Abstract
The mechanosensitive channel of large conductance MscL from Escherichia coli has been reconstituted into sealed vesicles, and the effects of lipid structure on the flux of the fluorescent molecule calcein through the open channel have been studied. The channel was opened by reaction of the G22C mutant of MscL with the reagent [2-(triethylammonium)ethyl]methanethiosulfonate (MTSET) which introduces five positive charges within the pore constriction. Flux through the channel was small when the lipid was phosphatidylcholine, but addition of the anionic lipids phosphatidylglycerol, phosphatidic acid, or cardiolipin up to 50 mol % resulted in increases in the amplitudes and rates of release of calcein. Similar effects were seen when either wild-type MscL or the G22C mutant was opened by osmotic pressure difference; rates of release of calcein were very slow in the absence of anionic lipid but increased with increasing concentrations of phosphatidylglycerol to 50 mol %. The observed partial release of trapped calcein following activation of MscL was attributed to the formation of a long-lived subconductance state of MscL following channel opening. Effects of anionic lipid were attributed to an increase in the rate of the transition from closed to fully open state and to a decrease in the rate of the transition from the fully open state to the subconductance state. Higher concentrations of anionic lipid led to a decrease in the rate and amplitude of release of calcein, possibly due to a decreased rate of flux through the open channel. In mixtures with anionic lipids, phosphatidylethanolamine resulted in lower rates and amplitude of release than phosphatidylcholine.
The bacterial mechanosensitive channel of large conductance MscL,1 reconstituted into simple lipid bilayers, opens when membrane tension is increased, showing that the only interactions important for channel opening are those between the channel and the surrounding lipid molecules (1-3). These interactions are likely to be dependent on the headgroup structure of the lipid molecules, hydrogen-bonding interactions, for example, being important for interactions with zwitterionic lipids such as phosphatidylcholines and phosphatidylethanolamines, with charge interactions being more important for anionic lipids such as phosphatidic acid, phosphatidylglycerol, and cardiolipin (4, 5). Interactions between anionic lipids and a membrane protein might be expected to be particularly strong if the protein contained clusters of positively charged residues, and indeed, MscL from Mycobacterium tuberculosis contains a cluster of three positively charged residues, Arg-98, Lys-99, and Lys-100, that act as a hot spot for binding anionic lipid (6). The functional importance of interactions with lipid headgroups is suggested by the fact that loss of function mutants in MscL are concentrated on either side of the transmembrane domain, in regions that could interact with the lipid headgroups (7). Despite these expectations, Moe and Blount (8) found that the tension required to open the MscL channel was not affected by the addition of anionic lipids. However, channel opening is a complex event involving a number of subconductance states (1, 9), and it is possible that lipid composition could affect properties of the channel other than the tension required for channel opening.
Studies of the effects of lipid structure on MscL function are best performed in reconstituted systems where the lipid composition can be varied at will and where the lipid composition is fully defined. Studies of MscL function in reconstituted membranes using patch clamping techniques are, however, technically difficult and require the determination of the curvature of the membrane patch to allow the calculation of membrane tension from the applied pressure (8). An alternative fluorescence technique for studying function has been introduced by Kocer et al. (10, 11) in which the fluorescent dye calcein is trapped at high concentrations within the lumen of reconstituted vesicles containing MscL; at high concentrations, the intensity of calcein fluorescence is reduced by concentration quenching. Opening of the MscL channel leads to the release of calcein into the external medium with a large increase in fluorescence intensity. In these experiments, Escherichia coli MscL was activated using a mutant in which a Cys residue (G22C) was introduced within the pore constriction (12); labeling with [2-(triethylammonium)ethyl]methanethiosulfonate bromide (MTSET) introduced five positively charged choline groups at the constriction site, forcing the channel into an open state (13-15). This technique has the advantage that no tension needs to be applied to open the channel. Here, we use this approach to show that the rate and level of efflux of calcein through the MscL channel when activated by introduction of charge is affected very markedly by the lipid composition of the membrane.
MATERIALS AND METHODS
Calcein and N-(1-pyrenyl)maleimide were obtained from Sigma, and [2-(trimethylammonium)ethyl]methanethiosulfonate bromide (MTSET) and sodium (2-sulfonatoethyl)methanethiosulfonate (MTSES) were obtained from Toronto Research Chemicals. The detergents octyl β-d-glucoside (OG) and octa(ethylene glycol) n-dodecyl ether (C12E8) were obtained from Anatrace and Calbiochem, respectively. Dioleoylphosphatidylcholine (DOPC), dioleoylphosphatidylethanolamine (DOPE), dioleoylphosphatidic acid (DOPA), dioleoylphosphatidylglycerol (DOPG), tetraoleoylcardiolipin (CL), and azolectin (soybean total lipid extract) were obtained from Avanti Polar Lipids. DOPC and DOPG were brominated to give di(9,10-dibromostearoyl)phosphatidylcholine (BrPC) and di(9,10-dibromostearoyl)phosphatidylglycerol (BrPG), as described in East and Lee (16).
E. coli strain BL21(λDE3)pLysS and plasmid pET-28a were obtained from Novagen. The E. coli mscL gene, EcmscL, with a poly-His epitope at the N-terminus was the generous gift of Professor B. Martinac. The E. coli mscL gene with a poly-His epitope at the C-terminus was generated by cloning the gene into plasmid pET-28a, using restriction sites HindIII and NcoI.
Mutation and Expression
Site-directed mutagenesis was performed using the QuikChange protocol from Stratagene. E. coli BL21(λDE3)pLysS transformants carrying the pET-28a plasmid (Novagen) with the G22C-EcmscL-6×His gene were generally grown in 6 L of Luria broth to midlog phase (OD600 = 0.6) and then induced for 3 h in the presence of isopropyl β-d-thiogalactopyranoside (IPTG; 1 mM). MscL was purified as described by Powl et al. (17) and stored at −80 °C until use. The presence of any endogenous nonmutated MscL is assumed to have no significant effect because of the large excess of the mutated channel produced following induction.
Reconstitution into Sealed Vesicles
Lipid (6.7 μmol) was dried down from solution in chloroform onto the walls of a thin glass vial. Buffer (1 mL; 20 mM Hepes, 100 mM KCl, 40 mM OG, 50 mM calcein, pH 7.2) was added. The sample was sonicated to optical clarity in a bath sonicator. MscL (67 nmol of monomer) was then added to the sonicated suspension to give a 500:1 molar ratio of lipid:MscL pentamer, unless otherwise stated. Detergent was removed by the addition of 200 mg of washed SM2 Bio-Beads (mesh size 20–50; Bio-Rad) followed after 1 h by a second addition of 200 mg of Bio-Beads. After a further hour the sample of reconstituted vesicles was removed from the Bio-Beads. Unencapsulated dye was then removed by passage through two G-50 Sephadex columns (100–300 μm), washed and eluted in buffer (20 mM Hepes, 100 mM KCl, 0.5 M sucrose, pH 7.2); the eluted sample was kept on ice until use. The same procedure was used for reconstituting vesicles in the absence of calcein, except that the column step was not required.
Vesicle diameters were determined using a Coulter N4 Plus particle sizer that measures particle size by light scattering.
Calcein Efflux Assay
A 50 μL aliquot of the reconstituted vesicles was diluted into 2.95 mL of buffer (20 mM Hepes, 100 mM KCl, 0.5 M sucrose, pH 7.2) in a stirred fluorescence cuvette at 25 °C, unless otherwise stated. A baseline was recorded for 50 s, followed by the addition of 1 mM MTSET from a freshly prepared stock solution of 300 mM MTSET in buffer (20 mM Hepes, 100 mM KCl, pH 7.2), after which the fluorescence was monitored for up to 30 min. Vesicles were finally burst by the addition of 200 μM C12E8 to determine the fluorescence intensity when all of the trapped calcein had been released. Fluorescence was recorded at 520 nm on an SLM 8100C fluorometer (Urbana, IL) with excitation at 490 nm. Traces from single experiments are shown in the following figures, but results are reproducible between samples.
Extent of Labeling by MTSET
Samples of vesicles reconstituted as above were reacted with MTSET for about 1 min and then diluted and rapidly frozen. The samples were then freeze-dried and extracted with a chloroform/methanol mixture to remove most of the lipid. Samples were analyzed by nanoelectrospray mass spectrometry (LCT orthogonal acceleration-TOF instrument; Micromass) and confirmed a 1:1 molar ratio of labeling of MscL by MTSET under these conditions.
Fluorescence Quenching by Brominated Phospholipids
A mutant of E. coli MscL containing a Trp residue at position 93 (F93W) was reconstituted into lipid bilayers by the dilution method by mixing lipid and MscL in cholate at a 100:1 molar ratio of lipid to MscL monomer, followed by dilution into buffer to decrease the concentration of cholate below its critical micelle concentration, as described (17). F93W was also reconstituted into sealed vesicles using the Bio-Beads protocol described above. Trp fluorescence intensities were measured at 325 nm with excitation at 280 nm, for 0.98 μM MscL in buffer (20 mM Hepes, 200 mM KCl, 1 mM EGTA, pH 7.2) at 25 °C, using an SLM 8100 fluorometer. Intensities were corrected for light scatter by subtraction of a blank consisting of lipid alone in buffer.
