Abstract
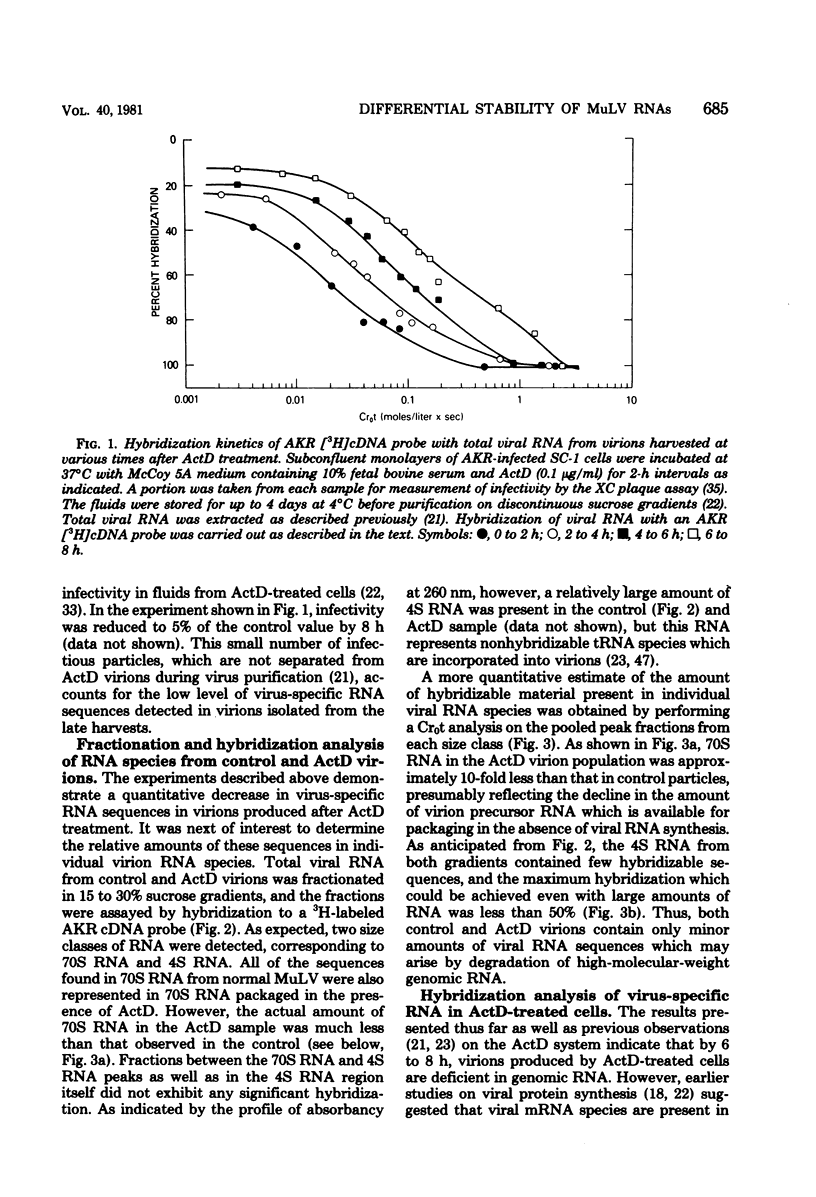
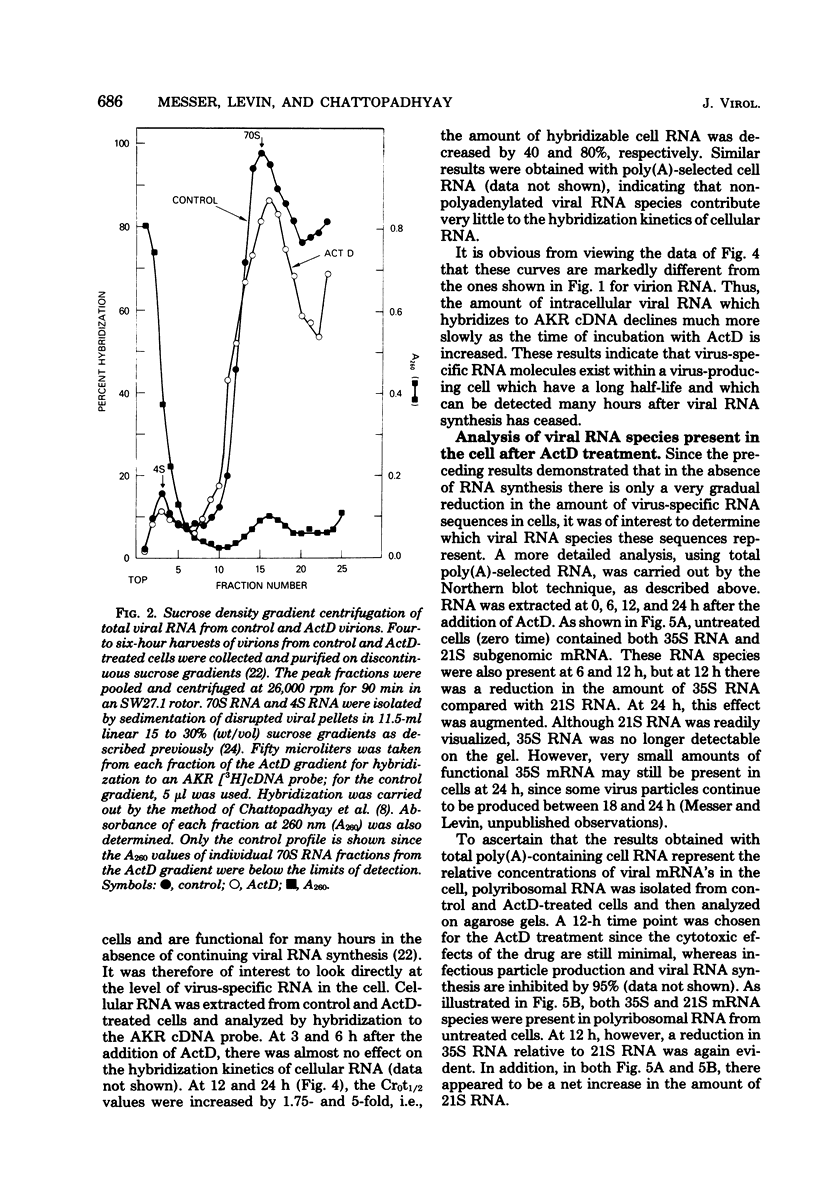
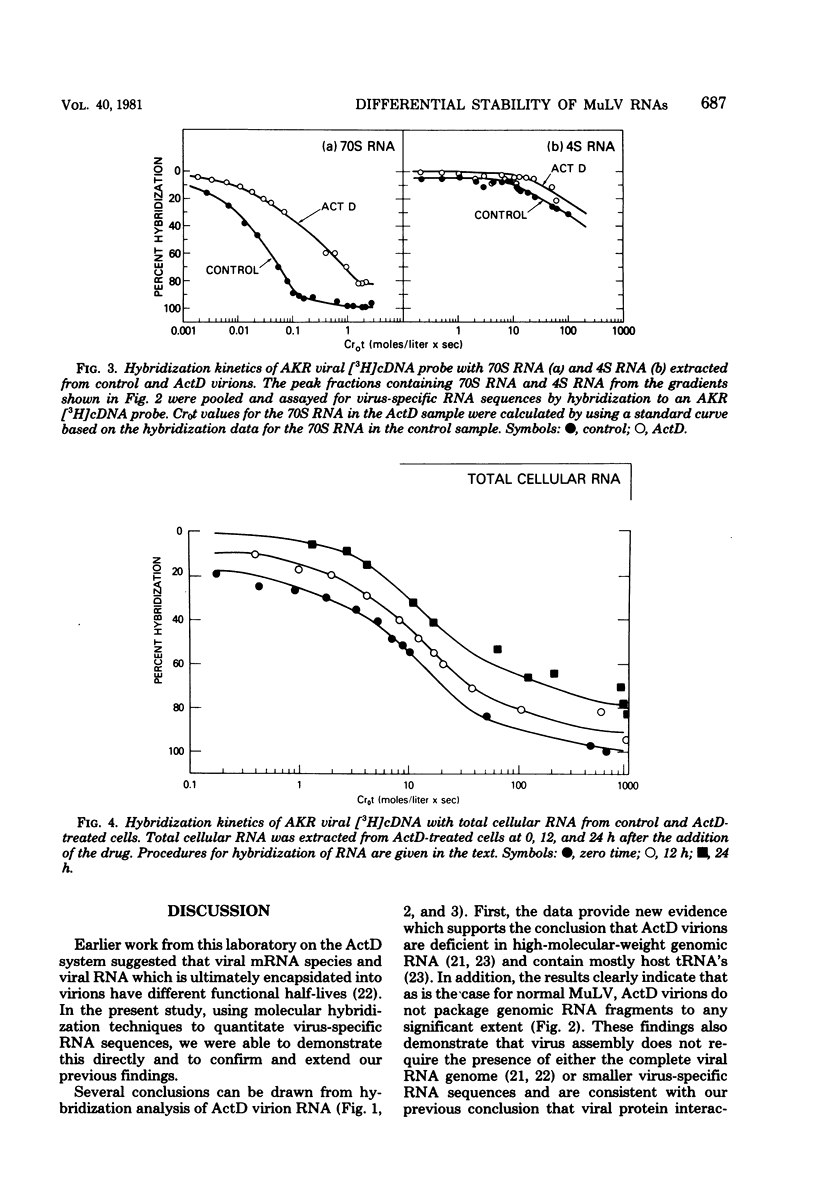
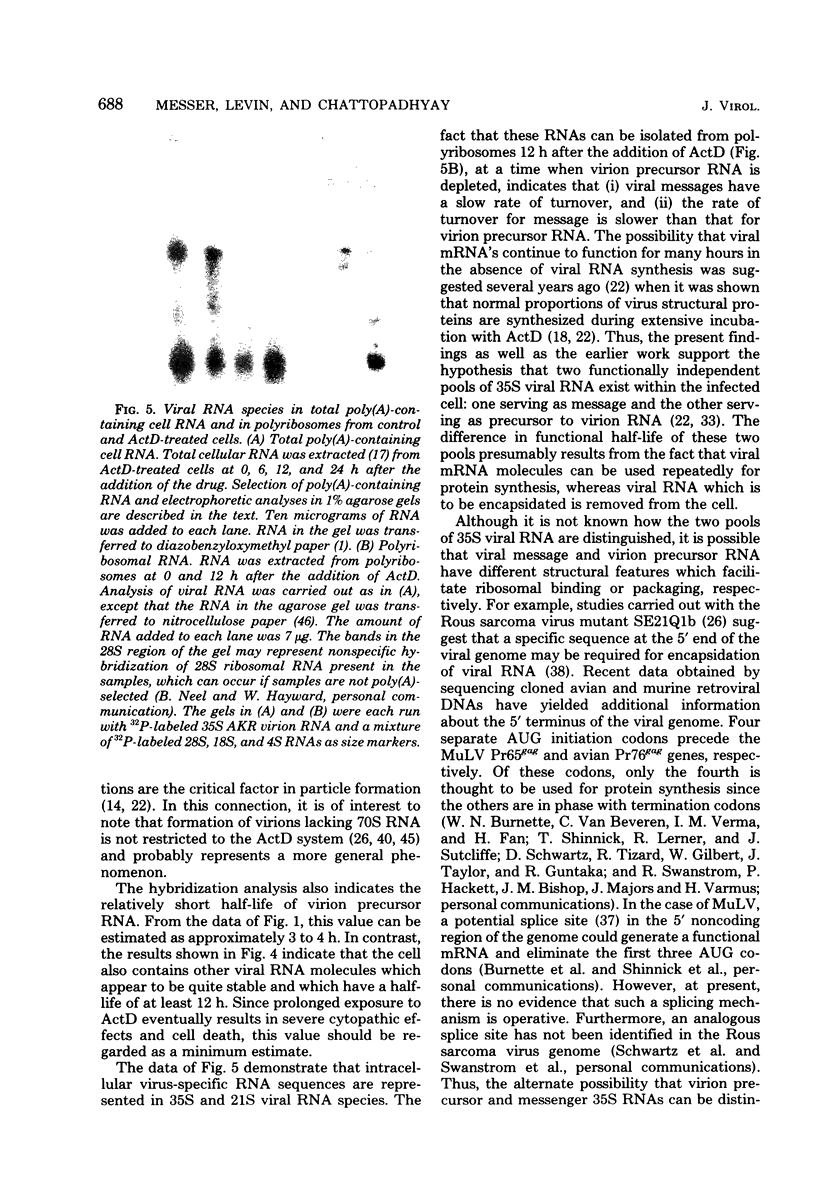
Molecular hybridization techniques were used to examine the stability of viral message and virion precursor RNA in murine leukemia virus-infected cells treated with actinomycin D. Under the conditions used, viral RNA synthesis was inhibited, but viral protein synthesis continued, and the cells produced noninfectious particles (actinomycin D virions) lacking genomic RNA (J. G. Levin and M. J. Rosenak, Proc. Natl. Acad. Sci. U.S.A. 73:1154-1158, 1976). Analysis of total RNA in virions revealed that the amount of hybridizable viral RNA decreased steadily after the addition of actinomycin D and by 8 h was 10% of the control value. Studies on fractionated viral RNA showed that this low level of hybridization is due to residual 70S RNA in the virion population. The results indicated that viral RNA which is destined to be encapsidated into virions has a half-life of approximately 3 to 4 h. In contrast, other intracellular virus-specific RNA molecules appeared to be quite stable and persisted for a long period of time, with a half-life of at least 12 h. These observations support the idea that two independent functional pools of 35S viral RNA exist within the infected cell: one serving as message and the other as precursor to virion RNA. The existence of two viral RNA pools was further documented by the finding that 12 h after the addition of actinomycin D, when virion precursor RNA was depleted, 35S and 21S viral nRNA species could be identified in polyribosomal RNA as well as in total polyadenylated cell RNA. Surprisingly, 35S and mRNA declined more rapidly than did 21S mRNA, which appeared to be increased in amount.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader J. P. Synthesis of the RNA of RNA-containing tumor viruses. I. The interval between synthesis and envelopment. Virology. 1970 Mar;40(3):494–504. doi: 10.1016/0042-6822(70)90192-3. [DOI] [PubMed] [Google Scholar]
