Abstract
Background
In patients with severe malaria, acute respiratory distress syndrome usually develops after the start of drug treatment and is a major cause of death. Its pathogenesis is not well understood.
Methods
Respiratory symptom, spirometry, and gas transfer analyses were performed longitudinally in adults in Papua, Indonesia, with uncomplicated (n = 50) and severe (n = 30) falciparum malaria; normal values were derived from 109 control subjects. Gas transfer was partitioned into its alveolar-capillary membrane (DM) and pulmonary vascular (Vc) components, to characterize the site of impaired gas transfer.
Results
Cough was frequent in both patients with uncomplicated malaria (50%) and those with severe malaria (30%) and resolved by day 14. Reduced midexpiratory flow indicated obstruction of the small airways. Gas transfer was significantly impaired in patients with severe malaria. DM was reduced in patients with severe malaria but not in those with uncomplicated malaria and only returned to normal levels after 2 weeks. In patients with uncomplicated malaria, Vc was reduced at presentation but improved thereafter. In patients with severe malaria, Vc decreased with treatment and was lowest at day 7.
Conclusions
Our results suggest that pulmonary vascular occlusion occurs in both patients with uncomplicated malaria and those with severe malaria, likely from sequestration of both red blood cells (RBCs) and white blood cells. There was also impaired alveolar-capillary membrane function in patients with severe malaria but not in those with uncomplicated malaria. Persistent impairment long after clearance of parasitized RBCs suggests prolonged posttreatment inflammatory alveolar-capillary injury.
Acute respiratory distress syndrome (ARDS) is a major cause of death in adults with severe malaria [1, 2]. In hospital case series of severe malaria, 9%-23% of patients developed pulmonary edema [3-5]. Without ventilatory support for affected patients, the mortality for malaria-associated ARDS reaches at least 80% [2], which is higher than the 15%-30% overall case-fatality rate for severe malaria [1]. Despite this finding, the pathogenesis of ARDS in patients with severe malaria is poorly understood; no previous studies have examined altered pulmonary physiology in patients with severe malaria [2]. Microvascular sequestration of parasitized red blood cells (RBCs) underlies most extrapulmonary organ-specific manifestations of severe falciparum malaria [6, 7]. However, ARDS usually commences during the first 5 days after the start of treatment, when peripheral parasitemia has decreased or disappeared [1, 8-10]. It has therefore been hypothesized that lung injury following treatment of malaria may be predominantly an inflammatory response, rather than a purely microvascular mechanical obstructive phenomenon [11].
In recent pilot studies, disease-related changes in pulmonary function were defined in returned travellers with uncomplicated malaria [11]. Altered lung function was common in both patients with uncomplicated falciparum malaria and those with vivax malaria and included obstruction of the small airways, reduced alveolar ventilation, reduced alveolar gas transfer, and increased pulmonary phagocytic cell activity [11]. Although we have hypothesized that lung function would be impaired to a greater extent in patients with severe malaria, there are no data on the nature, site, or magnitude of impaired lung function in patients with severe malaria. The extent to which impaired gas transfer results from microvascular obstruction, pathological abnormality at the alveolar membrane, or both in patients with uncomplicated malaria and those with severe malaria is not known. Also, there are no data on the effects of malaria on lung function in adult residents of malaria-endemic areas who have past exposure to malaria, nor are there data on whether noninvasive assessments of lung physiology are feasible and reproducible in malaria-endemic settings.
We thus undertook more-detailed studies, including respiratory symptom, spirometry, and gas transfer analyses, in larger numbers of adult subjects with and without severe falciparum malaria in a malaria-endemic area of Papua, Indonesia. We partitioned gas transfer into its alveolar-capillary membrane and pulmonary vascular components, to characterize more closely the site of impairment in gas transfer in patients with uncomplicated malaria and in those with severe malaria. Because lung injury in patients with malaria most commonly develops after the start of antimalarial treatment, we also performed longitudinal assessments of lung function, to examine whether gas transfer would deteriorate with treatment.
PATIENTS, MATERIALS, AND METHODS
The present study was a prospective, longitudinal observational study of altered lung physiology in malaria. It was conducted at Mitra Masyarakat Hospital (RSMM) in Timika, Papua, Indonesia, a lowland region of unstable malaria transmission in which both Plasmodium falciparum and Plasmodium vivax are endemic. All research was conducted in accordance with national and institutional guidelines for human experimentation. The study was explained in Indonesian language, using local health providers and, when necessary, local language translators. Individuals who agreed to participate were provided with a written information sheet in Indonesian and signed a consent form.