Sidedness of Reconstituted MscL
The sidedness of MscL in the reconstituted vesicles was determined by measuring the rate of labeling of Cys-containing mutants of MscL by N-(1-pyrenyl)maleimide, making use of the fact that N-(1-pyrenyl)maleimide is nonfluorescent before reaction with a Cys residue. M. tuberculosis MscL mutants L69C and Y94C containing Cys residues on the periplasmic and cytoplasmic sides of the protein, respectively, were used in these experiments. L69C and Y94C were reconstituted into sealed vesicles as described above, and 50 μL aliquots of the reconstituted vesicles were diluted into 2.95 mL of buffer (20 mM Hepes, 100 mM KCl, pH 7.2) at 25 °C. A 5-fold molar excess of N-(1-pyrenyl)maleimide was then added (7 μM), and the fluorescence intensity was recorded at 392 nm with excitation at 343 nm. After a steady signal had been achieved, 200 μMC12E8 was added to burst the vesicles and allow the N-(1-pyrenyl)maleimide to label those Cys residues previously sequestered in the internal space of the vesicles. Labeling of the Cys residues was complete as shown by the observation that addition of further N-(1-pyrenyl)maleimide had no affect.
Data Analysis
Vesicles reconstituted as described above have diameters of ca. 100 nm. The mass of lipid in one vesicle is given by 4πρ[(ri + δ)3 − ri3]/3, where ρ is the density of the lipid, assumed to be 1 g/mL, ri is the internal radius of the vesicle, and δ is the thickness of the bilayer, taken to be 4 nm. A vesicle of external diameter 100 nm will then contain ca. 9.3 × 104 lipid molecules and at a lipid: MscL channel molar ratio of 500:1, ca. 190 channels. The internal volume of such a vesicle will be ca. 4.1 × 10−19 L, and if the calcein concentration within the vesicles is 50 mM, the vesicle will contain 1.2 × 104 molecules of calcein.
Calcein fluorescence data were fitted to single exponentials using the nonlinear least-squares routine in the SigmaPlot package (SPSS, Chicago, IL). Simulations of calcein release were performed using the program FACSIMILE (UKEA).
RESULTS
Details of the Method of Reconstitution
As discussed by van den Bogaart et al. (12), electrophysiological recordings from reconstituted MscL generally show much lower numbers of active channels than expected from the amount of protein used in the reconstitution. One possible explanation for such observations could be that the reconstitutions were of low efficiency, much of the MscL not incorporating into the reconstituted membranes. In these electrophysiological studies, the method of reconstitution was generally to add MscL in the presence or absence of detergent to preformed lipid vesicles (8, 12, 18). An alternative approach, more likely to result in complete mixing of lipid and protein, is to mix the lipid and protein while both are in suspension in detergent and then to remove the detergent to re-form membranes. One method for removing detergent is to dilute the detergent mixture into buffer, dropping the concentration of detergent below its critical micelle concentration, leading to membrane re-formation. This approach has been shown to result in highly efficient reconstitution of a wide variety of membrane proteins (19-22), including MscL (17, 23). The simple dilution approach is, however, not applicable here, since the membranes produced by dilution are generally not in the form of sealed vesicles. Sealed vesicles can, however, be produced starting from lipid and protein mixed in detergent, using absorbent Bio-Beads to slowly remove detergent (24, 25). This is the procedure used here.
The efficiency of reconstitution of MscL into the vesicles was determined using a fluorescence quenching assay. A short distance between a Trp residue and a bromine atom results in quenching of the fluorescence of the Trp residue, the level of quenching depending on the distance between the Trp residue and the bromine atoms (17). Reconstitution of a mutant MscL containing a Trp residue in the transmembrane domain, into bilayers of phospholipids containing brominated fatty acyl chains, by the dilution method resulted in quenching of the fluorescence of the Trp residue, the data being consistent with a very high efficiency of reconstitution of MscL into the membranes (17, 26). As shown in Table 1, reconstitution of MscL containing a Trp residue at position 93 into bilayers of BrPC or BrPG by the Bio-Beads method results in levels of fluorescence quenching comparable to those observed on reconstituting by the dilution method. We conclude that the efficiency of reconstitution of MscL is high under the conditions used here. This is also consistent with the results of the experiments labeling Cys mutants of MscL with pyrene maleimide as described below. We note that studies of the open and closed structures of MscL in sealed vesicles using EPR spectroscopic methods by Perozo et al. (27) also rely on the efficiency of reconstitution being high.
Table 1. Fluorescence Quenching and Method of Reconstitutiona.
| fluorescence quenching, F/F0 |
||
|---|---|---|
| brominated lipid | dilution method | Bio-Beads method |
| BrPC | 0.34 ± 0.02 | 0.29 ± 0.02 |
| BrPG | 0.24 ± 0.02 | 0.28 ± 0.02 |
The F93W mutant of E. coli MscL was reconstituted into lipid bilayers either by dilution or using Bio-Beads as described in the Materials and Methods section. F0 and F are fluorescence intensities in nonbrominated and brominated lipid, respectively.
Details of the Calcein-Release Assay
E. coli MscL containing a C-terminal 6-His tag and a G22C mutation was reconstituted into sealed vesicles containing 50 mM calcein by mixing MscL and lipid in detergent solution containing calcein, followed by removal of detergent with Bio-Beads; calcein not trapped within the vesicles was removed on a Sephadex column. A very low fluorescence intensity was recorded when calcein-containing lipid vesicles composed of a 1:1 molar ratio of DOPG:DOPC, in the absence of MscL, were diluted into buffer (Figure 1A, trace a). However, a large increase in fluorescence intensity was seen when the vesicles were made leaky by the addition of 200 μM C12E8, due to the decrease in the concentration of calcein as it leaked out of the vesicles into the external medium, where it was no longer subject to concentration quenching of fluorescence. Addition of lipid vesicles or detergent to a solution of calcein in buffer had no observable effect on fluorescence intensity.
Figure 1.
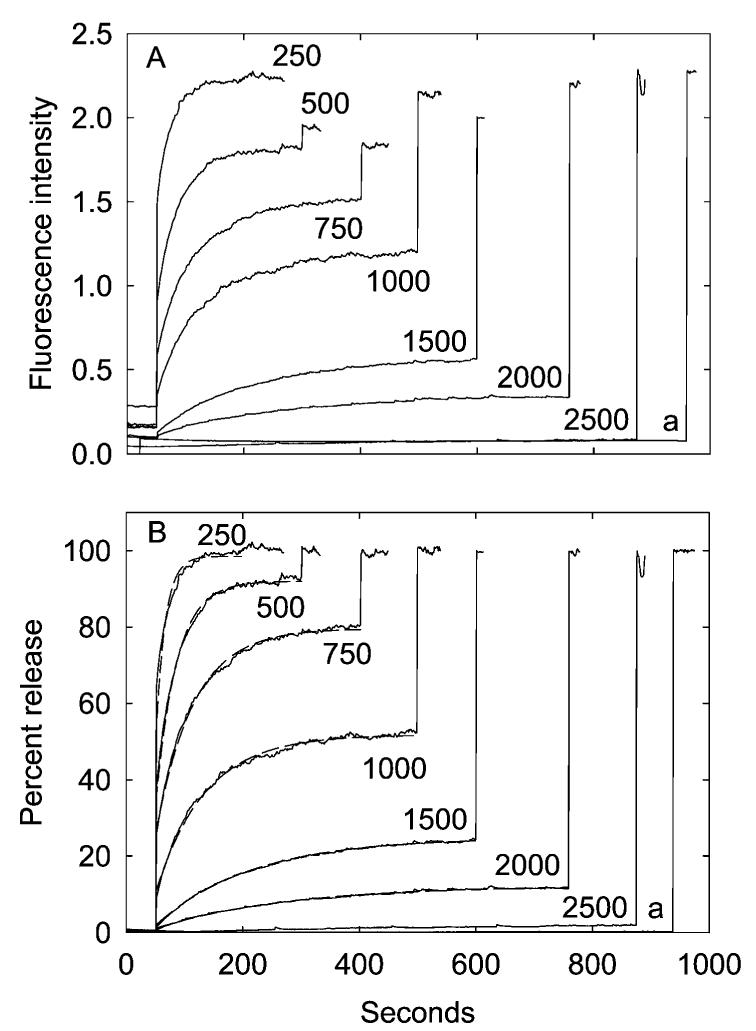
Effect of channel concentration on the efflux of calcein from reconstituted vesicles. The G22C mutant of E. coli MscL was reconstituted into calcein-containing vesicles containing a 1:1 molar ratio of DOPG:DOPC at the given molar ratios of total lipid to MscL. Samples were diluted into buffer, and a fluorescence baseline was recorded for 50 s, followed by the addition of 1 mM MTSET. Finally, 200 μMC12E8 was added to burst the vesicles and establish a value for the fluorescence intensity when all of the trapped calcein had been released. The amount of lipid was kept constant at 6.7 μmol. The trace labeled a shows an experiment for vesicles containing a 1:1 molar ratio of DOPG:DOPC in the absence of MscL. In (A), results are expressed as changes in absolute fluorescence intensities. In (B), results are expressed as a fraction of the observed change in fluorescence intensity between the initial value and that observed in the presence of 200 μM C12E8. The broken lines in (B) show best fits to a single exponential process.