- Baluda M. A., Nayak D. P. Incorporation of precursors into ribonucleic acid, protein, glycoprotein, and lipoprotein of avian myeloblastosis virions. J Virol. 1969 Nov;4(5):554–566. doi: 10.1128/jvi.4.5.554-566.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bases R. E., King A. S. Inhibition of Rauscher murine leukemia virus growth in vitro by actinomycin D. Virology. 1967 Jun;32(2):175–183. doi: 10.1016/0042-6822(67)90268-1. [DOI] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Benveniste R. E., Scolnick E. M. RNA in mammalian sarcoma virus transformed nonproducer cells homologous to murine leukemia virus RNA. Virology. 1973 Feb;51(2):370–382. doi: 10.1016/0042-6822(73)90436-4. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. K., Hartley J. W., Lander M. R., Kramer B. S., Rowe W. P. Biochemical characterization of the amphotropic group of murine leukemia viruses. J Virol. 1978 Apr;26(1):29–39. doi: 10.1128/jvi.26.1.29-39.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordell B., Weiss S. R., Varmus H. E., Bishop J. M. At least 104 nucleotides are transposed from the 5' terminus of the avian sarcoma virus genome to the 5' termini of smaller viral mRNAs. Cell. 1978 Sep;15(1):79–91. doi: 10.1016/0092-8674(78)90084-3. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H., Robinson W. S. Inhibition of mouse leukemia virus (MLV) replication by actinomycin D. Virology. 1967 Apr;31(4):742–746. doi: 10.1016/0042-6822(67)90211-5. [DOI] [PubMed] [Google Scholar]
- Fan H., Baltimore D. RNA metabolism of murine leukemia virus: detection of virus-specific RNA sequences in infected and uninfected cells and identification of virus-specific messenger RNA. J Mol Biol. 1973 Oct 15;80(1):93–117. doi: 10.1016/0022-2836(73)90235-0. [DOI] [PubMed] [Google Scholar]
