Abstract
Astrocyte dysfunction and death accompany cerebral ischemia/reperfusion and possibly compromise neuronal survival. Animal studies indicate that neuronal death, neurologic injury, and oxidative molecular modifications are worse in animals exposed to hyperoxic compared to normoxic ventilation during reperfusion after global cerebral ischemia. It is unknown, however, whether ambient O2 affects brain cell survival using in vitro ischemia paradigms where mechanisms of injury to specific cell types can be more thoroughly investigated. This study tested the hypothesis that compared with the supraphysiological level of 20% O2 normally used in cell culture, lower, more physiological O2 levels protect astrocytes from death following oxygen and glucose deprivation. Primary rat cortical astrocytes were cultured under either 7 or 20% O2, exposed to O2, and glucose deprivation for 4 h, and then exposed to normal medium under either 7 or 20% O2. Cell death and 3-nitrotyrosine and 8-hydroxy-2-deoxyguanosine immunoreactivities were assessed at different periods of reoxygenation. Astrocytes exposed to low levels of O2 during reoxygenation undergo less death and exhibit lower levels of protein nitration and nucleic acid oxidation when compared with those under high levels of O2 during reoxygenation. These results support the hypothesis that the 20% O2 normally used in cell culture exacerbates astrocyte death and oxidative stress in an in vitro ischemia/reperfusion model compared to levels that more closely approximate those that exist in vivo.
Keywords: oxidative stress, cerebral ischemia, nitrotyrosine, 8-hydroxydeoxyguanosine
INTRODUCTION
Several studies have demonstrated that when animals are hyperoxic during reperfusion following global cerebral ischemia, brain tissue oxidative stress markers, impaired cerebral energy metabolism, neuronal death, and neurologic impairment are greater than what is observed following normoxic reperfusion (Balan et al., 2006; Liu et al., 1998; Mickel et al., 1987; Richards et al., 2006; Vereczki et al., 2006). These findings have led to the concept that brain tissue oxygenation in excess of what is necessary to saturate metabolic O2 utilization is toxic during early reperfusion, when altered intracellular conditions, e.g., pH, [Ca2+], etc., may either promote the formation of reactive oxygen species (ROS) or inhibit their detoxification (Rosenthal and Fiskum, 2005). While injury to and death of neurons has been the focus of ischemic brain injury research, evidence indicates that astrocytes also undergo dysfunction and delayed death after global cerebral ischemia (Liu et al., 1999; Petito et al., 1998). Moreover, astrocyte death can occur prior to neuronal death and, like neurons, exhibits a spatial pattern of selective vulnerability (Ouyang et al., 2007). Experiments performed in vitro with primary cultures of cortical astrocytes exposed to oxygen and glucose deprivation (OGD) demonstrate that overexpression of copper/zinc superoxide dismutase substantially protects against delayed death (Wang et al., 2005), suggesting that oxidative stress contributes to the pathophysiology of astrocyte cell death during ischemia/reperfusion, as other studies have shown for neurons (Sugawara et al., 2002; Vereczki et al., 2006).
The relevance of studies performed with primary cultures of brain cells to acute brain injury disorders is limited by several factors, including the very different environments in which the cells exist. For instance, cell culture is typically performed under an atmosphere of 95% air and 5% CO2, which is ∼20% O2 (149 mm Hg), corresponding to >250 μM dissolved O2 present in the medium. The intraparenchymal O2 tension present in a normal brain ranges from ∼20–30 mm Hg (Erecinska and Silver, 2001), corresponding to 30–50 μM O2 dissolved O2 in the extracellular fluid. Since cells maintained under normal cell culture O2 tensions are in a severely hyperoxic environment compared with what exists in vivo and since hyperoxia can promote the generation of reactive O2 species (Kudin et al., 2004; Turrens et al., 1982), cells in culture may be particularly sensitive to conditions that promote oxidative stress. Alternatively, exposure of cells to hyperoxia can potentially cause upregulation of antioxidant gene expression, resulting in relative resistance to oxidative stress (Papaiahgari et al., 2004). In light of these considerations, this study was undertaken to determine if the death of cortical astrocytes is influenced by the ambient O2 concentration present either prior to or after exposure to OGD, as an in vitro model of cerebral ischemia. In addition to comparing outcomes at 20% O2 to those at a more physiologically realistic level of 7% O2, astrocytes were exposed to OGD using an ionic shifts solution, which contains protons, Ca2+, and other ions at levels that much more closely resemble those that exist during cerebral ischemia than those present in normal culture medium (Bondarenko and Chesler, 2001). The results of this study support those obtained in vivo with different levels of ventilatory O2 and demonstrate that hyperoxic reoxygenation promotes oxidative stress and exacerbates delayed death of astrocytes after transient O2 and glucose deprivation.