Three groups of patients ≥18 years old were enrolled from the outpatient clinic or emergency department: (1) patients with uncomplicated falciparum malaria, defined as P. falciparum parasitemia (>1000 asexual parasites/μL) with documented fever (axillary temperature ≥38°C) or self-reported history of fever during the preceding 48 h, with no other cause present; (2) patients with severe falciparum malaria, defined as P. falciparum parasitemia (>1000 asexual parasites/μL) with normal mental state and ≥1 modified World Health Organization criteria [12] (acute renal failure [creatinine level >265 μmol/L], hyperbilirubinemia with either renal impairment [creatinine level >130 μmol/L] or parasitemia of >100,000 asexual parasites/μL, blackwater fever, or hyperparasitemia [>10% parasitized RBCs]); and (3) healthy control subjects with no history of fever during the preceding 48 h, no parasitemia, no history of current or recent wheeze (within the preceding 12 months), and no history indicating chronic bronchitis (i.e., no cough productive of sputum on most days for ≥3 months/year for ≥2 years). Control subjects were usually well family members of patients. Pregnant women were excluded from all groups. Patients with uncomplicated malaria were required to have a hemoglobin concentration >8 g/dL.
Clinical assessments included those of respiratory signs and symptoms, the history of episodes of malaria, and the length of illness before presentation. Hemoglobin concentration and RBC and white blood cell (WBC) counts were determined by use of a Coulter counter, and parasite counts were determined by use of Giemsa-stained thick film. Cough was recorded as being present on the basis of patient history (either volunteered or elicited in response to direct questioning) with or without observed cough. Treatment of falciparum malaria included 7 days of quinine, in accordance with the standard Indonesian Ministry of Health and RSMM guidelines, either oral (for uncomplicated malaria) or intravenous (for severe malaria). Patients with uncomplicated malaria underwent repeat assessments, at 7 and 14 days after enrollment. Patients with severe malaria were reviewed at days 4, 7, 14, and 28.
Each assessment included questions relating to current cough, recent tobacco consumption, and the measurement of capillary hemoglobin concentration (HemoCue). Tests of lung function at each review included spirometry and measurement of gas transfer (DLCOSB), including its partitioning into membranous (DM) and vascular (Vc) components (see below). Local medical staff (T.H. and E.K.) were trained in the performance and quality assurance of lung function testing; ongoing distant supervision of electronic test data was provided by a respiratory physician (G.P.M.).
Lung function was measured using a pulmonary function testing station (TT544; Morgan Transflow System; Morgan Medical) that had been adapted for portable use. Twice-daily calibration of the flow detector and gas analyzers was performed using a known concentration of a gas mixture of He, CO, and O2.
Spirometry was performed using American Thoracic Society criteria for technique and reproducibility [13]. Values were converted to normal body temperature (37°C), ambient pressure, saturated with water vapor [13]. Forced midexpiratory flow (FEF25-75), forced expiratory flow in 1 s (FEV1), and forced vital capacity (FVC) were measured. Gas transfer and lung volumes were measured using the single-breath apnea technique (DLCOSB) corrected for hemoglobin [14, 15]. This required patients to inhale a known concentration and volume of a mixture of He, CO, and O2, hold their breath for 10 s, and then exhale. The composition of the exhaled mixture was then analyzed.
DLCOSB was partitioned into a pulmonary membrane (DM) and capillary component (encompassing the uptake rate for CO [θCO] and pulmonary capillary volume [Vc]), according to the Roughton-Forster relationship: 1/DLCO = 1/DM+1/(θCO × Vc) [15, 16]. To do this required the measurement of DLCOSB using a gas mixture of high and low concentrations of oxygen, with the mixture containing a high concentration of oxygen being preceded by 5 min of breathing 100% oxygen. Analyses of gas transfer using low concentrations of oxygen were performed at least twice. Results of gas transfer analyses using high and low concentrations of oxygen were only included for analysis if these duplicates agreed to within 10% and if the time holding breath was between 9 and 10 s.
Data were analyzed using Intercooled Stata (version 7.0; Stata Corp). Normal values for spirometry and gas transfer analyses were derived from control subjects, using multivariate linear regression modelling that incorporated age, sex, and height (authors’ unpublished data). Although the majority of malaria cases were seen in highlanders, sufficient numbers of control subjects from both the highlands and the lowlands were recruited, to enable the calculation of predicted values for lung function in each ethnic group.
For spirometry analyses, there was no significant independent association with ethnicity (highland vs. lowland), and ethnicity was not incorporated. Gas transfer regression models, however, also included significant independent associations with ethnicity (highland vs. lowland) and tobacco consumption within the preceding 24 h (authors’ unpublished data). Univariate statistics were used to compare calculated normal lung function values with those for patients with malaria, using Student’s t test for normally distributed data and the Wilcoxon rank sum test for nonparametric data. For univariate analysis of categorical variables, the χ2 test or Fisher’s exact test was used, as appropriate. All statistical tests were 2-sided; P < .05 was taken to indicate statistical significance.