- Fan H., Mueller-Lantzsch N. RNA metabolism of murine leukemia virus. III. Identification and quantitation of endogenous virus-specific mRNA in the uninfected BALB/c cell line JLS-V9. J Virol. 1976 May;18(2):401–410. doi: 10.1128/jvi.18.2.401-410.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Verma I. M. Size analysis and relationship of murine leukemia virus-specific mRNA's: evidence for transposition of sequences during synthesis and processing of subgenomic mRNA. J Virol. 1978 May;26(2):468–478. doi: 10.1128/jvi.26.2.468-478.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerwin B. I., Levin J. G. Interactions of murine leukemia virus core components: characterization of reverse transcriptase packaged in the absence of 70S genomic RNA. J Virol. 1977 Nov;24(2):478–488. doi: 10.1128/jvi.24.2.478-488.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gielkens A. L., Salden M. H., Bloemendal H. Virus-specific messenger RNA on free and membrane-bound polyribosomes from cells infected with Rauscher leukemia virus. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1093–1097. doi: 10.1073/pnas.71.4.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley J. W., Rowe W. P. Clonal cells lines from a feral mouse embryo which lack host-range restrictions for murine leukemia viruses. Virology. 1975 May;65(1):128–134. doi: 10.1016/0042-6822(75)90013-6. [DOI] [PubMed] [Google Scholar]
- Hayward W. S. Size and genetic content of viral RNAs in avian oncovirus-infected cells. J Virol. 1977 Oct;24(1):47–63. doi: 10.1128/jvi.24.1.47-63.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamjoom G. A., Naso R. B., Arlinghaus R. B. Selective decrease in the rate of cleavage of an intracellular precursor to Rauscher leukemia virus p30 by treatment of infected cells with actinomycin D. J Virol. 1976 Sep;19(3):1054–1072. doi: 10.1128/jvi.19.3.1054-1072.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr I. M., Olshevsky U., Lodish H. F., Baltimore D. Translation of murine leukemia virus RNA in cell-free systems from animal cells. J Virol. 1976 May;18(2):627–635. doi: 10.1128/jvi.18.2.627-635.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzyzek R. A., Collett M. S., Lau A. F., Perdue M. L., Leis J. P., Faras A. J. Evidence for splicing of avian sarcoma virus 5'-terminal genomic sequences into viral-specific RNA in infected cells. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1284–1288. doi: 10.1073/pnas.75.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Grimley P. M., Ramseur J. M., Berezesky I. K. Deficiency of 60 to 70S RNA in murine leukemia virus particles assembled in cells treated with actinomycin D. J Virol. 1974 Jul;14(1):152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Rosenak M. J. Synthesis of murine leukemia virus proteins associated with virions assembled in actinomycin D-treated cells: evidence for persistence of viral messenger RNA. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1154–1158. doi: 10.1073/pnas.73.4.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Effect of polymerase mutations on packaging of primer tRNAPro during murine leukemia virus assembly. J Virol. 1981 Apr;38(1):403–408. doi: 10.1128/jvi.38.1.403-408.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. G., Seidman J. G. Selective packaging of host tRNA's by murine leukemia virus particles does not require genomic RNA. J Virol. 1979 Jan;29(1):328–335. doi: 10.1128/jvi.29.1.328-335.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Penman S. The metabolism of poly (A)+ and poly(A)-hnRNA in cultured Drosophila cells studied with a rapid uridine pulse-chase. Cell. 1977 May;11(1):105–113. doi: 10.1016/0092-8674(77)90321-x. [DOI] [PubMed] [Google Scholar]
- Linial M., Medeiros E., Hayward W. S. An avian oncovirus mutant (SE 21Q1b) deficient in genomic RNA: biological and biochemical characterization. Cell. 1978 Dec;15(4):1371–1381. doi: 10.1016/0092-8674(78)90062-4. [DOI] [PubMed] [Google Scholar]
- Lowy D. R., Rands E., Chattopadhyay S. K., Garon C. F., Hager G. L. Molecular cloning of infectious integrated murine leukemia virus DNA from infected mouse cells. Proc Natl Acad Sci U S A. 1980 Jan;77(1):614–618. doi: 10.1073/pnas.77.1.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellon P., Duesberg P. H. Subgenomic, cellular Rous sarcoma virus RNAs contain oligonucleotides from the 3' half and the 5' terminus of virion RNA. Nature. 1977 Dec 15;270(5638):631–634. doi: 10.1038/270631a0. [DOI] [PubMed] [Google Scholar]
