mice, and indicated that HMGB1 was present at concentrations anticipated to have proinflammatory activity (Figure 2D; 61.8 and 40.6 ng/ml in Scnn1b-Tg and WT littermates, respectively).
Figure 2.
High-mobility group box 1 (HMGB1) levels are elevated in the lungs of Scnn1b-transgenic (Scnn1b-Tg) mice. (A) Neutrophils and eosinophils are significantly increased in bronchoalveolar lavage fluid (BALF) samples obtained from Scnn1b-Tg mice compared with wild-type littermate controls. BALF samples were procured in a paired fashion at 5 weeks of life; P < 0.005, n = 15 per genotype. (B) HMGB1 was detected after loading 30 μl BALF aliquots and analyzed by Western blot using sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot with monoclonal antibody directed against HMGB1. HMGB1 has a calculated molecular weight of 24.9 kD and migrates as a 29-kD band under SDS-PAGE conditions. (C) Summary of data representing normalized densitometry of wild-type versus Scnn1b-Tg mice; *P < 0.05 by densitometry analysis, n = 10 per genotype. (D) HMGB1 was detected by ELISA; *P < 0.05, n = 8 wild-type and 7 Scnn1b-Tg mice. wt = wild type.
Chemotactic Activity of HMGB1 In Vitro
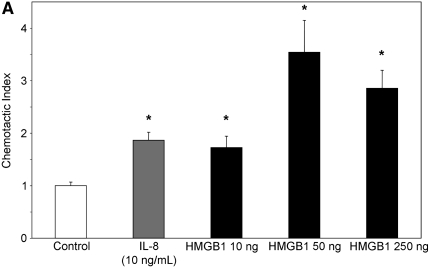
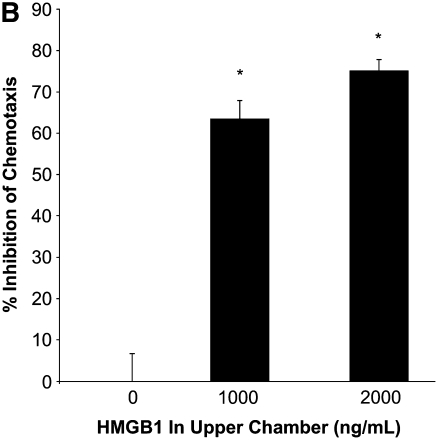
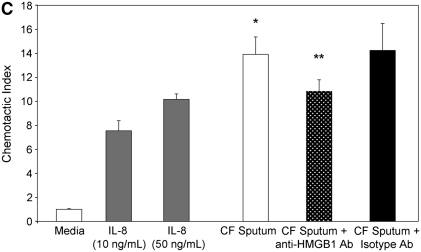
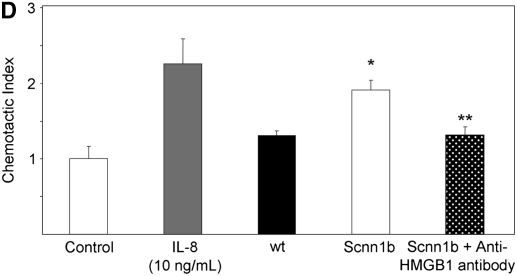
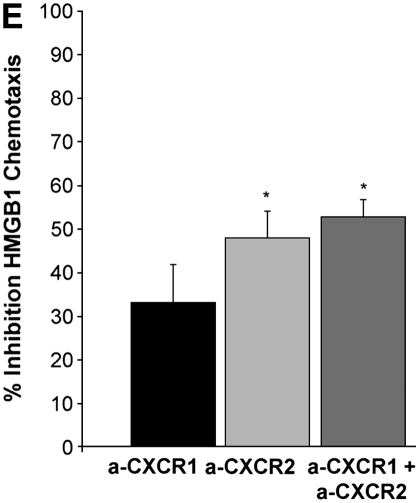
To evaluate whether the levels of HMGB1 detected in subjects with CF and Scnn1b-Tg mice contributed to neutrophil influx into the airways, we evaluated the chemotactic activity of recombinant HMGB1 for human neutrophils in vitro. Recombinant purified HMGB1 induced dose-dependent chemotaxis (as opposed to chemokinesis) of isolated human neutrophils (Figures 3A and 3B), but had no effect on chemotaxis of purified murine neutrophils (data not shown) despite significant homology between human and murine protein sequence (>95% homology between human and mouse HMGB1). Sputum from subjects with CF and APE was highly chemotactic for human neutrophils (14-fold more than control subjects) and was partially inhibited by preincubation with polyclonal anti-HMGB1 neutralizing antibody (Figure 3C). Cell-free BALF from Scnn1b-Tg mice also exhibited increased chemotactic activity for human neutrophils (although less potently than CF sputum) compared with WT BALF (Figure 3D). Preincubation of BALF from Scnn1b-Tg mice with anti-HMGB1 monoclonal antibody reduced neutrophil chemotaxis to levels seen with WT BALF. To more precisely define the mechanistic basis of HMGB1-induced neutrophil chemotaxis seen in human and murine biologic samples, we next tested chemotaxis induced by purified HMGB1 after blockade of CXCR1 and CXCR2 receptors. Human neutrophil chemotaxis for purified HMGB1 was significantly reduced after blockade of CXCR2 (but not CXCR1) receptors by neutralizing antibodies (Figure 3E), indicating the importance of this pathway in human samples. Greater chemotaxis seen by CF sputum compared with murine BALF may have been due in part to dilution, as total protein concentration in sputum were 2.99 ± 0.38 μg/ml compared with 0.73 ± 0.05 μg/ml and 0.63 ± 0.03 μg/ml in Scnn1b-Tg and WT mice, respectively.