RESULTS
Between January 2002 and February 2004, 80 patients with falciparum malaria were enrolled into the study; 30 had severe malaria. There were 109 control subjects. The proportion of patients who were assessed at baseline, were available for review on follow-up, and had adequate-quality results of lung function tests are listed in table 1. Compared with control subjects, patients with either severe or uncomplicated malaria were less able to perform gas transfer analyses (P < .01). Compared with patients with uncomplicated malaria, patients with severe malaria were also less able to perform spirometry analyses (P = .03), but there was no such difference with gas transfer analyses. Inability to have lung function testing performed was not associated with sex, ethnicity, anemia, or previous antimalarial treatment.
Table 1. Proportion of patients who achieved follow-up, as per the study protocol, and who were able to have adequate spirometry and gas transfer analyses performed.
| Patients with |
|||
|---|---|---|---|
| Variable | Control subjects | Uncomplicated malaria | Severe malaria |
| Reviews | ... | 93 (62) | 68 (45) |
| Spirometry | 80 (73) | 63 (68) | 38 (56) |
| Gas transfer | 67 (61) | 34 (37) | 24 (35) |
NOTE. Data are no. (%) of patients or control subjects.
Baseline demographics
Demographic and clinical details of the patients for whom data on cough or acceptable results of at least 1 lung function test were available are compared in table 2. Patients with malaria were 1.3-fold (95% confidence interval [CI], 1.1-1.5-fold) more likely to come from the highlands, compared with control subjects (P = .01). In total, 14% (3/21) of patients with severe malaria had a hemoglobin level <7 g/dL, compared with 1% (1/108) of control subjects and 4.2% (2/48) of patients with uncomplicated malaria (P = .008). None of the patients with malaria died or developed ARDS.
Table 2. Demographic and clinical characteristics, at presentation, of patients for whom data on cough or at results of least 1 acceptable respiratory function test were available.
| Patients with |
|||
|---|---|---|---|
| Characteristic | Control subjects (n = 109) | Uncomplicated malaria (n = 49) | Severe malaria (n = 28) |
| Age, median (range), years | 27 (18-56) | 23 (18-44) | 24 (17-45) |
| Sex, no. (%) female | 36 (33) | 21 (43) | 10 (36) |
| Ethnic group, no. (%) highlander | 75 (70) | 43 (88) | 26 (93) |
| Current smoker, no. (%) | 41 (38) | 16 (33) | 11 (39) |
| Height, mean (SD), cm | 157.8 (6.4) | 156.6 (6.3) | 157.1 (5.8) |
| Weight, mean (SD), kg | 58.2 (8.1) | 56.1 (8.1) | 55.2 (7.4) |
| Hemoglobin at presentation, mean (SD), g/dL | 12.6 (2.3) | 11.7 (2.2) | 10.0 (2.4) |
| Parasite density, geometric mean (range), parasites/μL | 0 | 14,294 (235-96,360) | 30,125 (19-373,500) |
| Days of fever before presentation, median (IQR), days | 0 | 3 (0-14) | 4 (1-14) |
| History of malaria, no. (%) | 80 (76) | 46 (94) | 16 (57) |
| Treatment before presentation, no. (%) | 31 (30) | 15 (31) | 4 (14) |
NOTE. All patients with severe malaria started quinine before testing for lung function. IQR, interquartile range.
Clinical evidence of respiratory involvement
At presentation, cough was present in 26% (45/175) of patients and control subjects. Smokers were 1.8-fold (95% CI, 1.1-2.9-fold) (P = .02) more likely to complain of a cough, but there was no significant difference in cough rates for other baseline characteristics. Patients with malaria were at a higher risk of reporting a cough than were control subjects. A total of 14% (15/105) of control subjects reported having a cough, compared with 49% (24/49) of patients with uncomplicated malaria and 29% (6/21) of patients with severe malaria (P < .001). The increased risk of cough in patients with uncomplicated malaria continued to day 7 but had resolved by day 14 (figure 1). By day 3, a greater proportion of patients with severe malaria (39%) than control subjects had a cough (P = .02). None of 105 control subjects had a respiratory rate >32 breaths/min, compared with 10% of patients with uncomplicated malaria and 38% of patients with severe malaria (P = .001). By day 3, only 12% (2/17) of patients with severe malaria had a respiratory rate >32 breaths/min, and, by day 7, this number had fallen to zero (0/12). Patients with malaria also had marginally lower room-air oxygen saturation levels at presentation, compared with control subjects (median, 98% [interquartile range {IQR}, 97%-98%] vs. 98% [IQR, 98%-99%]; P = .001). No patient had an oxygen saturation level <95%.
Figure 1.