- Naso R. B., Arcement L. J., Wood G., Saunders T. E., Arlinghaus R. B. The cell-free translation of Rauscher leukemia virus RNA into high molecular weight polypeptides. Biochim Biophys Acta. 1975 Mar 10;383(2):195–206. doi: 10.1016/0005-2787(75)90261-0. [DOI] [PubMed] [Google Scholar]
- Panet A., Gorecki M., Bratosin S., Aloni Y. Electron microscopic evidence for splicing of Moloney murine leukemia virus RNAs. Nucleic Acids Res. 1978 Sep;5(9):3219–3230. doi: 10.1093/nar/5.9.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paskind M. P., Weinberg R. A., Baltimore D. Dependence of Moloney murine leukemia virus production on cell growth. Virology. 1975 Sep;67(1):242–248. doi: 10.1016/0042-6822(75)90421-3. [DOI] [PubMed] [Google Scholar]
- Rothenberg E., Donoghue D. J., Baltimore D. Analysis of a 5' leader sequence on murine leukemia virus 21S RNA: heteroduplex mapping with long reverse transcriptase products. Cell. 1978 Mar;13(3):435–451. doi: 10.1016/0092-8674(78)90318-5. [DOI] [PubMed] [Google Scholar]
- Rowe W. P., Pugh W. E., Hartley J. W. Plaque assay techniques for murine leukemia viruses. Virology. 1970 Dec;42(4):1136–1139. doi: 10.1016/0042-6822(70)90362-4. [DOI] [PubMed] [Google Scholar]
- Salden M. H., Selten-Versteegen A. M., Bloemendal H. Translation of Rauscher murine leukemia viral RNA. A model for the function of virus-specific messenger. Biochem Biophys Res Commun. 1976 Sep 20;72(2):610–618. doi: 10.1016/s0006-291x(76)80084-8. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. The genome of human papovavirus BKV. Cell. 1979 Dec;18(4):963–977. doi: 10.1016/0092-8674(79)90209-5. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Linial M. Avian oncovirus mutant (SE21Q1b) deficient in genomic RNA: characterization of a deletion in the provirus. J Virol. 1980 Nov;36(2):450–456. doi: 10.1128/jvi.36.2.450-456.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam G., Bhaduri S., Green M. The virus-specific RNA species in free and membrane-bound polyribosomes of transformed cells replicating murine sarcoma-leukemia viruses. Biochem Biophys Res Commun. 1974 Feb 4;56(3):697–702. doi: 10.1016/0006-291x(74)90661-5. [DOI] [PubMed] [Google Scholar]