Figure 3.
High-mobility group box 1 (HMGB1) is a potent chemoattractant for isolated human neutrophils in vitro, contributes to polymorphonuclear leukocyte chemotaxis elicited by sputum from subjects with cystic fibrosis (CF) and from bronchoalveolar lavage fluid (BALF) of Scnn1b-transgenic (Scnn1b-Tg) mice, and can be partially inhibited by neutralizing antibody. (A) Chemotaxis of various concentrations of recombinant human HMGB1 was compared with medium (negative control) and IL-8 (positive control). Migration was measured by quantitative photomicrograph after 1 hour incubation and standardized to cell count found in medium control; *P < 0.005 versus medium control, n = 5, ±SEM. (B) HMGB1 is chemotactic rather than chemokinetic, as evidenced by inhibition of chemotaxis toward HMGB1 in the lower chamber (2,000 ng/ml) by placement of HMGB1 in the upper chamber; *P < 0.05 versus control, n = 3 per condition, ±SEM. (C) CF sputum was evaluated alone, with anti-HMGB1 neutralizing antibody or isotype control antibody. Medium and IL-8 positive control are provided for comparison; *P < 0.001 versus medium; **P < 0.01 versus CF sputum; n = 6, ±SEM. (D) BALF from Scnn1b-Tg mice was compared with wild-type littermate controls with and without the presence of anti-HMGB1 neutralizing antibody; *P < 0.01 versus control and **P < 0.05 versus Scnn1b-Tg mice, n = 5, ±SEM. (E) HMGB1 induces human neutrophil chemotaxis by a CXCR2-dependent mechanism, as evidenced by blockade of HMGB1 (2,000 ng/ml)-induced chemotaxis after preincubation of isolated human neutrophils with anti-CXCR2 antibody. Neutralization with both anti-CXCR1 and anti-CXCR2 antibodies also inhibited chemotaxis. Anti-CXCR antibodies (25 μg/ml) were preincubated with neutrophils after isolation for 45 minutes at 4°C, as described in Methods; *P < 0.05, n = 3 per condition, ±SEM. wt = wild type.
Collagen Fragmentation Related to In Vivo HMGB1 Activity
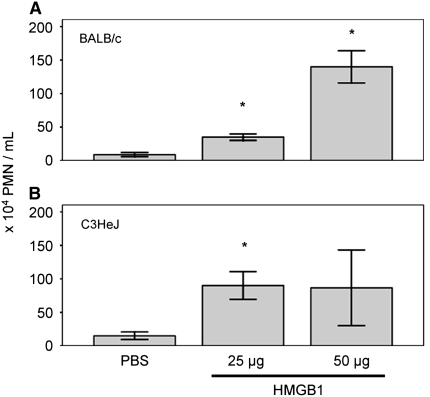
To determine whether purified HMGB1 sufficient to elicit an inflammatory response in the lung was associated with downstream effects relevant to CF pulmonary disease, we intratracheally instilled purified (prokaryotic derived) HMGB1 and evaluated the inflammatory PMN infiltrate by BAL. At 24 hours after instillation, HMGB1 induced dose-dependent influx of PMNs into the airways (Figure 4). As previously described, this finding was similar in both BALB/c (Figure 4A) and endotoxin (LPS)-resistant C3H/HeJ mice (Figure 4B), confirming that results were independent of possible LPS contamination (2). Macrophage or lymphocyte influx was not observed in either mouse strain.
Figure 4.
Intratracheal injection of recombinant human high-mobility group box 1 (HMGB1) induces dose-dependent influx of neutrophils in bronchoalveolar lavage fluid. HMGB1 neutrophil chemotactic activity was observed in (A) wild-type BALB/c mice or (B) endotoxin-resistant C3H/HeJ mice. Mice were killed 24 hours after HMGB1 instillation; *P < 0.05, n = 3–6 per condition, ±SEM. PMN = polymorphonuclear leukocytes.