MATERIALS AND METHODS
Materials
DMEM/F12 50/50 culture medium was purchased from Cellgro (Manassas, VA), Fetal bovine serum from HyClone (Logan, UT), penicillin-streptomycin solution from Gemini Bio-Products (West Sacramento, CA), mounting medium from Vector Laboratories, and phosphate buffered saline from Cambrex Bio Science (Walkersville, MD). All other reagents were obtained from Sigma-Aldrich (St. Louis, MO).
Primary Culture of Rat Cortical Astrocytes
Cortical astrocytes were prepared from brains of 1-day-old Sprague-Dawley rat pups as described by (Zielke et al., 1990) using a method based on a procedure by (Booher and Sensenbrenner, 1972) with minor modifications. The cell suspension (enriched in astrocytes) was seeded in 185 cm2 Nunc tissue flasks at a density of 30 mL cell suspension per flask. The cells were maintained in a culture incubator at 37°C in an atmosphere of 95% air/5% CO2, with 90% humidity. The culture medium was replaced after 4 days and twice weekly thereafter.
Experiments were performed on cultures with age between 18 and 21 days in vitro when they reach maximal sensitivity to cell death caused by the combination of O2 and glucose deprivation (Juurlink et al., 1992). Two days before the experiment the cells were replated at the density of 2–2.5 × 105 cells on 25 mm poly-l-lysine coated glass coverslips and maintained in DMEM/F12 media with 5 mM glucose, 10% serum, 1% Pen/Strep at either 20% O2 or 7% O2. All the cultures were grown in regular culture incubators (95% air and 5% CO2) before being replated on coverslips.
The purity of the astrocyte cultures was determined by immunocytochemical staining for glial fibrillary acidic protein (GFAP), a marker for astrocytes, ionized calcium binding adaptor molecule (IBA 1), a marker for microglia, and 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNPase) a marker for oligodendrocytes, and neuron nuclear antigen (NeuN), a marker for neurons. Cells on 25 mm poly-l lysine coated glass coverslips were rinsed twice with potassium phosphate-buffered saline, pH = 7.4, (KPBS) and fixed with 4% paraformaldehyde for 10 min. Fixed cells were incubated with 1% sodium borohydride for 20 min, and then rinsed multiple times until bubbles were eliminated. Cells were incubated with primary monoclonal anti-CNPase mouse antibody (Wako) and polyclonal anti-GFAP antibody rabbit antibody (DakoCytomation) in KPBS +0.4% Triton at 4°C for 48 h. Cells were rinsed and incubated with secondary donkey anti-mouse (Alexa Fluor 488, Invitrogen) and goat anti-rabbit (Alexa Fluor, 546, Invitrogen) 1 h at room temperature (RT). For IBA 1/GFAP co-staining, cells were incubated with primary rabbit anti-IBA1 antibody in KPBS +0.4%Triton at 4°C. After 48 h, the cells were rinsed and incubated with secondary goat anti-rabbit (Alexa Fluor 594, Invitrogen). Rinsed cells were blocked with 1% normal rabbit serum (KPL) for 1 h followed by incubation with goat F(ab′)2 anti-rabbit antibodies for 1 h at RT. The cells were incubated overnight with primary polyclonal anti-GFAP rabbit antibody (DakoCytomation). After being rinsed, the cells were incubated with secondary donkey anti-rabbit (Alexa Fluor 488, Invitrogen) for 1 h at RT. Coverslips were washed and mounted on glass slides using VectaShield mounting medium (Vector Laboratories). Contamination of the astrocyte cell cultures was determined to be <5% microglia and <2% oligodendrocytes or neurons and was not different when cells were cultured at 7 or 20% ambient O2 prior to the experiments.