Similar experiments were performed with reconstituted vesicles containing MscL. It is important in these experiments to minimize any osmotic imbalance across the membranes of the vesicles, since a large osmotic pressure difference across the membrane could lead to opening of the MscL channel and loss of the trapped calcein. The concentration of calcein trapped in the vesicles was 50 mM, but the osmolarity will be much higher than this since calcein contains five ionizable carboxyl groups. As described in more detail below, leak of calcein from the vesicles was prevented by the addition of 0.5 M sucrose to the buffer used on the Sephadex columns and by addition of 0.5 M sucrose to the buffer into which the reconstituted vesicles were diluted for the fluorescence assays. Under these conditions, only a small increase in fluorescence intensity was observed when an aliquot of reconstituted vesicles containing MscL at a molar ratio of lipid:channel of 250:1 was diluted into buffer (Figure 1A). However, addition of 1 mM MTSET to the MscL-containing vesicles led to a marked increase in fluorescence intensity, corresponding to release of essentially all the trapped calcein, since the subsequent addition of 0.2 mM C12E8 resulted in no further increase in fluorescence intensity, and the final fluorescence intensity was the same as that seen when lipid vesicles in the absence of MscL were made leaky by addition of C12E8 (Figure 1A). Both the extent and rate of the fluorescence change following addition of MTSET decreased with increasing molar ratio of lipid to MscL channel so that at a molar ratio of lipid:MscL channel of 2500:1 no significant change in fluorescence intensity was observed on addition of MTSET, although a large increase in intensity was observed on the subsequent addition of C12E8 to burst the vesicles (Figure 1A).
To account for differences in fluorescence intensity before addition of MTSET and for small differences in the recovery of sealed vesicles from the Sephadex column, it is convenient to express the fluorescence signal following addition of MTSET as a fraction of the total fluorescence change seen following the addition of both MTSET and C12E8 (Figure 1B). Because the total amount of calcein in the sample is constant in these experiments, the observed fractional change in fluorescence is equal to the fractional release of calcein. The observed changes in fluorescence intensity all fit to single exponential processes (Figure 1B). The rate of release of calcein observed at a molar ratio of lipid:MscL channel of 250:1 is too fast to fit accurately in these experiments, but, as shown in Figure 2, the rates and amplitudes of calcein release over the lipid:MscL channel molar ratio from 500:1 to 2500:1 depend on the mole fraction of MscL in the membrane in a close to linear manner.
Figure 2.

Rates (○) and amplitudes (△) of the fluorescence response following reaction with MTSET for MscL reconstituted in vesicles of a 1:1 molar ratio of DOPG:DOPC. The data were obtained by fitting the data in Figure 1 to single exponentials. The line shows a best fit of the rate data (○) to a straight line.
The unexpected result shown in Figure 1B is that vesicles containing a molar ratio of MscL channel:lipid of less than 1:250 show only partial release of the trapped calcein, even though they are calculated to contain a large number of channels. As described below, these reconstituted vesicles have a diameter of ca. 100 nm, and a lipid vesicle of diameter 100 nm will contain, on average, ca. 380 MscL channels at a lipid:channel molar ratio of 250:1 and ca. 40 MscL channels at a lipid:channel molar ratio of 2500:1. One possible explanation for the partial release of calcein observed here (Figure 1) is that the MscL is largely denatured, either during purification or during reconstitution, so that the number of active channels per vesicle is much smaller than expected. As described later, it is likely that the presence of a single open channel in a vesicle would be sufficient to result in complete loss of the trapped calcein within a few minutes, and thus partial release of calcein would imply the presence of some vesicles containing no active channels. This possibility can be put on a more quantitative basis. Since reconstitution starts from a mixed dispersion of lipid and MscL in detergent, the simplest assumption to make is that the distribution of active channels between the vesicles will be random, describable by the Poisson distribution:
| (1) |
where P(n) is the fraction of vesicles containing n active channels and λ is the average number of active channels per vesicle. The number P(0) of vesicles with no active channels is
| (2) |
The data in Figure 1 show that the fraction of calcein released is ca. 50% at a molar ratio of lipid:total MscL channels of 1000:1. If this were to mean that 50% of these vesicles lacked active channels, eq 2 would give an average number of active channels per vesicle of 0.7, compared to an average total number of MscL channels per vesicle of ca. 95, implying that only ca. 0.7% of the MscL present was active. With such a very high proportion of the total MscL being denatured during purification or reconstitution, the amount of active MscL remaining would be expected to be highly variable between preparations and between reconstitutions. This is not the case; the levels of release of calcein seen in the experiments reported here are highly reproducible, as shown by the examples in Table 2.
Table 2. Reproducibility of Levels of Calcein Releasea.
| magnitude of calcein release |
|||
|---|---|---|---|
| lipid | mean (%) | standard deviation | n |
| DOPG:DOPC, 1:1 | 92.2 | ±3.6 | 6 |
| DOPA:DOPC, 1:4 | 32.7 | ±4.6 | 3 |
| DOPA:DOPC, 1:1 | 92.0 | ±5.9 | 4 |
| DOPA | 12.5 | ±2.5 | 3 |
Percentage levels of calcein release after reaction of the G22C mutant of MscL with MTSET were calculated as illustrated in Figure 1B for MscL reconstituted at a molar ratio of lipid:MscL channel of 500:1 for the lipid compositions shown. The table shows the mean values and standard deviations obtained from the given number n of independent reconstitutions.
The argument can be taken further. With 0.7% of the total MscL being functional, as required to fit the data at a molar ratio of lipid:total MscL channels of 1000:1, eq 2 gives a proportion of vesicles containing no active channels of 25% at a molar ratio of lipid:total MscL channels of 500:1, but only about 10% of the calcein is observed experimentally to be retained at this molar ratio of lipid:MscL channels (Figure 1). Further, an explanation for partial release of calcein in terms of a Poissonian distribution of a small number of active channels between vesicles would predict that changing the molar ratio of lipid:total MscL channels from 1000:1 to 500:1 would have only a small effect on the rate of release. This is because the major effect of decreasing the molar ratio of lipid:total MscL channels would be to decrease the number of vesicles containing no active channels, with only a small increase in the number of vesicles containing more than one active channel. The rate of release of calcein from the vesicles containing active channels would increase linearly with the number of active channels in the vesicle. Using eq 1 to calculate the fraction of vesicles containing 1, 2, 3 etc. active channels when only 0.7% of the total MscL is active shows that the rate of release of calcein at a molar ratio of lipid:total MscL channels of 500:1 would be 1.3 times that at a molar ratio of 1000:1. In fact, the observed rate is about double, the rate of release increasing roughly linearly with increasing mole fraction of MscL in the membrane (Figure 2). Similar problems arise in trying to fit the data at other molar ratios of lipid:MscL channels. Thus the results shown in Figure 1 do not fit well with the suggestion that a very large proportion of the MscL present in the reconstituted membranes is present in a denatured form. We note that the studies of the open and closed structures of MscL in sealed vesicles using EPR spectroscopic methods by Perozo et al. (27) imply that the MscL is present largely in a native, nondenatured form.
If, therefore, the efficiency of reconstitution of MscL is high and if the MscL is present largely in a native, nondenatured form, the partial release of calcein seen in these experiments following addition of MTSET would imply that, after an initial period of activity following reaction with MTSET, the MscL channels transform into a form unable to allow the flux of calcein out of the vesicles. Although the possibility of inactivation of MscL has been little discussed in the literature, Hase et al. (18) have shown that, in patches of reconstituted MscL where the number of active channels is low, MscL activity occurs in bursts, followed by long inactive periods and that, following this inactivation, channels cannot be recovered either by changing voltage or by application of pressure.
Effect of Anionic Lipid on the Rate and Amplitude of Calcein Release
MscL was reconstituted into vesicles containing mixtures of DOPC and anionic lipid, at a fixed molar ratio of lipid:MscL channel of 500:1. In vesicles containing just DOPC and MscL, there was no detectable release of calcein following addition of MTSET (Figure 3A). However, with increasing DOPG content up to 50 mol % both the rate and amplitude of release increased, but both decreased with further increases in DOPG content to 100% (Figure 3A). Similar effects were seen in mixtures of DOPC and DOPA (Figure 3B) or DOPC and CL (Figure 3C). For DOPA, as for DOPG, maximum rates and amplitudes of release were observed at ca. 50 mol % of anionic lipid. In experiments with CL, the mole fraction of CL was calculated on a fatty acyl chain basis, to account for the fact that CL contains four chains whereas DOPC contains two. The maximum rates and amplitude of release in mixtures with CL were also observed at 50 mol %, when calculated on a chain basis. In all cases the data fitted to a single exponential process; rates and amplitudes of release are plotted as a function of mole fraction of anionic lipid in the Supporting Information. Maximal rates of release in bilayers containing DOPA were less than in bilayers containing DOPG or CL, and higher concentrations of DOPA had a larger inhibitory effect on the amplitude of release than observed with either DOPG or CL.