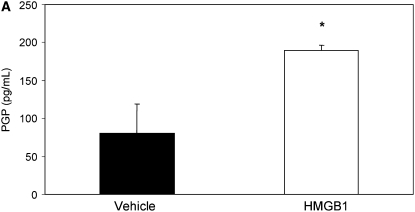
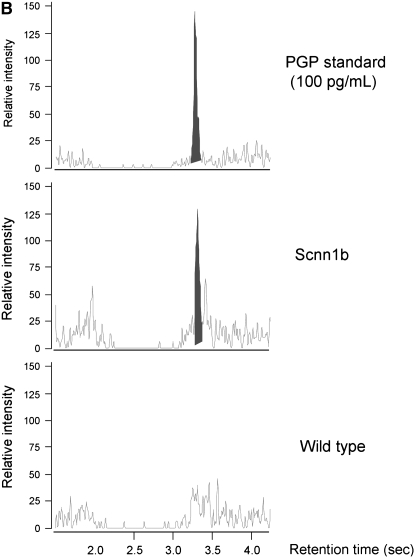
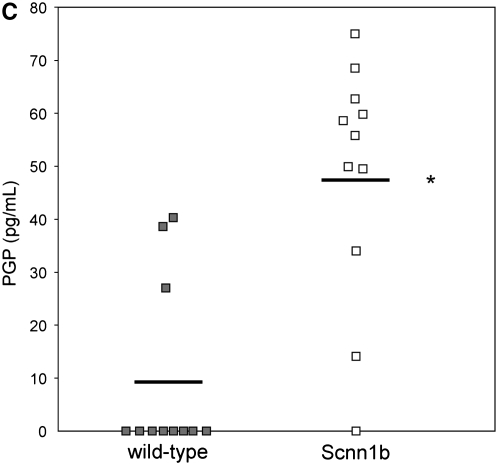
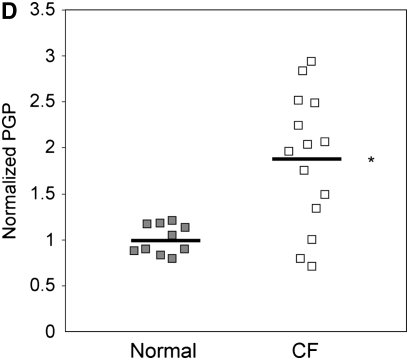
Because the influx of neutrophils and induction of inflammation by HMGB1 might be expected to induce extracellular matrix turnover by activation of NF-κB (signaling) (33) and the release of matrix metalloproteinases 8 and 9 into the airway (25, 34), thus contributing to chronic bronchitis and structural damage seen in CF, we evaluated BALF of C3H/HeJ mice administered purified HMGB1 for the collagen breakdown product PGP. PGP is a tripeptide collagen fragment that results from lung matrix degradation and has been shown to be an IL-8 cogener that induces neutrophil chemotaxis through a CXCR-dependent mechanism (22). PGP levels detected using electron ionization–liquid chromatography tandem mass spectrometry were 2.3-fold greater in C3H/HeJ mice administered 25 μg HMGB1 compared with animals administered PBS (Figure 5A, 189.7 vs. 80.7 pg/ml in C3H/HeJ). PGP levels were also significantly elevated in BALF samples from Scnn1b-Tg mice, whereas in WT littermates, PGP levels were much lower and often below the limit of detection (Figures 5B and 5C; 48.0 vs. 9.6 pg/ml in Scnn1b-Tg mice and WT controls, respectively). Using a protocol adapted to measure PGP in human serum, we also found elevated levels of PGP in subjects with CF and APE (1,076 pg/ml) compared with non-CF control subjects (563 pg/ml) (Figure 5D).
Figure 5.
Intratracheal instillation of high-mobility group box 1 (HMGB1) triggers matrix collagen degradation and release of proline-glycine-proline (PGP) peptide, which is also found to be elevated in Scnn1b-transgenic (Scnn1b-Tg) mice bronchoalveolar lavage fluid (BALF) and in the serum of patients with cystic fibrosis (CF). (A) Instillation of HMGB1 at dosage sufficient to induce airway polymorphonuclear leukocyte influx also induces production of the collagen fragment PGP. Recombinant human HMGB1 (25 μg) was instilled in C3H/HeJ mice, which were then killed 24 hours later for BALF; *P < 0.05, n = 3 per condition, ±SEM. (B) Using electrospray ionization–liquid chromatography followed by tandem mass spectrometry, PGP was detected as a peak with retention time at 3.1 seconds and molecular weight splits of 270/70 and 270/116 (270/70 split shown in top panel; bold peak represents PGP standard [100 pg/ml]). The same peak was observed in BALF of Scnn1b-Tg mice (middle panel, representative section) but not in the BALF from wild-type littermate controls (bottom panel). (C) PGP was detected by electrospray ionization–liquid chromatography followed by tandem mass spectrometry and quantified by the relative standard curve method. BALF samples were obtained in a paired fashion from 5-week-old, sex-matched mice; *P < 0.005, n = 11 per genotype. (D) Serum samples obtained from normal nonsmoking individuals and subjects with CF hospitalized for acute pulmonary exacerbation were analyzed for PGP. Results were normalized to the expression seen in normal subjects; *P < 0.02, n = 10 normal subjects, 14 subjects with CF, ±SEM.