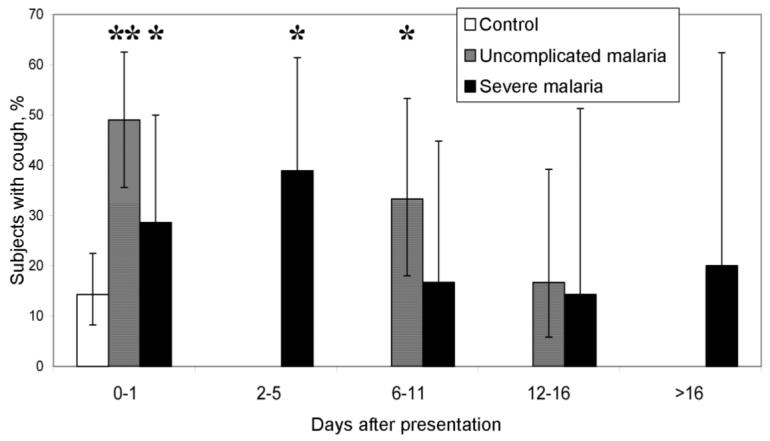
Proportion of patients with falciparum malaria who had cough over time, compared with control subjects. The whiskers denote 95% confidence intervals. *P < .05; **P < .01 (χ2 test).
Lung function tests
Adequate spirometry [13] was performed at presentation on 80 control subjects, 30 patients with uncomplicated malaria, and 12 patients with severe malaria. FEF25-75 in patients with uncomplicated malaria (85% of that predicted from control data [95% CI, 71%-98%]) was significantly lower than that in control subjects (P = .03). By day 7, this difference had resolved (figure 2). There were no significant differences in FEV1 or FVC (data not shown).
Figure 2.
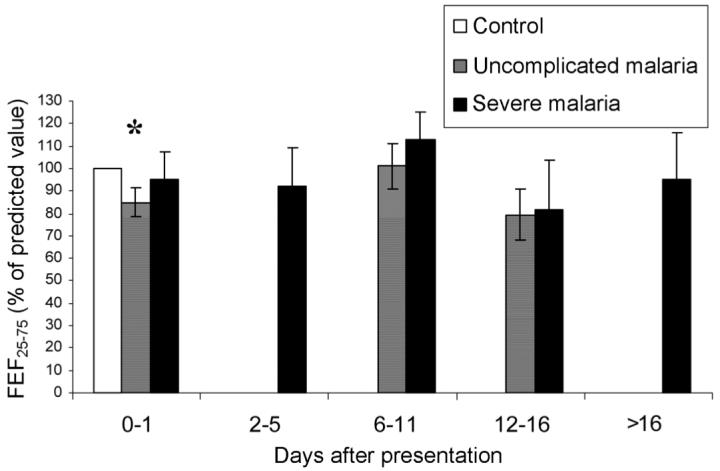
Forced midexpiratory flow (FEF25-75) over time, for patients with falciparum malaria. Whiskers denote SEs. *P < .05.
At presentation, patients with severe malaria had a significant reduction in DLCO (mean, 78% of that predicted from control data [95% CI, 62%-94%]; P = .001), and this difference persisted until after day 14. There was a smaller reduction in DLCO in patients with uncomplicated malaria, which became significant on day 7 and persisted until after day 14 (figure 3A).
Figure 3.
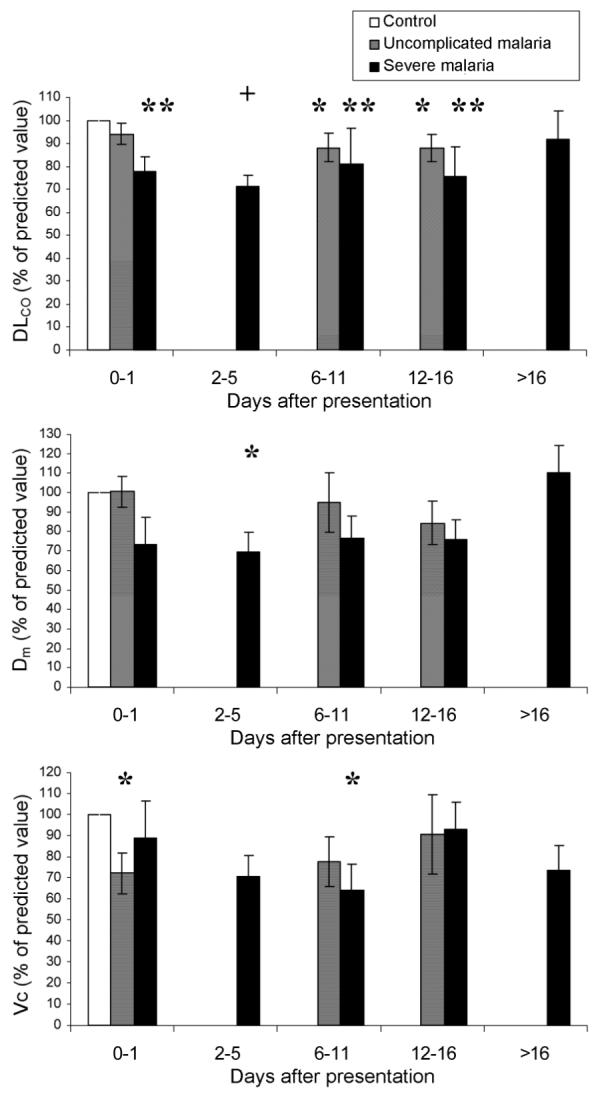
Gas transfer measures over time, for patients with falciparum malaria. Whiskers denote SEs. DLCO, gas transfer; DM, alveolar-capillary membranous component of gas transfer; Vc, pulmonary capillary vascular component of gas transfer. *P < .05; **P < .01; and +P < .001.