Oxygen and Glucose Deprivation (OGD)
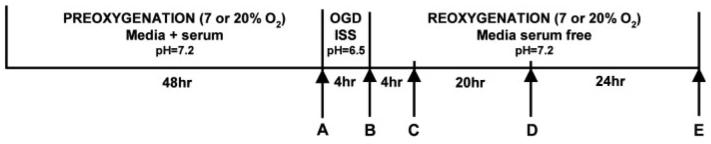
Primary cultures of rat cortical astrocytes were deprived of O2 and glucose by changing the culture medium to glucose-free pH 6.55 “ionic shift” solution (ISS), containing NaCl (39 mM), Na-gluconate (11 mM), K-gluconate (65 mM), n-methyl-D-glucamine-Cl (38 mM), NaH2PO4 (1 mM), CaCl2 (0.13 mM), MgCl2 (1.5 mM), Bis-Tris (10.5 mM) with HCl used to adjust the pH to 6.55. The ISS was deoxygenated by 24-h preincubation in the anaerobic chamber. The Bis-Tris was used as buffer considering its high buffer capacity at pH 6.5 (pKa = 6.5 ± 0.15). Compared with normal culture medium, ISS has reduced pH, lower concentrations of Ca2+, Na+, Cl−, and increased K+, to model changes in the extracellular milieu during ischemia, as described by (Bondarenko and Chesler, 2001).The coverslips were placed in an anaerobic chamber (Forma Scientific Model 1025) under an atmosphere of 10% H2, 5% CO2, and 85% N2. The levels of O2 and H2 inside the chamber were monitored by using a Monitor Analyzer (Coy Laboratory Products) and values of <1 part O2 per million and H2 at between 5 and 6% were considered acceptable. The dissolved O2 concentration of the ISS solution was measured using CHEMet test (CHEMetrics, VA) that employs the Rhodazine D method. The values of the dissolved oxygen in deoxygenated ISS were between 10 and 40 parts per billion, equivalent to 0.32–1.28 μM. After washing the cells twice with deoxygenated ISS, they were incubated in the anaerobic chamber for 4 h. At the end of this period, cells were removed from the anaerobic environment, the ISS was replaced with serum free medium (DMEM/F12) containing 5 mM glucose, and the cultures placed in an incubator under either 95% air (20% O2)/5% CO2 or 7% O2/5% CO2/88% N2. In addition to varying the O2 tension following OGD, in some experiments cells were also maintained in culture for 48 h prior to OGD at 7% O2. Control experiments were performed with cells maintained under identical conditions before, during, and after OGD except that they were maintained during the sham OGD in serum free medium that contained 5 mM glucose. The experimental paradigm is shown schematically in Fig. 1.
Fig. 1.
Astrocyte oxygen/glucose deprivation (OGD) paradigm. Rat cortical astrocyte cultures were replated onto 25-mm glass coverslips and maintained for a further 48 h at either 20 or 7% oxygen. Cells were then exposed to OGD in a glucose free ionic shift solution (ISS) with a medium free [O2] as described in Methods. OGD was terminated by removing the cultures from the anaerobic chamber, replacing the ISS with DMEM/F12 serum free medium containing 5 mM glucose and transferring the cells to incubators at either 7 or 20% O2. The experiments were terminated at 4 h reoxygenation for measurements of protein and DNA/RNA oxidation, or at 0, 24, or 48 h reoxygenation for measurements of cell death. Control cultures were exposed to serum free medium under 7 or 20% O2 during the same period as OGD cultures. In some experiments, cells were exposed to only glucose deprivation or O2 deprivation during the same period as OGD cultures. A–E represents the times when the cells were collected and analyzed.