DISCUSSION
We show that the acute inflammatory mediator HMGB1 is significantly elevated in the sputum of humans with CF, a disease dominated by chronic neutrophilic inflammation of the airways, and in a murine model of the disease. At concentrations equivalent to those observed in airway secretions, HMGB1 induced neutrophil chemotactic activity through a CXCR-dependent pathway, and a neutralizing antibody against HMGB1 caused significant reduction in chemotaxis induced by human CF sputum and BALF from Scnn1b-Tg mice. Intratracheal instillation of HMGB1 in mice induced significant airway neutrophilia and was accompanied by lung matrix degradation, as evidenced by the production of PGP, a bioactive collagen degradation product also shown to be elevated in Scnn1b-Tg mice BALF and human CF serum. Elevated PGP was previously reported in CF sputum (35). Although quantification of HMGB1 in serum and airway secretions has been reported to be difficult due to its potential to bind albumin and other extracellular proteins (36), we were able to quantify HMGB1 in abundance in biologic samples using both immunoblotting (compared with protein standard) and ELISA methods.
Previous reports of HMGB1 activity have focused on its importance as a mediator of acute inflammation of the lung, including its role in sepsis, hemorrhagic shock, ARDS, and ventilator-associated injury (1–3, 9, 14). Our study is the first to suggest that HMGB1 also has a significant role in neutrophilic inflammation in the CF lung and complements findings reported by Kokkola and colleagues (16, 37), which showed a substantial presence of HMGB1 in the synovial fluid of a rheumatoid arthritis rat model, a disease also mediated by long-standing neutrophilic inflammation. Notably, we demonstrate that HMGB1 acts as a direct neutrophil chemoattractant, and our research supports observations reported by Orlova and colleagues (38) who showed that HMGB1 induced neutrophil chemotaxis dependent on Mac-1 (CD116/CD18) and RAGE through activation of the NF-κB pathway. Our findings extend these results and establish that neutrophil chemotaxis is also induced directly by HMGB1 in a mechanism dependent on CXCR2. Given that mouse neutrophils, like human PMNs, expressed CXCR2, but did not exhibit chemotaxis in response to purified HMGB1 (Figure 3E), suggests that HMGB1 likely contributes to inflammation in Scnn1b-Tg mice predominantly through indirect mechanisms, such as the release of TNF-α and KC (through activation of inflammation by Toll-like receptor [TLR]-2, TLR-4, and RAGE [2]) or by augmenting other proinflammatory cytokines, as suggested by Sha and colleagues (39). In contrast, in humans, HMGB1 exhibits both direct (CXCR2-dependent) and indirect proinflammatory effects.
Our study is the first report of inhibition of HMGB1-mediated chemotaxis by antibody inhibition in biologic specimens that also supports the potential for in vivo blockade of chemoattractant activity. HMGB1-dependent chemotactic activity shown in these studies suggests that exaggerated levels, possibly promoted by poor mucociliary clearance of necrotic cells (40), have deleterious effects in the CF airway that may be self-sustaining. Direct activation of PMNs by HMGB1 would be anticipated to further contribute to airway destruction and progression of bronchiectasis. These findings may have implications in other proinflammatory airway diseases where neutrophils are prominent, including chronic obstructive pulmonary disease (41) or ischemic/reperfusion injury of the transplanted lung (42).
Beyond the intrinsic chemotactic properties of HMGB1, these data show that modest levels of HMGB1 in the lung can lead to collagen matrix degradation and production of bioactive proinflammatory mediators, thus linking influx and activation of PMNs by HMGB1 using a readily obtained, noninvasive measure of lung injury. The finding that HMGB1 is also detected in the Scnn1b-Tg model of chronic bronchitis, at levels anticipated to activate neutrophils (33) and sufficient to induce PMN chemotaxis, provides a mechanism by which HMGB1 could contribute to neutrophil migration and activation, lung matrix degradation, and liberation of PGP. We speculate that neutrophil influx by HMGB1 through CXCR2 and activation mediated through the NF-κB pathway may contribute to the release of bioactive enzymes, including neutrophil elastase and matrix metalloproteinase 8 and 9, each previously reported to be present in PMNs highly active in the CF lung (25, 43). Collagen degradation induced by activation of innate immunity in the inflamed airway results in liberation of PGP, a collagen fragment reported by our laboratory to have proinflammatory effects through both CXCR1 and CXCR2 receptors present in neutrophils (22). Combined with HMGB1, release of this peptide fragment provides a positive feedback mechanism that contributes to persistent neutrophilic inflammation in the CF lung.