Gas transfer was partitioned into its constituent membranous and vascular components (figure 3B and 3C). In patients with uncomplicated malaria, the alveolar-capillary membrane component was not significantly impaired at any stage. In contrast, in patients with severe malaria, the alveolar-capillary membrane component was significantly reduced on day 3 (mean, 69% of that predicted from control data [95% CI, 43%-95%]; P = .02) and returned slowly to normal levels thereafter (figure 3B). In patients with uncomplicated malaria, the pulmonary capillary vascular component was reduced at presentation (mean, 72% of that predicted from control data [95% CI, 51%-93%]; P = .03), but, by day 7, it was not significantly lower than that in control subjects. In contrast, in patients with severe malaria, Vc decreased after treatment and was significantly impaired on day 7, with a gradual improvement apparent on later assessment (figure 3C). There was no significant relationship between WBC count or parasitemia and gas transfer variables in multivariate analysis.
DISCUSSION
Cough was common in both patients with uncomplicated malaria and those with severe malaria. Although cough and respiratory symptoms of falciparum malaria have been well recognized in children residing in malaria-endemic areas [17, 18] and in adult returned travellers [11], the present study has demonstrated that cough is also a dominant presenting symptom in adults residing in malaria-endemic areas. Cough occurred with similar frequency in patients with severe malaria and those with uncomplicated malaria and resolved within 2 weeks of treatment. The respiratory rate at presentation was significantly increased in both patients with uncomplicated malaria and those with severe malaria. This tachypnoea may have been due to several factors, including fever, metabolic acidosis, and lung injury. Lack of hypoxemia is unsurprising, since no patients developed ARDS. On many occasions, patients with severe malaria were too sick to have lung function testing, particularly spirometry, performed. This tended to exclude patients with the most-severe disease. As a result of this, the abnormalities reported are likely to underestimate the derangement in lung function in patients with severe malaria.
The only significant spirometric abnormality was a modest reduction in FEF25-75 in patients who had uncomplicated malaria on presentation that resolved within 7 days. This finding is consistent with our earlier study [11] and indicates the likelihood of pathological abnormality selectively involving and obstructing the small airways [19, 20]. Midexpiratory flow was not statistically significantly lower in patients with severe malaria. This may be explained by the smaller number of patients with severe malaria who were able to perform acceptable spirometry.
A reduction in single-breath diffusion capacity was found in both patients with uncomplicated malaria and those with severe malaria. Cough did not predict gas transfer impairment, suggesting that cough may be associated with irritation of the larger airways, rather than the more-distal pathology that caused the abnormalities of the gas exchanging units noted in the present study.
A range of potential factors may influence gas transfer, including alterations in pulmonary capillary blood flow and hemoglobin concentration, injury to capillary endothelial or alveolar epithelial cells, infiltration of the alveolar space or lung interstitium, and reduced alveolar ventilation [15]. Many of these factors may be operating within the context of falciparum malaria to cause a reduction in gas transfer. Ventilation-perfusion mismatch and matched ventilation and perfusion defects are the most likely functional explanations. Fever alone is an unlikely factor, since the abnormality persisted well after the patients became afebrile and the overall diffusing capacity was not greatly influenced by temperature [21]. Increased cardiac output, as a result of an acute febrile illness, will increase DLCO (including both Vc and DM) [15] and, therefore, does not explain our results. Anemia, which was common in these patients, was taken into account with a correction factor applied in the derivation of DLCO. The abnormal ventilatory capacity, as reflected in the reduction in FEF25-75, suggested an abnormality in the small airways and, thus, some maldistribution in ventilation and ventilation/blood-flow imbalance. However, the airway dysfunction was very mild and, therefore, unlikely to explain the reduction in diffusing capacity. In patients with asthma, in whom airflow obstruction is greater than that in patients with malaria, diffusing capacity and pulmonary capillary blood volume remain normal [22].
The pattern of uncoupling of DM and Vc in patients with uncomplicated malaria has not previously been noted in patients with other diseases [15]. We speculate that the changes in Vc in both patients with uncomplicated malaria and those with severe malaria represent encroachment of the vascular compartment in patients with malaria, causing reduced pulmonary capillary blood flow. This could be caused by pulmonary microvascular sequestration of cytoadherent blood cells, either parasitized RBCs or leukocytes. Leukocyte cytoadherence to the vascular endothelium is unlikely to be the predominant cause of this impairment in Vc in patients with uncomplicated malaria, since this should also cause a parallel decrease in DM [15], which was not found in patients with uncomplicated malaria.