Figure 3.

Effect of anionic lipid on calcein flux through MscL. The G22C mutant of E. coli MscL was reconstituted into lipid vesicles containing DOPC and the given mole percent anionic lipid: (A) DOPG, (B) DOPA, and (C) CL. After 50 s, 1 mM MTSET was added, followed finally by 200 μMC12E8 to burst the vesicles. The broken lines show best fits to a single exponential process. In (C), mole fractions of CL are calculated on a fatty acyl chain basis to account for the fact that CL contains four chains whereas DOPC contains two.
A variety of experiments showed that labeling with MTSET was fast and complete under these conditions. Samples of MscL reconstituted in vesicles of DOPC, of DOPG, or of a 1:1 molar ratio of DOPG:DOPC were allowed to react with MTSET for about 1 min and then analyzed by mass spectrometry; no unlabeled MscL was observed, as shown in Figure 4 for MscL in vesicles of DOPC. Yoshimura et al. (14) deduced from electrophysiological experiments that the Cys residue in the G22C mutant was accessible to MTSET from the periplasmic side in both the open and closed states but accessible to MTSET from the cytoplasmic side only when the channel was open. As described below, MscL is present in the reconstituted vesicles with a random orientation. Efficient labeling of MscL in the reconstituted vesicles could therefore involve initial labeling of those MscL molecules with their “periplasmic” sides exposed to the external medium, this labeling leading to opening of these channels, allowing access of the MTSET to the inside of the vesicles and the labeling of the remaining MscL molecules. Alternatively, any osmotic pressure differences across the vesicle membrane might result in sufficient opening of the MscL molecules with their “cytoplasmic” faces externally exposed for reaction with MTSET to occur.
Figure 4.

Analysis of MTSET labeling of MscL by electrospray mass spectrometry. The G22C mutant of E. coli MscL was reconstituted into lipid vesicles containing DOPC and reacted with MTSET as described under Materials and Methods. (A) and (B) show electrospray mass spectra for MscL unreacted (A) and after reaction with MTSET (B). In (A) the peaks at molecular masses 16492 and 16361 correspond to MscL and MscL lacking the N-terminal Met residue, respectively. In (B) the peaks at molecular masses 16611 and 16480 correspond to labeled MscL and labeled MscL lacking the N-terminal Met residue, respectively, and the peaks at 16627 and 16496 correspond to single Met oxidations of MscL (MscL is Met-rich). No unlabeled MscL is observed in spectrum B.
Fast labeling by MTSET under the experimental conditions used here is also suggested by the observation that increasing or decreasing the concentration of MTSET from 1 to 2 or 0.5 mM, respectively, had no effect on the observed rate or amplitude of release (data not shown): if the rate of reaction of MTSET with MscL was affecting the rate of the observed increase in fluorescence intensity, changing the concentration of MTSET would have changed this observed rate. Finally, addition of a second aliquot of MTSET following the initial addition led to no further change in fluorescence intensity.
The same effects of anionic lipid on efflux through the MscL channel were observed when MscL was labeled with MTSET in detergent suspension before reconstitution into the lipid vesicles. Thus when MscL was prelabeled with MTSET and then reconstituted into vesicles of DOPC, large quantities of calcein were retained that could be released by addition of C12E8 to burst the vesicles (Figure 5, trace b). In contrast, when prelabeled MscL was reconstituted into vesicles of a 1:1 molar ratio of DOPG:DOPC, the vesicles retained very little calcein (Figure 5, trace d), mirroring the effects of anionic lipid seen when unlabeled MscL was labeled within the vesicle by addition of MTSET.
Figure 5.
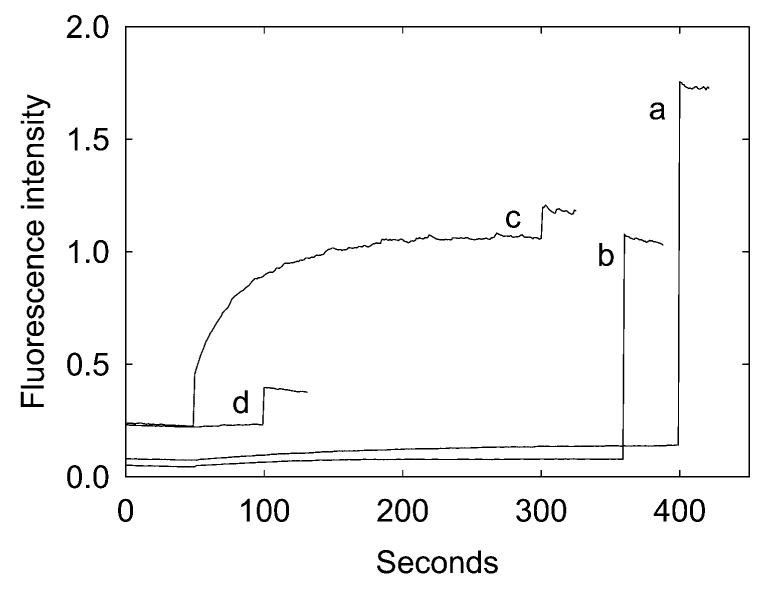
Effect of labeling MTSET before reconstitution. MscL was labeled with MTSET either before or after reconstitution into lipid vesicles. (a) DOPC vesicles were reconstituted with unlabeled MscL. Addition of 1 mM MTSET after 50 s had little effect, so that addition of 200 μM C12E8 after 400 s resulted in a large release of calcein. (b) MscL was labeled with 1 mM MTSET in OG micelles prior to reconstitution and then reconstituted with DOPC. As shown by the large release of calcein observed on addition of C12E8 after 350 s, the vesicles retained a large concentration of calcein. The experiments were repeated for vesicles containing a 1:1 molar ratio of DOPG:DOPC (c, d). When unlabeled MscL was reconstituted into a 1:1 mixture of DOPG:DOPC, addition of MTSET after 50 s resulted in a large release of calcein, so that subsequent addition of C12E8 resulted in only a small release (c). When labeled MscL was reconstituted into vesicles containing a 1:1 molar ratio of DOPG:DOPC, the vesicles retained only a small amount of calcein, as shown by the small response on addition of C12E8 after 100 s (d).
Effect of Phosphatidylethanolamine on the Rate and Amplitude of Calcein Release
The rate and extent of calcein release were markedly depressed when DOPC in mixtures with anionic lipid was replaced by DOPE (Figure 6). This effect of DOPE was observed in mixtures with DOPG, DOPA, or CL. The native E. coli membrane contains a mixture of phosphatidylethanolamine, phosphatidylglycerol, and cardiolipin. Reconstitution of MscL into a mixture of DOPE, DOPG, and CL, representing the native composition, resulted in a low amplitude and rate of release (Figure 7, trace b), but replacing the DOPE in the mixture with DOPC resulted in an increase in both amplitude and rate of release (Figure 7, trace a). The amplitude and rate of release in azolectin, a mixture of lipids from soybean, were also low (Figure 7, trace c).
Figure 6.

Effect of phosphatidylethanolamine on calcein flux through MscL. The G22C mutant of E. coli MscL was reconstituted into lipid vesicles containing 1:1 molar ratios of the following lipids: (a) DOPC:DOPA, (b) DOPE:DOPA, (c) DOPC:DOPG, (d) DOPE:DOPG, (e) DOPC:CL, and (f) DOPE:CL. After 50 s, 1 mM MTSET was added, followed finally by 200 μM C12E8 to burst the vesicles.
Figure 7.
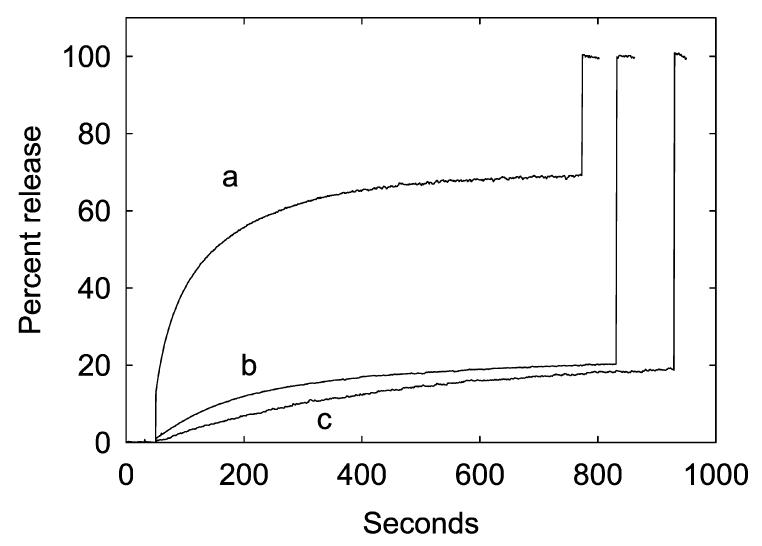
Effect of lipid mixtures on calcein flux through MscL. The G22C mutant of E. coli MscL was reconstituted into lipid vesicles containing the following lipid mixtures: (a) DOPC:DOPG: CL (70:25:5 mol %), (b) DOPE:DOPG:CL (70:25:5 mol %), and (c) azolectin. After 50 s, 1 mM MTSET was added, followed finally by 200 μM C12E8 to burst the vesicles.