Estimates of HMGB1 levels in human sputum were inversely correlated with the duration of intravenous antibiotics in patients with CF and APE (Figure 1D). Previous studies indicate that appropriate antimicrobial therapy is associated with a reduction in markers of inflammation in sputum and serum (particularly IL-8, IL-6, and total neutrophil counts), although not all studies have been consistent (44–46). The inverse correlation with antimicrobial therapy in our study further indicates the importance of HMGB1 during periods of disease exacerbation, and suggests that HMGB1 may serve as a biomarker for the activation of innate immunity. Moreover, HMGB1 was intermediately elevated in outpatients with CF, in whom neutrophilic inflammation is also evident (but to a lesser extent), and was inversely correlated with lung function when both outpatients and inpatients with CF were considered, which supports the notion that extracellular HMGB1 levels are correlated with overall disease activity and are particularly sensitive to the presence of acute lung inflammation. The potential indicators of CF APE are an area of significant interest because they may be useful biomarkers for novel CF therapeutics and assist in the clinical management of subjects with CF (47, 48). The potential to combine the detection of upstream mediators, such as HMGB1, IL-8, and other proteases indicative of acute inflammation, with downstream events, such as the liberation of PGP, might allow the monitoring of both early and late pathways underlying lung inflammation and improve the characterization of CF APE beyond typical clinical criteria. The potential of HMGB1 to serve as a disease biomarker deserves further attention (such as evaluation of paired samples before and after therapeutic intervention) to confirm our initial observations and assess response to systemic antimicrobial therapy.
Inhibitors of HMGB1 are currently being developed for application in sepsis and other inflammatory diseases. In CF, an intervention that blocks the dysregulated activation of the innate immune system could potentially ameliorate excessive chronic neutrophilic inflammation that contributes to disease progression (18, 20, 49). Elevated HMGB1 levels in the Scnn1b-Tg mouse model, without acquisition of P. aeruginosa, suggests its importance in chronic inflammation that is due to mucus stasis, possibly precipitated by the presence of necrotic cellular debris detectible in the airways of these mice (21). Inhibition of inflammatory mediators that contribute to the CF inflammatory phenotype before the onset of bacterial colonization may be a more suitable therapeutic target than conventional cytokines and other mediators that become elevated primarily after the onset of chronic infection, when immune suppression could carry additional risks (50). If inhibition of this pathway could reduce infection-independent inflammation, inhibitors of HMGB1 may have a more favorable toxicity profile, thus avoiding the potential worsening of infection associated with potent inhibition of proinflammatory (and antiinfective) pathways, as reported with LTB4 antagonists (51, 52).
Although P. aeruginosa infection is not required, as evidenced by chemotaxis in both CF sputum and murine BALF sample (Figure 3), these data do not rule out a modulatory role for bacterial infection on the activity of HMGB1-mediated chemotaxis. Additional studies are needed that examine the role of P. aeruginosa infection and resultant HMGB1 release, to better define this pathway in CF inflammation. Recent reports by Tian and colleagues (53) and Rouhiainen and colleagues (54) indicate that immune activation and cytokine release induced by HMGB1 is significantly enhanced after tight binding to bacterial DNA, prokaryotic lipids, and other bacterial substances that are present in high quantities in infected and colonized CF sputum (55, 56). Not surprisingly, whereas blocking HMGB1 signaling caused a significant reduction in chemotaxis, our in vitro observations indicate that this is not the only pathway relevant to neutrophil influx (∼75% of neutrophil influx remained unabated) and may indicate the importance of other inflammatory mediators such as IL-8 or PGP in these specimens.
In summary, our study shows that HMGB1 is elevated in subjects with CF and in an animal model of the disease, that it is directly chemotactic for neutrophils through a CXCR-dependent mechanism, and that HMGB1 activity can be blocked by antibody binding in vitro. HMGB1 also induces collagen matrix degradation, resulting in a positive feedback loop that is expected to perpetuate and propagate neutrophilic inflammation through liberation of the proinflammatory polypeptide PGP. HMGB1 appears to be an important inflammatory modulator in airway secretions of subjects with CF and a murine model of CF inflammation. Studies using neutralizing anti-HMGB1 antibodies or other antagonists are warranted to confirm its pathogenic role in sterile airway inflammation in Scnn1b-Tg mice. If confirmed, neutralization of HMGB1 and/or PGP may represent potential therapeutic targets to treat the persistent chronic inflammation that is prominent in CF.