The decrease in Vc with treatment in patients with severe malaria occurred at a time when parasitized RBCs had been cleared and, thus, was unlikely to reflect significant residual microvascular obstruction of pulmonary capillaries by parasitized RBCs. Instead, it likely reflected a posttreatment inflammatory response with enhanced pulmonary vascular WBC sequestration, causing reduced pulmonary vascular blood volume and reduced pulmonary capillary blood flow after the start of treatment. This finding is supported by our earlier studies, which showed a significant increase in WBC phagocytic activity in patients with uncomplicated malaria within 2 days of the start of treatment [11] and by the few autopsy series that have reported detailed pulmonary intravascular histopathological findings after death from severe malaria [6, 8, 23-27]. Intravascular monocytes are seen to a much greater degree in alveolar capillaries than in other organ microvasculature [6, 8, 24] or peripheral blood [24]. Similar findings have also been reported in hamster models of malaria lung injury [28]. Intravascular neutrophils were also seen [6], and further histopathological studies are needed to clarify the relative importance of monocytes and neutrophils in malarial ARDS. The mechanisms leading to this infiltration of leukocytes remain unclear. Parasite-induced endothelial cell activation may cause binding of WBCs to pulmonary endothelial cell-adhesion molecules. Subsequent and additional leukocyte chemotaxis may occur as part of an inflammatory response to parasite products circulating through the lung after the death of parasites in both the systemic extrapulmonary and the local pulmonary microvasculature. Intravascular sequestration of parasitized RBCs is less marked in the lungs than in other organs, at least by the time of death [6, 24]. In contrast to the mature stages predominating in other organs, those found in pulmonary capillaries are more frequently small rings and trophozoites [6, 24]. The reduced Vc was slow to return to normal, which is consistent with a prolonged subclinical inflammatory response in the lungs after recovery from clinical symptoms of severe malaria. Other contributing mechanisms to impaired Vc in malaria could include decreased pulmonary capillary flow from the known reduction in RBC deformability and increased RBC viscosity in severe malaria [29, 30].
Although membrane changes were minimal, compared with changes in Vc in patients with uncomplicated malaria, DM was significantly abnormal in patients with severe malaria and appeared to parallel changes in Vc in patients with severe malaria, with initial deterioration during treatment followed by slow improvement. The DM data indicate pathological abnormality at the alveolar-capillary membrane and that changes in gas transfer in patients with severe malaria are not exclusively caused by intravascular obstruction and reduced pulmonary capillary blood flow. Potential explanations for the reduced DM include endothelial injury and alterations in endothelial permeability that result in interstitial edema. Electron-microscopic findings in patients who died from malaria include endothelial swelling, interstitial edema, and adherence of intravascular monocytes to the capillary endothelium [25, 26]. Cytoadherence of leukocytes to pulmonary vascular endothelium [11] would reduce DM, as well as Vc. Deterioration in DM after treatment is, again, consistent with the inflammatory response being exacerbated by antimalarial treatment in patients with severe malaria and mirrors the time course of lung injury in clinical disease [31]. It is unlikely that the abnormality in DM seen in patients with severe malaria is a consequence of ventilation maldistribution that causes ventilation-perfusion mismatch, since, in patients with uncomplicated malaria, in whom the reduction in FEF25-75 was similar, DM was not reduced.
After consideration of the partitioning of DLCO in patients with malaria, there remains some uncertainty relating to the assumption implied in the derivation of θ, the reaction rate of CO with the red cell. The regression constants proposed by Roughton and Forster for the relationship between θ and oxygen tension for normal blood [16] were used in the present study. However, θ may be influenced by changes in internal RBC viscosity, membrane physicochemical properties [32], RBC shape, and alterations in hemoglobin. To what extent these factors alter θ in falciparum malaria and the influence they would have on DLCO and its partitioned constituents are not known. Further elucidation of this gas transfer defect will require a more detailed understanding of the effect of malaria on CO-RBC kinetics.
It is also not known to what extent differences in endogenous pulmonary CO production may have influenced DLCO in patients with malaria. Expression of heme oxygenase (HO)-1, the enzyme that produces CO, is increased in multiple organs, including the lungs, of children dying from severe malaria [33]. However, levels of carboxyhemoglobin in children admitted to the hospital with uncomplicated malaria were modest and not different from levels found in patients with cerebral malaria (or with other infective and noninfective causes for admission), suggesting that there may not necessarily be an increase in systemic HO activity/CO production with increasing malaria disease severity [34].