Effects of Labeling with the Negatively Charged Label MTSES
To show that the effects of anionic lipid on calcein release observed here with MTSET were not related in some way to the positive charge on MTSET, we also studied calcein release induced by reaction of the G22C mutant of MscL with the negatively charged reagent MTSES. Release of calcein following reaction with 1 mM MTSES is slower than that observed following reaction with 1 mM MTSET, but the effects of anionic lipid were the same (Figure 8). In vesicles of DOPC containing 25 mol % DOPG, reaction of MscL with MTSES led to a very low level of release of calcein, the level of release increasing when the level of DOPG was increased to 50 mol %, the rate of release of calcein then decreasing at higher mole fractions of DOPG (Figure 8). Increasing the concentration of MTSES to 5 mM had no significant effect on the rate or amplitude of calcein release (data not shown) so that the slow release of calcein following reaction with MTSES is unlikely to be the result of a slower reaction with MTSES. It is possible that the slower release of calcein following reaction with MTSES than with MTSET is related to the introduction of negative charge into the channel by labeling with MTSES.
Figure 8.

Calcein flux following reaction of MscL with MTSES. The G22C mutant of E. coli MscL was reconstituted into lipid vesicles containing DOPC and the given mole percent DOPG. After 50 s, 1 mM MTSES was added, followed finally by 200 μM C12E8 to burst the vesicles.
Diameters of Reconstituted Vesicles
Vesicle diameters were determined by light scatter. As shown in Table 3, reconstitution of MscL into DOPC resulted in a preparation in which 70% of the vesicles had diameters of 87 ± 13 nm, with 30% having diameters of 180 ± 30 nm. Reconstitution of MscL into a 1:1 mixture of DOPC and DOPG resulted in a preparation in which all of the vesicles had diameters of 101 ± 18 nm. Similar results were obtained for vesicles in the absence of MscL (Table 3), and vesicle sizes were found to be independent of the anionic lipid employed (data not shown). Reconstitution of Ca2+-ATPase into sealed vesicles using the procedure employed here has been reported similarly to give vesicles of diameter 100 nm (28) or 120 ± 30 nm, with no significant effect of anionic lipid on vesicle diameters (25).
Table 3. Diameters of Reconstituted Vesiclesa.
| diameter (nm) |
||
|---|---|---|
| lipid composition | −MscL | +MscL |
| DOPC | 60%, 56 ± 9 | 70%, 87 ± 13 |
| 40%, 329 ± 50 | 30%, 182 ± 30 | |
| DOPG:DOPC 1:1 | 76 ± 28 | 101 ± 18 |
Vesicle diameters were determined by light scatter. For vesicles containing MscL the molar ratio of lipid:MscL channel was 500:1.
Sidedness of MscL in the Reconstituted Vesicles
Reconstitution of a variety of membrane proteins by mixing lipid and protein in detergent followed by removal of detergent with Bio-Beads has been shown to result in a random orientation of the protein in the membrane with half of the protein molecules having a “right-side-out” orientation and half having a “wrong-side-out” orientation (24, 25). To confirm that this was the case with MscL, we made use of two mutants of M. tuberculosis MscL, L69C and Y94C available from previous studies (26), containing single Cys residues on the periplasmic and cytoplasmic sides of the protein, respectively. N-(1-Pyrenyl)maleimide is nonfluorescent but becomes fluorescent on reaction with a Cys residue. Addition of N-(1-pyrenyl)maleimide to reconstituted vesicles resulted in a rapid increase in fluorescence intensity which about doubled on addition of C12E8 to burst the vesicles, for both L69C and Y94C, in vesicles of DOPC or in vesicles of a 1:1 molar ratio of DOPG:DOPC (Figure 9). We conclude therefore that insertion of MscL in the reconstituted vesicles is close to random and is unaffected by the presence of anionic lipid. We note that these experiments are also consistent with a high efficiency for reconstitution of MscL under the conditions employed here, since the presence of a large proportion of unreconstituted MscL would have resulted in a ratio of fluorescence labeling in the presence and absence of detergent very different to 1:1.
Figure 9.

Sidedness of MscL reconstituted into lipid vesicles. L69C-TbMscL (A) and Y94C-TbMscL (B) with Cys residues located on the periplasmic and cytoplasmic sides of the channel, respectively, were reconstituted into vesicles of DOPC (solid line) or DOPG: DOPC (molar ratio, 1:1; dashed line). Cys residues were then reacted with 7 μM N-(1-pyrenyl)maleimide. Vesicles were burst by the addition of 200 μM C12E8.
Effects of Osmotic Shock
The osmotic pressure difference ΔP across a membrane due to solute A is given by
where cAin and cAout are the concentrations of the solute on the two sides of the membrane. With a value for R of 0.08206 L • atm • mol−1 K−1, a difference in concentration of 100 mM corresponds to a pressure difference of 2.4 atm. Large unilamellar lipid vesicles can maintain a concentration gradient of solute up to about 200 mOsm without leaking, but at higher gradients transient pores form, leading to a leak of some of the vesicle content, until the osmotic pressure across the vesicle membrane has fallen sufficiently for the vesicles to reseal (29, 30). The pressure difference across the membrane is related to tension by Laplace's law [tension = (pressure × radius)/2]. For a vesicle of radius 50 nm, 1 atm of pressure difference corresponds to a tension of 2.5 dyn/cm. The membrane tension required to open the wild-type E. coli MscL channel is ca. 7–12 dyn/cm, depending on lipid composition (1, 8), suggesting that the MscL channel could open under achievable osmotic pressure differences in reconstituted vesicles.
To test the ability of lipid vesicles to resist the osmotic pressure arising from trapped calcein, vesicles of 1:1 DOPA: DOPC containing 50 mM calcein were reconstituted from detergent solution using Bio-Beads as described under Materials and Methods but in the absence of MscL. On dilution into buffer containing either 100 mM KCl or 100 mM KCl plus 0.5 M sucrose, the same low fluorescence intensity was observed; addition of C12E8 to burst the vesicles resulted in a large increase in fluorescence intensity (Figure 10A). The same experiment was repeated for vesicles containing the G22C mutant of MscL. Dilution into buffer containing 100 mM KCl plus 0.5 M sucrose resulted in a low fluorescence intensity that was stable for up to 800 s (Figure 10B, trace a); addition of MTSET resulted in a large increase in fluorescence intensity (Figure 10B, trace c). However, dilution of the vesicles into buffer containing just 100 mM KCl resulted in a higher initial fluorescence that was stable for at least 400 s, with a smaller change on addition of MTSET, so that the overall fluorescence change was comparable to that seen in buffer in the presence of sucrose (Figure 10B, trace b). We conclude that, in the absence of sucrose in the external buffer, the osmotic pressure effect of the trapped calcein is sufficient to open the MscL channel, releasing calcein into the external medium, until the concentration of trapped calcein has dropped to a level where the osmotic pressure is not sufficient to hold open the MscL channel when release of calcein stops.
Figure 10.

Effect of osmotic pressure on release of calcein. (A) Vesicles of a 1:1 mixture of DOPA:DOPC containing 50 mM calcein were diluted into buffer containing 100 mM KCl (a) or 100 mM KCl plus 0.5 M sucrose (b). At the time shown by the arrows the vesicles were burst by the addition of 200 μM C12E8. (B) Vesicles of a 1:1 mixture of DOPA:DOPC with the G22C mutant of MscL containing 50 mM calcein were diluted into buffer containing 100 mM KCl plus 0.5 M sucrose and either burst with 200 μM C12E8 at 800 s (a) or first reacted with MTSET at 50 s and then burst with 200 μM C12E8 at 800 s (c). The same preparation of vesicles was also diluted into buffer containing 100 mM KCl and no sucrose, followed by addition of MTSET after 400 s, and then burst with 200 μM C12E8 at 800 s (b).