Acknowledgments
The authors thank Ms. Meenakshi Sthanam and Mr. Albert Tousson for their technical assistance with experiments. The authors also thank Drs. R. Boucher and S. Randell for providing infrastructure support for maintenance of the Scnn1b-Tg mouse colony.
Supported by National Institutes of Health grants 1K23DK075788-01 (S.M.R.), 1P30DK072482-01A1 (E.J.S.), R01HL077783 (J.E.B.), R01HL090999 (J.E.B.), 1P01HL068743 (E.A.), P50GM049222 (E.A.), P30CA13148 (University of Alabama at Birmingham Comprehensive Cancer Center Core Support Grant); and Cystic Fibrosis Foundation grants R464-CR02 (J.E.B. and E.J.S.), GAGGAR07A0 (A.G.), LIVRAG04I0 (A.L.), and ONEAL07GO (W.O.). This project was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases and the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases; National Heart, Lung, and Blood Institute; or the National Institutes of Health.
Originally Published in Press as DOI: 10.1164/rccm.200712-1894OC on July 24, 2008
Conflict of Interest Statement: S.M.R. received $147,000 for a research grant with Novartis. P.L.J. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. M.H. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.L. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. G.M.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. D.B.M. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. B.D.N. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. A.G. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.P.C. has received honorarium for grant reviews from the Cystic Fibrosis Foundation, and reimbursement from the National Institute of Health for serving on the Rare Disease Network Data Safety Monitoring Board. W.O. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.J.S. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. E.A. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript. J.E.B. does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Wang H, Bloom O, Zhang M, Vishnubhakat JM, Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et al. HMG-1 as a late mediator of endotoxin lethality in mice. Science 1999;285:248–251. [DOI] [PubMed] [Google Scholar]
- 2.Abraham E, Arcaroli J, Carmody A, Wang H, Tracey KJ. HMG-1 as a mediator of acute lung inflammation. J Immunol 2000;165:2950–2954. [DOI] [PubMed] [Google Scholar]
- 3.Kim JY, Park JS, Strassheim D, Douglas I, Diaz del Valle F, Asehnoune K, Mitra S, Kwak SH, Yamada S, Maruyama I, et al. HMGB1 contributes to the development of acute lung injury after hemorrhage. Am J Physiol Lung Cell Mol Physiol 2005;288:L958–L965. [DOI] [PubMed] [Google Scholar]
- 4.Gardella S, Andrei C, Ferrera D, Lotti LV, Torrisi MR, Bianchi ME, Rubartelli A. The nuclear protein HMGB1 is secreted by monocytes via a non-classical, vesicle-mediated secretory pathway. EMBO Rep 2002;3:995–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, Rubartelli A, Agresti A, Bianchi ME. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J 2003;22:5551–5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of Toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem 2004;279:7370–7377. [DOI] [PubMed] [Google Scholar]
- 7.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol 2005;5:331–342. [DOI] [PubMed] [Google Scholar]
- 8.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature 2002;418:191–195. [DOI] [PubMed] [Google Scholar]
- 9.Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med 2004;255:320–331. [DOI] [PubMed] [Google Scholar]
- 10.Bell CW, Jiang W, Reich CF III, Pisetsky DS. The extracellular release of HMGB1 during apoptotic cell death. Am J Physiol Cell Physiol 2006;291:C1318–C1325. [DOI] [PubMed] [Google Scholar]
- 11.Sappington PL, Yang R, Yang H, Tracey KJ, Delude RL, Fink MP. HMGB1 B box increases the permeability of Caco-2 enterocytic monolayers and impairs intestinal barrier function in mice. Gastroenterology 2002;123:790–802. [DOI] [PubMed] [Google Scholar]
- 12.Yang R, Harada T, Mollen KP, Prince JM, Levy RM, Englert JA, Gallowitsch-Puerta M, Yang L, Yang H, Tracey KJ, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med 2006;12:105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suda K, Kitagawa Y, Ozawa S, Saikawa Y, Ueda M, Ebina M, Yamada S, Hashimoto S, Fukata S, Abraham E, et al. Anti-high-mobility group box chromosomal protein 1 antibodies improve survival of rats with sepsis. World J Surg 2006;30:1755–1762. [DOI] [PubMed] [Google Scholar]