In conclusion, the results of the present study suggest that lung injury is likely to be a continuum from subclinical involvement in uncomplicated malaria and severe malaria through to frank ARDS in severe malaria. The pathophysiology of impaired lung function in falciparum malaria is likely to involve more than just pulmonary microvascular obstruction by parasitized RBCs. We speculate that, in patients with severe malaria without acute lung injury, the impairment in gas transfer results from ventilation-perfusion mismatch caused by endovascular obstruction from WBCs, parasitized RBCs, and RBCs with reduced deformability, with or without endothelial injury and interstitial edema. Worsening and persisting abnormality in gas transfer after treatment and beyond the expected time of clearance of parasitized RBCs is consistent with a prolonged posttreatment inflammatory response. Further clarification of the mechanisms of lung injury will require correlation of physiological indices with inflammatory mediators and markers of endothelial/epithelial activation and injury. Greater understanding of the underlying pathophysiology may lead to new treatment options for this grave complication of falciparum malaria and may allow the early identification of patients who may progress from subclinical impairment to ARDS. The noninvasive techniques for assessing pulmonary function used in the present study may prove to be useful in monitoring organ-specific responses to future adjunctive therapies, to prevent and treat lung injury in patients with malaria.
Acknowledgments
We thank Drs. Jeanne Rini and Paulus Sugiarto, for their support and permission to undertake this study at Mitra Masyarakat Hospital.
Financial support: Wellcome Trust-National Health and Medical Research Council (Practitioner Fellowship to N.A. and International Collaborative Research Grant); Tudor Foundation.
Footnotes
Potential conflicts of interest: none reported.
References
- 1.World Health Organization Severe falciparum malaria. Trans R Soc Trop Med Hyg. 2000;94(Suppl 1):S1–90. [PubMed] [Google Scholar]
- 2.Taylor WR, White NJ. Malaria and the lung. Clin Chest Med. 2002;23:457–68. doi: 10.1016/s0272-5231(02)00004-7. [DOI] [PubMed] [Google Scholar]
- 3.Lichtman A, Mohrcken S, Engelbrecht M, Bigalke M. Pathophysiology of severe forms of falciparum malaria. Crit Care Med. 1990;18:666–8. doi: 10.1097/00003246-199006000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Aursudkij B, Wilairatana P, Vannaphan S, Walsh DS, Gordeux VR, Looareesuwan S. Pulmonary edema in cerebral malaria patients in Thailand. Southeast Asian J Trop Med Public Health. 1998;29:541–5. [PubMed] [Google Scholar]
- 5.Bruneel F, Hocqueloux L, Alberti C, et al. The clinical spectrum of severe imported falciparum malaria in the intensive care unit: report of 188 cases in adults. Am J Respir Crit Care Med. 2003;167:684–9. doi: 10.1164/rccm.200206-631OC. [DOI] [PubMed] [Google Scholar]
- 6.Macpherson GG, Warrell MJ, White NJ, Looareesuwan S, Warrell DA. Human cerebral malaria: a quantitative ultrastructural analysis of parasitized erythrocyte sequestration. Am J Pathol. 1985;119:385–401. [PMC free article] [PubMed] [Google Scholar]
- 7.Marchiafava E, Bignami A. On summer-autumnal fever. London: The New Sydenham Society; 1894. [Google Scholar]
- 8.Brooks CMH, Kiel LCFW, Sheehy LCTW, Barry LCKG. Acute pulmonary edema in falciparum malaria. N Engl J Med. 1968;279:732–7. [Google Scholar]
- 9.James MF. Pulmonary damage associated with falciparum malaria: a report of ten cases. Ann Trop Med Parasitol. 1985;79:123–38. doi: 10.1080/00034983.1985.11811899. [DOI] [PubMed] [Google Scholar]
- 10.Martell RW, Kallenbach J, Zwi S. Pulmonary oedema in the falciparum malaria. BMJ. 1979;1:1763–4. doi: 10.1136/bmj.1.6180.1763-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anstey NM, Jacups SP, Cain T, et al. Pulmonary manifestations of uncomplicated falciparum and vivax malaria: cough, small airways obstruction, impaired gas transfer, and increased pulmonary phagocytic activity. J Infect Dis. 2002;185:1326–34. doi: 10.1086/339885. [DOI] [PubMed] [Google Scholar]
- 12.Tran TH, Day NP, Nguyen HP, et al. Controlled trial of artemether or quinine in Vietnamese adults with severe falciparum malaria. N Engl J Med. 1996;335:76–83. doi: 10.1056/NEJM199607113350202. [DOI] [PubMed] [Google Scholar]