Figure 11 shows the response of MscL reconstituted in a 1:1 mixture of DOPA:DOPC to osmotic gradient. Whereas dilution of vesicles containing 50 mM calcein into buffer containing 100 mM KCl and 0.5 M sucrose results in no release of calcein, as described above, dilution into buffer containing 100 mM KCl and 0.45 M sucrose does lead to calcein release, the extent of release not changing when the concentration of sucrose was dropped to 0.2 M, although some further release was observed when the sucrose was removed entirely. A drop in sucrose concentration from 0.5 to 0.45 M corresponds to a change in osmotic pressure of 1.2 atm or to a change in membrane tension of 3 dyn/m for a vesicle of radius 50 nm. The tension required for half-maximal opening of wild-type MscL in phosphatidylcholine-containing vesicles has been reported by Moe and Blount (8) to be ca. 7 dyn/cm, the results of Yoshimura et al. (31) suggesting that the tension required for half-maximal opening of the G22C mutant of MscL will be ca. 10 dyn/cm.
Figure 11.

The osmotic pressure difference required to open MscL. The G22C mutant of E. coli MscL was reconstituted into vesicles of a 1:1 mixture of DOPA:DOPC containing 50 mM calcein and diluted into buffer containing 100 mM KCl and the following concentrations of sucrose: (a) 0.5 M; (b) 0.45 M; (c) 0.4 M; (d) 0.2 M; (e) 0 M. After 150–250 s, 200 μM C12E8 was added to burst the vesicles.
Figure 12A shows the effect of anionic lipid content on the osmotic sensitivity of vesicles containing the G22C mutant of MscL. As shown, the higher the DOPG content of the reconstituted vesicles, the higher the initial fluorescence intensity and the smaller the effect seen on subsequent addition of MTSET; the final fluorescence intensity is similar in all the experiments. Figure 12B shows the corresponding experiments with wild-type MscL. Again, the initial fluorescence intensity observed after osmotic shock is low when the vesicles contain DOPC as the only lipid, with the initial fluorescence intensity increasing with increasing DOPG content.
Figure 12.
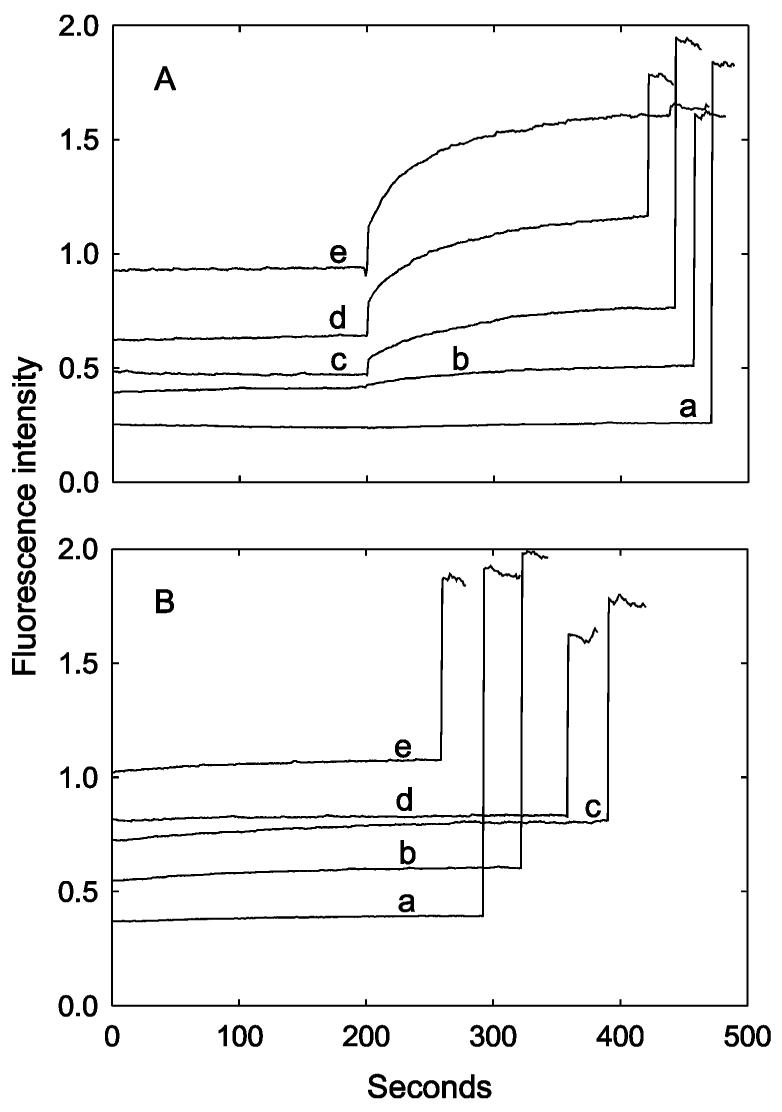
Effect of anionic lipid on osmotic sensitivity. The G22C mutant of E. coli MscL (A) or wild-type E. coli MscL (B) was reconstituted into vesicles containing mixtures of DOPG and DOPC with 50 mM calcein and diluted into buffer containing 100 mM KCl. In (A), 1 mM MTSET was added after 200 s, followed by 200 μM C12E8 to burst the vesicles at about 500 s. In (B), 200 μM C12E8 was added to burst the vesicles after about 300 s. The mole percent of DOPG in the vesicles was as follows: (a) 0; (b) 20%; (c) 30%; (d) 40%; (e) 50%.
These experiments in which the MscL channel is opened by the osmotic pressure difference across the membrane are more complex than those in which the mutant channel is opened by reaction with MTSET, in that the osmotic pressure difference that is keeping the channel open will decrease with time, as a result of the release of calcein through the open channels. Thus the final fluorescence intensity level observed following the osmotic shock could reflect the point at which the osmotic pressure difference is no longer sufficient to keep the channel open or could reflect transformation of the channel into a subconductance state. Nevertheless, the important result is that anionic lipid has comparable effects on release through the MscL channel whether the wild-type channel is opened by osmotic shock or the mutant channel is opened by reaction with MTSET. In both cases the extent of release of calcein is very low in the absence of anionic lipid.
DISCUSSION
The MscL channel opens when the tension in the surrounding membrane increases, and because MscL is fully functional in lipid bilayers in the absence of any other proteins, membrane tension must be transduced directly from the lipid bilayer to the protein (1, 2). It might therefore be expected that MscL function would be dependent on the structure of the surrounding lipid molecules. Indeed, Moe and Blount (8) found using patch clamp techniques that replacing phosphatidylcholine by phosphatidylethanolamine in the surrounding bilayer increased the tension required to open the channel. However, Moe and Blount (8) found no effect of anionic phospholipids on the tension required to open the MscL channel. In contrast, we report here that the presence of anionic lipid is essential for flux of the fluorescent dye calcein through the MscL channel. We suggest that the effects we observe could arise from effects of anionic lipid on the rate of the transition from the closed to the fully open state and on the rate of the transition from the fully open state to a subconductance state unable to pass calcein.
A convenient method for activating the MscL channel makes use of the G22C mutant of MscL, where the Cys residue is located within the pore constriction; reaction with MTSET introduces five positive charges into the pore constriction, forcing the channel into an open state (13-15). Channel opening is detected by release of calcein, trapped at high concentration in the lumen of reconstituted vesicles containing MscL; release of calcein results in a large increase in calcein fluorescence due to the relief of concentration quenching. Unexpectedly, although addition of MTSET to vesicles containing MscL reconstituted in bilayers containing anionic lipid does result in the release of calcein, the release is only partial, a large proportion of the calcein being retained, depending on the MscL concentration in the membrane (Figure 1).
At a molar ratio of MscL channel to lipid of 1:2500, an average vesicle is expected to contain ca. 40 channels and 1.2 × 104 molecules of calcein when the lumenal concentration of calcein is 50 mM. The rate of flux of a solute through a simple channel is proportional to the cross-sectional area of the pore and so to the square of the radius of the pore (32). An open pore diameter of ca. 25–30 Å has been suggested for MscL (33, 34). The equations given by Hille (32) suggest a rate of unidirectional flux for a monovalent ion through such a pore of ca. 108 ions/s, in close agreement with the estimate of Steinbacher et al. (35) for the rate of water flux through MscL of ca. 2 × 108 molecules/s. Diffusion coefficients of solutes are inversely related to the diameter of the solute. The diameter of the calcein molecule is ca. 12 Å, based on the crystal structure of the related dye fluorescein (36). This is ca. 6 times that of a water molecule, so that the rate of diffusion of a calcein molecule through the pore would be ca. 3 × 107 molecules/s. Thus one open channel per vesicle would be expected to lead to release of all the trapped calcein molecules within 1 s. For the reasons discussed in the Results section, we believe that the efficiency of reconstitution of MscL into the vesicles is high and that the MscL will be present in a largely native form. The partial release of calcein shown in Figure 1 therefore suggests that over a time period of several hundreds of seconds following reaction with MTSET, the initially open channels transform into a state with a lifetime of many hundreds of seconds, which is unable to allow the passage of calcein. The possibility of inactivation of MscL has been discussed by Hase et al. (18), who showed that, in patches of reconstituted MscL where the number of active channels was low, MscL activity occurred in bursts followed by long inactive periods and that, following this inactivation, channels could not be recovered either by changing voltage or by application of pressure.