- 14.Ogawa EN, Ishizaka A, Tasaka S, Koh H, Ueno H, Amaya F, Ebina M, Yamada S, Funakoshi Y, Soejima J, et al. Contribution of high-mobility group box-1 to the development of ventilator-induced lung injury. Am J Respir Crit Care Med 2006;174:400–407. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Ochani M, Li J, Qiang X, Tanovic M, Harris HE, Susarla SM, Ulloa L, Wang H, DiRaimo R, et al. Reversing established sepsis with antagonists of endogenous high-mobility group box 1. Proc Natl Acad Sci USA 2004;101:296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kokkola R, Li J, Sundberg E, Aveberger AC, Palmblad K, Yang H, Tracey KJ, Andersson U, Harris HE. Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum 2003;48:2052–2058. [DOI] [PubMed] [Google Scholar]
- 17.Andersson U, Tracey KJ. HMGB1 as a mediator of necrosis-induced inflammation and a therapeutic target in arthritis. Rheum Dis Clin North Am 2004;30:627–637. [DOI] [PubMed] [Google Scholar]
- 18.Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 2005;352:1992–2001. [DOI] [PubMed] [Google Scholar]
- 19.Ratjen F, Doring G. Cystic fibrosis. Lancet 2003;361:681–689. [DOI] [PubMed] [Google Scholar]
- 20.Sagel SD, Accurso FJ. Monitoring inflammation in CF. Cytokines. Clin Rev Allergy Immunol 2002;23:41–57. [DOI] [PubMed] [Google Scholar]
- 21.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med 2004;10:487–493. [DOI] [PubMed] [Google Scholar]
- 22.Weathington NM, van Houwelingen AH, Noerager BD, Jackson PL, Kraneveld AD, Galin FS, Folkerts G, Nijkamp FP, Blalock JE. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat Med 2006;12:317–323. [DOI] [PubMed] [Google Scholar]
- 23.Jackson PL, Livraghi A, O'Neal WK, Noerager BD, Solomon GM, McQuaid DB, Liu G, Gaggar A, Clancy JP, Sorscher EJ, et al. Novel mediators of persistent neutrophilic inflammation in cystic fibrosis [abstract]. Pediatr Pulmonol Suppl 2007;42:229. [Google Scholar]
- 24.Rowe SM, Solomon GM, Livraghi A, Jackson PL, O'Neal WK, McQuaid DB, Gaggar A, Clancy JP, Sorscher EJ, Abraham EA, et al. Evidence for pathogenic role of HMGB1 in cystic fibrosis [abstract]. Am J Respir Crit Care Med 2007;175:A448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaggar A, Li Y, Weathington N, Winkler M, Kong M, Jackson P, Blalock JE, Clancy J. Matrix Metalloprotease-9 dysregulation in lower airway secretions of cystic fibrosis patients. Am J Physiol Lung Cell Mol Physiol 2007;293:L96–L104. [DOI] [PubMed] [Google Scholar]
- 26.Yum HK, Arcaroli J, Kupfner J, Shenkar R, Penninger JM, Sasaki T, Yang KY, Park JS, Abraham E. Involvement of phosphoinositide 3-kinases in neutrophil activation and the development of acute lung injury. J Immunol 2001;167:6601–6608. [DOI] [PubMed] [Google Scholar]
- 27.Yamada S, Inoue K, Yakabe K, Imaizumi H, Maruyama I. High mobility group protein 1 (HMGB1) quantified by ELISA with a monoclonal antibody that does not cross-react with HMGB2. Clin Chem 2003;49:1535–1537. [DOI] [PubMed] [Google Scholar]
- 28.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol 2004;75:604–611. [DOI] [PubMed] [Google Scholar]
- 29.Miller AP, Feng W, Xing D, Weathington NM, Blalock JE, Chen YF, Oparil S. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation 2004;110:1664–1669. [DOI] [PubMed] [Google Scholar]
- 30.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr 1998;132:589–595. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med 1994;331:637–642. [DOI] [PubMed] [Google Scholar]
- 32.Livraghi A, Randell SH. Cystic fibrosis and other respiratory diseases of impaired mucus clearance. Toxicol Pathol 2007;35:116–129. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Wang H, Czura CJ, Tracey KJ. The cytokine activity of HMGB1. J Leukoc Biol 2005;78:1–8. [DOI] [PubMed] [Google Scholar]
- 34.Verma RP, Hansch C. Matrix metalloproteinases (MMPs): chemical-biological functions and (Q)SARs. Bioorg Med Chem 2007;15:2223–2268. [DOI] [PubMed] [Google Scholar]
- 35.Gaggar A, Jackson PL, Noerager BD, O'Reilly PJ, McQuaid DB, Rowe SM, Clancy JP, Blalock JE. A novel proteolytic cascade generates an extracellular matrix-derived chemoattractant in chronic neutrophilic inflammation. J Immunol 2008;180:5662–5669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Urbonaviciute V, Furnrohr BG, Weber C, Haslbeck M, Wilhelm S, Herrmann M, Voll RE. Factors masking HMGB1 in human serum and plasma. J Leukoc Biol 2007;81:67–74. [DOI] [PubMed] [Google Scholar]