In some experiments, astrocytes were exposed to either glucose deprivation (GD) or O2 deprivation (OD) alone. For GD, cells were cultured only under normal 95% air/5% CO2 before, during, and after a 4 h exposure to ISS minus glucose. All other conditions were the same as for the OGD experiments. For OD, cells were cultured before and after OD under 95% air/5% CO2. During OD, the ISS medium contained 10 mM glucose. All other conditions were the same as for the OGD experiments.
Cell Death Measurements
Astrocyte cell death was assessed by using the membrane impermeable fluorescent dye propidium iodide (PI; 50 μg/mL) to label dead cells, and the cell permeable fluorescent dye Hoechst 33258 (35 μg/mL) to label all cells. Astrocytes were exposed to both dyes for 20 min at 37°C, fixed in ice-cold paraformaldehyde for 10 min, washed with phosphate buffered saline, and mounted with VectaShield mounting medium. Fluorescence was observed with a 20× objective lens using a Nikon Eclipse E800 fluorescence microscope and images were captured with a SPOT camera. Merged PI/Hoechst images were used for cell counting performed on three random fields per coverslip (between 500 and 700 cells/field). Nuclei of viable cells were observed as blue intact nuclei. Red round nuclei (PI-positive cells) and fragmented (or condensed) blue nuclei were considered as dead cells. The number of dead cells is expressed as percentage of the total Hoechst-stained cells.
Oxidative Modification of Protein and Nucleic Acids
Fluorescence immunocytochemistry was performed on cell cultures on coverslips. After 4 h reoxygenation, the coverslips were rinsed twice with 0.05 M potassium phosphate buffered saline (KPBS), pH 7.4, and fixed with 4% paraformaldehyde for 10 min. Fixed cells were incubated with 1% sodium borohydride solution for 20 min and then rinsed multiple times until bubbles were eliminated. Rinsed cells were incubated with primary rabbit anti-3-nitrotyrosine antibody (Upstate, NY) or goat 8-hydroxydeoxy-2-guanosine (8OHdG) antibody (Chemicon) in KPBS +0.4% Triton at 4°C. After 48 h, cells were rinsed and incubated with secondary goat anti-rabbit (Alexa Fluor 555, Invitrogen) and donkey anti-goat antibody (Alexa Fluor 546, Invitrogen) 1 h at RT. The coverslips were washed, and then incubated in Hoechst and mounted on glass slides using VectaShield mounting medium (Vector laboratories, CA). The fluorescence was observed with a 20× objective lens, using a computer-assisted image analyzer consisting of a Nickon Eclipse E800 fluorescence microscope, a CCD digital camera (Biovision Technologies) and an IBM computer. Images were acquired on a Spot Advanced camera. The excitation wavelength range was 530–550 nm and fluorescence emission was measured at 565 nm.
For quantification, the coverslips double stained with antibodies against anti-3-nitrotyrosine or 8OHdG and Hoechst were analyzed with a computer-assisted image analyzer described above using the MetaMorph software. Three independent images were obtained from each individual coverslip, with 5–7 coverslips per experimental condition. The relative fluorescence for each image was calculated by dividing the integrated intensity by the number of cells present in the field. The value for each of the 5–7 experiments represents the average of the values for the three images.
Statistics
Results are presented as means ± SEM for three to seven independent experiments. Where indicated, statistical analyses used analysis of variance (ANOVA) followed by Student-Newman-Keuls or Holm-Sidak tests for multiple comparisons, with P < 0.05 considered significant.
RESULTS
Astrocyte Cell Death Following O2 and Glucose Deprivation
Pilot experiments using different periods of O2 and glucose deprivation established that 4 h exposure to OGD resulted in no immediate cell death but substantial delayed death (30–40%) of astrocytes at 24–48 h after reoxygenation plus glucose (see Fig. 2). This 4 h period was used in all subsequent experiments to model the primarily delayed brain cell death that occurs following transient global cerebral ischemia.
Fig. 2.