Effect of Phosphatidylethanolamine
Substitution of DOPC by DOPE in mixtures with anionic lipid results in a decrease in both the rate and amplitude of release (Figure 6). Moe and Blount (8) have reported that substitution of DOPC by DOPE results in an increase in the membrane tension required to open the MscL channel. Molecular dynamics simulations have suggested that differences in hydrogen bonding between MscL and the phosphatidylcholine and phosphatidylethanolamine lipid headgroups could result in significant changes in MscL structure (37, 38). These results are, perhaps, surprising given that the native E. coli membrane contains phosphatidylethanolamine as its zwitterionic lipid rather than phosphatidylcholine. MscL activity was also low in a mixture of 70% DOPE, 25% DOPG, 5% CL (Figure 7) that mimics the composition of the native E. coli membrane (39, 40).
Calcein Flux and Subconductance States of MscL
The results of the calcein flux experiments can be compared to the results of electrophysiological studies of MscL following reaction with MTSET. Labeling G22C with MTSET, or mutation of Gly-22 to a charged residue, results in a channel that opens at low or zero tension but which lingers most of the time in a subconductance state with about one-tenth of the full unitary conductance, similar to the conductance of the first substate for the wild-type channel (14, 31). Since flux through a pore is related to the square of the radius of the pore (32), a 10-fold decrease in conductance would correspond to a 3-fold or more decrease in radius. If the diameter of the fully open state is 25–30 Å (33, 34), then that of the subconductance state would be ca. 8–10 Å, too small to allow the free passage of a calcein molecule of diameter ca. 12 Å. Thus transformation of MscL from the fully open state to this subconductance state would stop efflux of calcein from the reconstituted vesicles.
A variety of subconducting states have been observed for MscL, and it has been suggested that the subconductance state seen on introduction of a charge at position 22 in MscL could correspond to the first subconductance state of the native protein (9, 14, 31). The modified channel exists largely in the subconductance state at zero tension but some at least of the modified channels open to a fully conducting state resembling the normal open state at high tension (9). Thus it is possible that the effect of introducing charge is to stabilize the normal first subconductance state of MscL relative to the closed state. The alternative possibility is, however, that the subconductance state is a new partially opened state of the channel, created as a result of the introduction of charge.
Anionic Lipid Stabilizes the Fully Open State
In bilayers of DOPC, reaction of the G22C mutant of MscL with MTSET resulted in no detectable release of calcein, but release increased in amplitude and rate with increasing anionic lipid content up to 50 mol %, beyond which the rates and amplitudes of release again decreased (Figure 3). The effects of anionic lipid are not related to direct effects on the rate or extent of reaction with MTSET: reaction with MTSET is fast on the time scale of calcein release, as shown by the fact that the rate of calcein release did not change with changes in the concentration of MTSET, and the extent of labeling was 100% as shown by mass spectrometric analysis of MscL in the presence or absence of anionic lipid (Figure 4). Further, the same results were observed if MscL was first labeled with MTSET in detergent solution and then reconstituted into lipid vesicles; vesicles of DOPC reconstituted with labeled MTSET were able to retain calcein whereas vesicles containing anionic lipid and labeled MTSET were not (Figure 5).
The effects of anionic lipid are not related in any way to differences in the sidedness of MscL in the reconstituted vesicles. The reconstitution procedure adopted here results in a random orientation of MscL molecules in the membrane, in either the presence or absence of anionic lipid (Figure 9). Effects of anionic lipid also cannot be related to changes in vesicle size. For example, as shown in Table 3, 70% of vesicles reconstituted with DOPC have diameters similar to those for vesicles reconstituted with a 1:1 mixture of DOPC: DOPG. Even if the 30% of larger vesicles seen with DOPC (Table 3) released calcein more slowly than the smaller vesicles, this would not explain the essentially total lack of calcein release observed for the DOPC vesicles (Figure 3). Finally, the effect of anionic lipid on calcein release is not related to the fact that the MTSET label introduces a positive charge into the channel, since comparable effects of anionic lipid are seen on calcein release induced by reaction with the negatively charged MTSES (Figure 8).
As shown in Supporting Information, the effects of anionic lipid observed here can be simulated in terms of the following three-state model, in which the channel is in one of the three states, C, O, or S, corresponding to closed, fully open, and substate, respectively:
The observed increase in rate of flux with increasing anionic lipid content up to 50 mol % is consistent with an increase in the rate of the C to O transition in the presence of anionic lipid, and the observed increase in amplitude of release with increasing anionic lipid content up to 50 mol % is consistent with a decrease in the rate of the O to S transition in the presence of anionic lipid. The decrease in rate and amplitude of release observed at concentrations of anionic lipid above 50 mol % can be simulated in terms of a decrease in the rate of flux through the open channel at high anionic lipid content. This could be a direct effect on the channel or an indirect effect of the high negative charge on the bilayer at high anionic lipid content.
Importantly, the results observed in experiments where release of calcein was induced by reaction with MTSET agree with experiments in which release was induced by osmotic pressure. As shown in Figure 12, the osmotic pressure effect of 50 mM calcein is sufficient to open both the G22C mutant of MscL and wild-type MscL and allow release of calcein, unless balanced by the presence of sufficient sucrose in the external medium (Figure 11). For both the G22C mutant and wild-type MscL, the higher the DOPG content of the reconstituted vesicles, the greater the level of release of calcein (Figure 12). The very similar results obtained for the G22C mutant of MscL and for wild-type MscL are consistent with the observation that although the pressure required to open the G22C mutant is higher than that required to open the wild-type protein, the behavior of the G22C mutant once opened is normal (14, 31). The observation that anionic lipid has comparable effects on release through the MscL channel whether the wild-type channel is opened by osmotic shock or the mutant channel is opened by reaction with MTSET argues that the effects of anionic lipid reported here are not unique to a channel modified within the pore constriction by introduction of charged residues. Rather, limited flux mediated by MscL in the absence of anionic lipid seems to be a general phenomenon, independent of how the channel is opened. This could then provide an alternative explanation for the observation that electrophysiological recordings for reconstituted MscL generally show much lower numbers of active channels than expected from the amount of protein used in the reconstitution (12); it is possible that, rather than reflecting a low efficiency of reconstitution, this could follow from an effect of lipid composition on the balance between open and subconductance or inactivated states of the channel.
The effects of anionic lipid on flux through the MscL channel could follow from effects of lipid composition on some general physical property of the membrane or could follow from direct interaction between anionic lipid and the channel. The observation that effects of anionic lipid depend on lipid headgroup structure (Figure 3) would argue for the importance of direct interaction with the channel. We have shown that anionic lipids bind strongly to a cluster of three positively charged residues on the cytoplasmic side of MscL (6). If effects of anionic lipid binding on channel function required binding to more than one of these clusters in the homopentameric channel, effects of anionic lipid content on function would be cooperative, as suggested by the data shown in Figure 1 in the Supporting Information.
ACKNOWLEDGMENT
We thank Professor B. Martinac for the gift of the MscL clone, J. Neville Wright for performing the mass spectral analyses, and Professor B. Poolman and Dr. A. Koçer for very helpful discussions of the calcein flux assay.
Footnotes
This work was supported by the Wellcome Trust.
Abbreviations: MscL, mechanosensitive channel of large conductance; MTSET, [2-(triethylammonium)ethyl]methanethiosulfonate; MTSES, sodium (2-sulfonatoethyl)methanethiosulfonate; OG, octyl β-d-glucoside; C12E8, octa(ethylene glycol) n-dodecyl ether; DOPC, dioleoylphosphatidylcholine; DOPE, dioleoylphosphatidylethanolamine; DOPA, dioleoylphosphatidic acid; DOPG, dioleoylphosphatidylglycerol; CL, tetraoleoylcardiolipin; BrPC, di(9,10-dibromostearoyl)phosphatidylcholine; BrPG, di(9,10-dibromostearoyl)phosphatidylglycerol.