- 13.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 14.American Thoracic Society Single-breath carbon monoxide diffusing capacity (transfer factor): recommendations for a standard technique—1995 update. Am J Respir Crit Care Med. 1995;152:2185–98. doi: 10.1164/ajrccm.152.6.8520796. [DOI] [PubMed] [Google Scholar]
- 15.Hughes JM, Bates DV. Historical review: the carbon monoxide diffusing capacity (DLCO) and its membrane (DM) and red cell (Theta.Vc) components. Respir Physiol Neurobiol. 2003;138:115–42. doi: 10.1016/j.resp.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Roughton FJW, Forster RE. Relative importance of diffusion and chemical reaction in determining rate of exchange of gases in the human lung. J Appl Physiol. 1957;11:290–302. doi: 10.1152/jappl.1957.11.2.290. [DOI] [PubMed] [Google Scholar]
- 17.O’Dempsey TJ, McArdle TF, Laurence BE, Lamont AC, Todd JE, Greenwood BM. Overlap in the clinical features of pneumonia and malaria in African children. Trans R Soc Trop Med Hyg. 1993;87:662–5. doi: 10.1016/0035-9203(93)90279-y. [DOI] [PubMed] [Google Scholar]
- 18.Redd SC, Bloland PB, Kazembe PN, Patrick E, Tembenu R, Campbell CC. Usefulness of clinical case-definitions in guiding therapy for African children with malaria or pneumonia. Lancet. 1992;340:1140–3. doi: 10.1016/0140-6736(92)93160-o. [DOI] [PubMed] [Google Scholar]
- 19.Macklem P. The physiology of small airways. Am J Respir Crit Care Med. 1998;157:S181–3. doi: 10.1164/ajrccm.157.5.rsaa-2. [DOI] [PubMed] [Google Scholar]
- 20.Bates D. Respiratory function in disease. 3rd ed. Philadelphia: W.B. Saunders; 1989. [Google Scholar]
- 21.Cander L, Hanowell E. The effects of fever on the pulmonary diffusing capacity and pulmonary mechanics in man. Fed Proc. 1960;19:374. doi: 10.1152/jappl.1963.18.6.1065. [DOI] [PubMed] [Google Scholar]
- 22.Kaminsky DA, Lynn M. Pulmonary capillary blood volume in hyperpnea-induced bronchospasm. Am J Respir Crit Care Med. 2000;162:1668–73. doi: 10.1164/ajrccm.162.5.9911053. [DOI] [PubMed] [Google Scholar]
- 23.Applebaum IL, Shrager J. Pneumonitis associated with malaria. Arch Intern Med. 1944;74:155–62. [Google Scholar]
- 24.Carr RA, Lucas S, Liomba N, et al. Lung pathology in fatal pediatric malaria. Am J Trop Med Hyg. 1999;61(Suppl 1):269–70. [Google Scholar]
- 25.Duarte MI, Corbett CE, Boulos M, Amato Neto V. Ultrastructure of the lung in falciparum malaria. Am J Trop Med Hyg. 1985;34:31–5. doi: 10.4269/ajtmh.1985.34.31. [DOI] [PubMed] [Google Scholar]
- 26.Mackenzie C, Carr RA, Das A, et al. Electron microscopy of the lung in fatal pediatric cerebral malaria. Am J Trop Med Hyg. 1999;61(Suppl 1):269. [Google Scholar]
- 27.Spitz S. The pathology of acute falciparum malaria. Milit Surg. 1946;99:555–72. [PubMed] [Google Scholar]
- 28.MacCallum DK. A study of macrophage-pulmonary vascular bed interactions in malaria-infected hamsters. J Reticendoth Soc. 1969;6:253–70. [PubMed] [Google Scholar]
- 29.Dondorp AM, Angus BJ, Hardeman MR, et al. Prognostic significance of reduced red blood cell deformability in severe falciparum malaria. Am J Trop Med Hyg. 1997;57:507–11. doi: 10.4269/ajtmh.1997.57.507. [DOI] [PubMed] [Google Scholar]
- 30.Dondorp AM, Kager PA, Vreeken J, White NJ. Abnormal blood flow and red blood cell deformability in severe malaria. Parasitol Today. 2000;16:228–32. doi: 10.1016/s0169-4758(00)01666-5. [DOI] [PubMed] [Google Scholar]
- 31.Brooks MH, Kiel FW, Sheehy TW, Barry KG. Acute pulmonary edema in falciparum malaria. N Engl J Med. 1968;279:732–7. [Google Scholar]
- 32.Dondorp AM, Pongponratn E, White NJ. Reduced microcirculatory flow in severe falciparum malaria: pathophysiology and electron-microscopic pathology. Acta Trop. 2004;89:309–17. doi: 10.1016/j.actatropica.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Clark IA, Awburn MM, Harper CG, Liomba NG, Molyneux ME. Induction of HO-1 in tissue macrophages and monocytes in fatal falciparum malaria and sepsis. Malar J. 2003;2:41. doi: 10.1186/1475-2875-2-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunnington AJ, Kendrick SF, Wamola B, Lowe B, Newton CR. Carboxyhemoglobin levels in Kenyan children with Plasmodium falciparum malaria. Am J Trop Med Hyg. 2004;71:43–47. [PubMed] [Google Scholar]