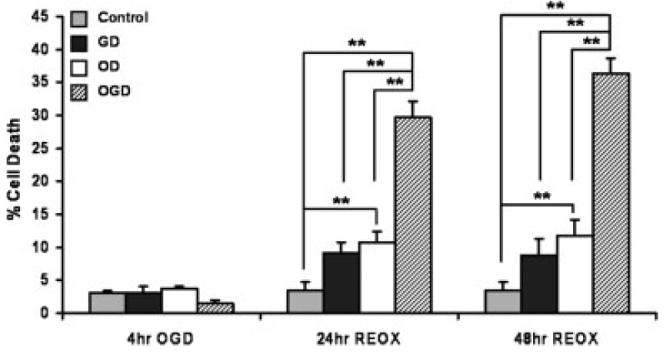
Effect of glucose deprivation (GD), oxygen deprivation (OD) and oxygen/glucose deprivation (OGD) on astrocyte cell death. Astrocytes maintained at 20% O2 were incubated for 4 h under 20% O2 in ISS medium with no glucose (GD), under anaerobic conditions in deoxygenated ISS plus 10 mM glucose (OD), or in deoxygenated ISS with no glucose (OGD). The percentage of cells that died was determined after the initial 4 h exposure and at 24 and 48 h of subsequent culture under 20% O2 in serum-free normal medium containing glucose. Results are expressed as means ± SEM from n = 4–7 different cell culture preparations. Data were analyzed using one-way ANOVA and Student Newman-Keuls post-hoc test. **P < 0.001
To investigate the effect of OGD on astrocyte cell survival, we first compared cell death after OGD to that observed after either O2 deprivation (OD) or glucose deprivation (GD) alone (see Fig. 2). Less than 5% cell death was observed immediately following 4 h OD, GD, or OGD, which was not significantly different than the death observed in the control cells. At both 24 and 48 h reoxygenation after OGD, 30–40% cell death was observed, which was significantly greater that death in control cells (<5%), cells exposed to GD (10%), or to cells following OD (10–15%). Although a small but significant increase in cell death occurred 24–48 h after 4 h OD but not GD, it is clear that the much greater death seen after OGD is the consequence of combined O2 and glucose deprivation rather than either condition alone.
Reducing O2 During Reoxygenation Protects Astrocytes from Death Following O2 and Glucose Deprivation
Establishment of an astrocyte OGD model that results in substantial delayed cell death with little or no acute death allows for testing the role of O2 levels present before and after OGD on outcome. Studies by Vereczki et al. (2006) and Balan et al. (2006) using an animal model of cardiac arrest and resuscitation indicate that early, postischemic hyperoxia aggravates neuronal injury and cell death, compared to what is observed using normoxic resuscitation. We therefore tested the hypothesis that the level of ambient O2 to which astrocytes are exposed in vitro affects cell death after OGD. We performed experiments using groups representing four sets of conditions. For two groups, we maintained the same O2 concentration before the insult and during reoxygenation as follows: (20/20%) and (7/7%). For another two groups, we changed the level of O2 during reoxygenation compared to what was present prior to OGD: (20/7%) and (7/20%).
As shown in Fig. 3, when astrocytes were exposed to 7% O2 before and after OGD (7/7), cell death measured at 48 h was significantly reduced by more than 50% compared to that observed with cells exposed to 20% O2 before and after OGD (20/20). As it is possible that the levels of O2 present for 48 h prior to OGD could be responsible for this difference, we also compared death observed in the 20/7 and 7/20 groups. When compared to the ∼60% death observed in the 20/20 group, the death in the 20/7 group was significantly lower whereas the death in the 7/20 group was not significantly different. While there was a trend toward cytoprotection in the 7/7 group compared with the 7/20 group, the difference was not statistically significant. Although not shown, the acute death observed after OGD alone was not different for cells preincubated at either 20 or 7% O2. In summary, these data indicate that the level of O2 present during reoxygenation after OGD is the primary determinant of the extent of cell death rather than the O2 present prior to the insult.
Fig. 3.