SUPPORTING INFORMATION AVAILABLE
Plots of rates and amplitudes of the fluorescence response following reaction with MTSET as a function of mole fraction of anionic lipid and further details of the simulations of calcein release described in the text. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.Sukharev SI, Sigurdson WJ, Kung C, Sachs F. Energetic and spatial parameters for gating of the bacterial large conductance mechanosensitive channel, MscL. J. Gen. Physiol. 1999;113:525–539. doi: 10.1085/jgp.113.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamill OP, Martinac B. Molecular basis of mechanotransduction in living cells. Physiol. Rev. 2001;81:685–740. doi: 10.1152/physrev.2001.81.2.685. [DOI] [PubMed] [Google Scholar]
- 3.Blount P, Iscla I, Moe PC, Li Y. MscL: the bacterial mechanosensitive channel of large conductance. Curr. Top. Membr. 2007;58:201–233. [Google Scholar]
- 4.Lee AG. Lipid-protein interactions in biological membranes: a structural perspective. Biochim. Biophys. Acta. 2003;1612:1–40. doi: 10.1016/s0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- 5.Lee AG. Lipid interactions with ion channels. Future Lipidol. 2006;1:103–113. [Google Scholar]
- 6.Powl AM, East JM, Lee AG. Heterogeneity in the binding of lipid molecules to the surface of a membrane protein: hot-spots for anionic lipids on the mechanosensitive channel of large conductance MscL and effects on conformation. Biochemistry. 2005;44:5873–5883. doi: 10.1021/bi047439e. [DOI] [PubMed] [Google Scholar]
- 7.Maurer JA, Dougherty DA. Generation and evaluation of a large mutational library from the Escherichia coli mechanosensitive channel of large conductance, MscL—Implications for channel gating and evolutionary design. J. Biol. Chem. 2003;278:21076–21082. doi: 10.1074/jbc.M302892200. [DOI] [PubMed] [Google Scholar]
- 8.Moe P, Blount P. Assessment of potential stimuli for mechano-dependent gating of MscL: effects of pressure, tension, and lipid headgroups. Biochemistry. 2005;44:12239–12244. doi: 10.1021/bi0509649. [DOI] [PubMed] [Google Scholar]
- 9.Anishkin A, Chiang CS, Sukharev S. Gain-of-function mutations reveal expanded intermediate states and a sequential action of two gates in MscL. J. Gen. Physiol. 2005;125:155–170. doi: 10.1085/jgp.200409118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kocer A, Walko M, Meijberg W, Feringa BL. A light-actuated nanovalve derived from a channel protein. Science. 2005;309:755–758. doi: 10.1126/science.1114760. [DOI] [PubMed] [Google Scholar]
- 11.Kocer A, Walko M, Feringa BL. Synthesis and utilization of reversible and irreversible light-activated nanovalves derived from the channel protein MscL. Nat. Protoc. 2007;2:1426–1437. doi: 10.1038/nprot.2007.196. [DOI] [PubMed] [Google Scholar]
- 12.van den Bogaart G, Krasnikov V, Poolman B. Dual-color fluorescence-burst analysis to probe protein efflux through the mechanosensitive channel MscL. Biophys. J. 2007;92:1233–1240. doi: 10.1529/biophysj.106.088708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batiza AF, Kuo MMC, Yoshimura K, Kung C. Gating the bacterial mechanosensitive channel MscL in vivo. Proc. Natl. Acad. Sci. U.S.A. 2002;99:5643–5648. doi: 10.1073/pnas.082092599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoshimura K, Batiza A, Kung C. Chemically charging the pore constriction opens the mechanosensitive channel MscL. Biophys. J. 2001;80:2198–2206. doi: 10.1016/S0006-3495(01)76192-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett JL, Li YZ, Blount P. Mechanosensitive channel gating transitions resolved by functional changes upon pore modification. Biophys. J. 2006;91:3684–3691. doi: 10.1529/biophysj.106.088062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.East JM, Lee AG. Lipid selectivity of the calcium and magnesium ion dependent adenosinetriphosphatase, studied with fluorescence quenching by a brominated phospholipid. Biochemistry. 1982;21:4144–4151. doi: 10.1021/bi00260a035. [DOI] [PubMed] [Google Scholar]
- 17.Powl AM, East JM, Lee AG. Lipid-protein interactions studied by introduction of a tryptophan residue: the mechanosensitive channel MscL. Biochemistry. 2003;42:14306–14317. doi: 10.1021/bi034995k. [DOI] [PubMed] [Google Scholar]
- 18.Hase CC, Le Dain AC, Martinac B. Purification and functional reconstitution of the recombinant large mechanosensitive ion channel (MscL) from Escherichia coli. J. Biol. Chem. 1996;270:18329–18334. doi: 10.1074/jbc.270.31.18329. [DOI] [PubMed] [Google Scholar]
- 19.Warren GB, Toon PA, Birdsall NJM, Lee AG, Metcalfe JC. Reconstitution of a calcium pump using defined membrane constituents. Proc. Natl. Acad. Sci. U.S.A. 1974;71:622–626. doi: 10.1073/pnas.71.3.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.O'Keeffe AH, East JM, Lee AG. Selectivity in lipid binding to the bacterial outer membrane protein OmpF. Biophys. J. 2000;79:2066–2074. doi: 10.1016/S0006-3495(00)76454-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pilot JD, East JM, Lee AG. Effects of bilayer thickness on the activity of diacylglycerol kinase of Escherichia coli. Biochemistry. 2001;40:8188–8195. doi: 10.1021/bi0103258. [DOI] [PubMed] [Google Scholar]
- 22.Williamson IM, Alvis SJ, East JM, Lee AG. Interactions of phospholipids with the potassium channel KcsA. Biophys. J. 2002;83:2026–2038. doi: 10.1016/S0006-3495(02)73964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Powl AM, East JM, Lee AG. Different effects of lipid chain length on the two sides of a membrane and the lipid annulus of MscL. Biophys. J. 2007;93:113–122. doi: 10.1529/biophysj.107.105130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rigaud JL, Pitard B, Levy D. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta. 1995;1231:223–246. doi: 10.1016/0005-2728(95)00091-v. [DOI] [PubMed] [Google Scholar]
- 25.Dalton KA, Pilot JD, Mall S, East JM, Lee AG. Anionic phospholipids decrease the rate of slippage on the Ca2+-ATPase of sarcoplasmic reticulum. Biochem. J. 1999;342:431–438. [PMC free article] [PubMed] [Google Scholar]
- 26.Powl AM, Wright JN, East JM, Lee AG. Identification of the hydrophobic thickness of a membrane protein using fluorescence spectroscopy: studies with the mechanosensitive channel MscL. Biochemistry. 2005;44:5713–5721. doi: 10.1021/bi047338g. [DOI] [PubMed] [Google Scholar]
- 27.Perozo E, Kloda A, Cortes DM, Martinac B. Physical principles underlying the transduction of bilayer deformation forces during mechanosensitive channel gating. Nat. Struct. Biol. 2002;9:696–703. doi: 10.1038/nsb827. [DOI] [PubMed] [Google Scholar]
- 28.Levy D, Gulik A, Bluzat A, Rigaud JL. Reconstitution of the sarcoplasmic reticulum Ca2+-ATPase—mechanisms of membrane protein insertion into liposomes during reconstitution procedures involving the use of detergents. Biochim. Biophys. Acta. 1992;1107:283–298. doi: 10.1016/0005-2736(92)90415-i. [DOI] [PubMed] [Google Scholar]
- 29.Shoemaker SD, Vanderlick TK. Stress-induced leakage from phospholipid vesicles: effect of membrane composition. Ind. Eng. Chem. Res. 2002;41:324–329. [Google Scholar]
- 30.Hull MC, Sauer DB, Hovis JS. Influence of lipid chemistry on the osmotic response of cell membranes: effect of non-bilayer forming lipids. J. Phys. Chem. B. 2004;108:15890–15895. [Google Scholar]
- 31.Yoshimura K, Batiza A, Schroeder M, Blount P, Kung C. Hydrophilicity of a single residue within MscL correlates with increased channel mechanosensitivity. Biophys. J. 1999;77:1960–1972. doi: 10.1016/S0006-3495(99)77037-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hille B. Ionic channels of excitable membranes. Sunderland, MA: Sinauer Associates; 2001. [Google Scholar]
- 33.Moe PC, Blount P, Kung C. Functional and structural conservation in the mechanosensitive channel MscL implicates elements crucial for mechanosensation. Mol. Microbiol. 1998;28:583–592. doi: 10.1046/j.1365-2958.1998.00821.x. [DOI] [PubMed] [Google Scholar]
- 34.Betanzos M, Chiang CS, Guy HR, Sukharev S. A large iris-like expansion of a mechanosensitive channel protein induced by membrane tension. Nat. Struct. Biol. 2002;9:704–710. doi: 10.1038/nsb828. [DOI] [PubMed] [Google Scholar]
- 35.Steinbacher S, Bass R, Strop P, Rees DC. Structures of the prokaryotic mechanosensitive channels MscL and MscS. Curr. Top. Membr. 2007;58:1–24. [Google Scholar]
- 36.Yamaguchi K, Tamura Z, Maeda M. Disodium fluorescein octahydrate. Acta Crystallogr. C. 1997;53:284–285. [Google Scholar]
- 37.Elmore DE, Dougherty DA. Molecular dynamics simulations of wild-type and mutant forms of the Mycobacterium tuberculosis MscL channel. Biophys. J. 2001;81:1345–1359. doi: 10.1016/S0006-3495(01)75791-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elmore DE, Dougherty DA. Investigating lipid composition effects on the mechanosensitive channel of large conductance (MscL) using molecular dynamics simulations. Biophys. J. 2003;85:1512–1524. doi: 10.1016/S0006-3495(03)74584-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harwood JL, Russell NJ. Lipids in Plants and Microbes. London: George Allen & Unwin; 1984. [Google Scholar]
- 40.de Kruijff B, Killian JA, Rietveld AG, Kusters R. Phospholipid structure and Escherichia coli membranes. Curr. Top. Membr. 1998;44:477–515. [Google Scholar]