- 37.Kokkola R, Sundberg E, Ulfgren AK, Palmblad K, Li J, Wang H, Ulloa L, Yang H, Yan XJ, Furie R, et al. High mobility group box chromosomal protein 1: a novel proinflammatory mediator in synovitis. Arthritis Rheum 2002;46:2598–2603. [DOI] [PubMed] [Google Scholar]
- 38.Orlova VV, Choi EY, Xie C, Chavakis E, Bierhaus A, Ihanus E, Ballantyne CM, Gahmberg CG, Bianchi ME, Nawroth PP, et al. A novel pathway of HMGB1-mediated inflammatory cell recruitment that requires Mac-1-integrin. EMBO J 2007;26:1129–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sha Y, Zmijewski J, Xu Z, Abraham E. HMGB1 develops enhanced proinflammatory activity by binding to cytokines. J Immunol 2008;180:2531–2537. [DOI] [PubMed] [Google Scholar]
- 40.Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med 2006;354:241–250. [DOI] [PubMed] [Google Scholar]
- 41.Barnes PJ. Chronic obstructive pulmonary disease. N Engl J Med 2000;343:269–280. [DOI] [PubMed] [Google Scholar]
- 42.Belperio JA, Keane MP, Burdick MD, Gomperts BN, Xue YY, Hong K, Mestas J, Zisman D, Ardehali A, Saggar R, et al. CXCR2/CXCR2 ligand biology during lung transplant ischemia-reperfusion injury. J Immunol 2005;175:6931–6939. [DOI] [PubMed] [Google Scholar]
- 43.Sagel SD, Kapsner RK, Osberg I. Induced sputum matrix metalloproteinase-9 correlates with lung function and airway inflammation in children with cystic fibrosis. Pediatr Pulmonol 2005;39:224–232. [DOI] [PubMed] [Google Scholar]
- 44.Ordonez CL, Kartashov AI, Wohl ME. Variability of markers of inflammation and infection in induced sputum in children with cystic fibrosis. J Pediatr 2004;145:689–692. [DOI] [PubMed] [Google Scholar]
- 45.Ordonez CL, Henig NR, Mayer-Hamblett N, Accurso FJ, Burns JL, Chmiel JF, Daines CL, Gibson RL, McNamara S, Retsch-Bogart GZ, et al. Inflammatory and microbiologic markers in induced sputum after intravenous antibiotics in cystic fibrosis. Am J Respir Crit Care Med 2003;168:1471–1475. [DOI] [PubMed] [Google Scholar]
- 46.Henig NR, Tonelli MR, Pier MV, Burns JL, Aitken ML. Sputum induction as a research tool for sampling the airways of subjects with cystic fibrosis. Thorax 2001;56:306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramsey BW. Outcome measures for development of new therapies in cystic fibrosis: are we making progress and what are the next steps? Proc Am Thorac Soc 2007;4:367–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mayer-Hamblett N, Ramsey BW, Kronmal RA. Advancing outcome measures for the new era of drug development in cystic fibrosis. Proc Am Thorac Soc 2007;4:370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koehler DR, Downey GP, Sweezey NB, Tanswell AK, Hu J. Lung inflammation as a therapeutic target in cystic fibrosis. Am J Respir Cell Mol Biol 2004;31:377–381. [DOI] [PubMed] [Google Scholar]
- 50.Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc 2007;4:406–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rao S, Grigg J. New insights into pulmonary inflammation in cystic fibrosis. Arch Dis Child 2006;91:786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Konstan MW, Doring G, Lands LC, Hilliard KA, Koker P, Bhattacharya S, Staab A, Hamilton AL. Results of a phase II clinical trial of BIIL 284 BS (an LTB4 receptor antagonist) for the treatment of CF lung disease. Ped Pulmonol Suppl 2005;28:125–126. [Google Scholar]
- 53.Tian J, Avalos AM, Mao SY, Chen B, Senthil K, Wu H, Parroche P, Drabic S, Golenbock D, Sirois C, et al. Toll-like receptor 9-dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat Immunol 2007;8:487–496. [DOI] [PubMed] [Google Scholar]
- 54.Rouhiainen A, Tumova S, Valmu L, Kalkkinen N, Rauvala H. Pivotal advance: analysis of proinflammatory activity of highly purified eukaryotic recombinant HMGB1 (amphoterin). J Leukoc Biol 2007;81:49–58. [DOI] [PubMed] [Google Scholar]
- 55.Rogers GB, Carroll MP, Serisier DJ, Hockey PM, Kehagia V, Jones GR, Bruce KD. Bacterial activity in cystic fibrosis lung infections. Respir Res 2005;6:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lambiase A, Raia V, Del Pezzo M, Sepe A, Carnovale V, Rossano F. Microbiology of airway disease in a cohort of patients with cystic fibrosis. BMC Infect Dis 2006;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]