- Shields A., Rosenberg N., Baltimore D. Virus production by Abelson murine leukemia virus-transformed lymphoid cells. J Virol. 1979 Aug;31(2):557–567. doi: 10.1128/jvi.31.2.557-567.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer R. H., Penman S. Stability of HeLa cell mRNA in actinomycin. Nature. 1972 Nov 10;240(5376):100–102. doi: 10.1038/240100a0. [DOI] [PubMed] [Google Scholar]
- Stacey D. W., Hanafusa H. Nuclear conversion of microinjected avian leukosis virion RNA into an envelope-glycoprotein messenger. Nature. 1978 Jun 29;273(5665):779–782. doi: 10.1038/273779a0. [DOI] [PubMed] [Google Scholar]
- Stoltzfus C. M., Kuhnert L. K. Evidence for the identity of shared 5'-terminal sequences between genome RNA and subgenomic mRNA's of B77 avian sarcoma virus. J Virol. 1979 Nov;32(2):536–545. doi: 10.1128/jvi.32.2.536-545.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoltzfus C. M., Montgomery J. A. Selective inhibition of avian sarcoma virus protein synthesis in 3-deazaadenosine-treated infected chicken embryo fibroblasts. J Virol. 1981 Apr;38(1):173–183. doi: 10.1128/jvi.38.1.173-183.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo C., Bissell M. J. Antiviral action of a rifamycin derivative: formation Rous sarcoma virus particles deficient in 60 to 70S RNA. J Virol. 1978 Mar;25(3):944–947. doi: 10.1128/jvi.25.3.944-947.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters L. C., Mullin B. C. Transfer RNA into RNA tumor viruses. Prog Nucleic Acid Res Mol Biol. 1977;20:131–160. doi: 10.1016/s0079-6603(08)60471-7. [DOI] [PubMed] [Google Scholar]
- Yakobson E., Weiss R. A. Mutant retrovirus particles package vesicular stomatitis virus mRNA during mixed infection. Virology. 1981 Feb;109(1):183–187. doi: 10.1016/0042-6822(81)90484-0. [DOI] [PubMed] [Google Scholar]