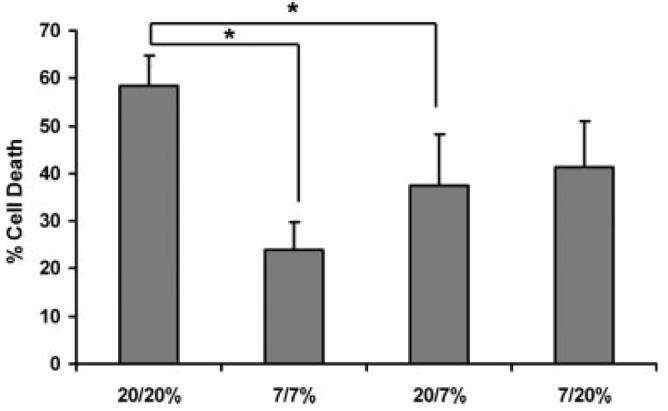
Effect of different atmospheric oxygen levels present before and after OGD on delayed astrocyte cell death. Astrocytes were maintained at 20% O2 or exposed to 7% O2 48 h before 4 h OGD. Reoxygenation was performed at 7 or 20% O2, generating four experimental combinations of pre- and post-OGD oxygenation conditions: 7/7, 7/20, 20/7, 20/20. Death of control cells was between 3.5 and 6.9% for all four experimental groups (not shown). Values are the means ± SEM from 3–5 independent experiments. Data were analyzed using one-way ANOVA and Holm-Sidak post hoc test. *P < 0.01
Reduced Ambient O2 Protects Astrocytes from Oxidative Modifications to Proteins and DNA
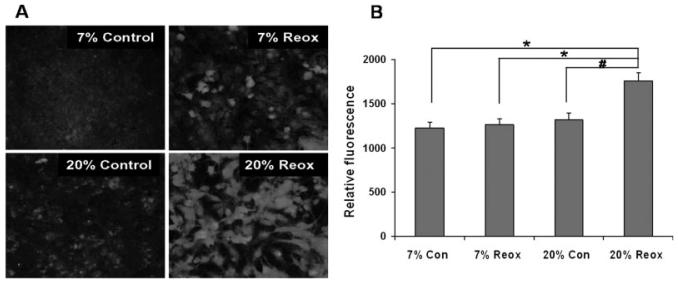
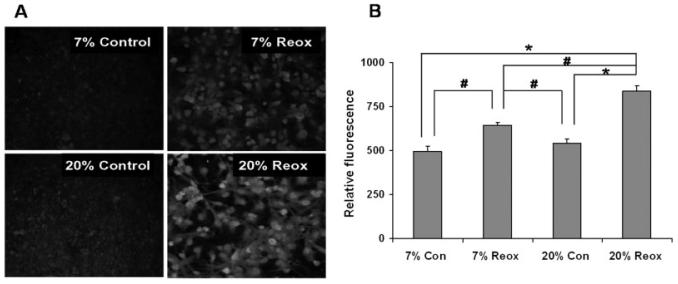
To test the hypothesis that differences in oxidative stress accompany the effects of reoxygenation O2 levels on astrocyte cell death after OGD, we used immunocytochemical staining for 3-nitrotyrosine (3-NT) as a marker of oxidative protein alteration and staining for 8-hydroxy-2-deoxyguanosine (8OHdG) as a marker of DNA/RNA oxidation. Figures 4 and 5 provide both representative images and quantification of 3-NT and 8-OHdG immunostaining, respectively, of astrocytes cultured under 7 or 20% O2 both before and after 4 h OGD. 3-NT immunostaining in cells cultured normally at 7 or 20% O2 was not significantly different (Fig. 4B). Immunostaining was also not different at 4 h reoxygenation after OGD under 7% O2 compared to normal cells under 7% O2. Immunostaining was significantly elevated, however, after reoxygenation under 20% O2, compared to reoxygenation at 7% O2 or to normal cells under 20% O2. As shown in Fig. 5B, the 8OHdG immunoreactivity observed in normal astrocytes cultured under 7 and 20% O2 was not different. In contrast to 3-NT immunostaining, 8OHdG immunoreactivity at 4 h reoxygenation under 7% O2 was greater than that observed with normal cells at 7% O2. As expected, this elevation after reoxygenation was also observed with cells under 20% O2. Most importantly, 8OHdG immunostaining after reoxygenation was significantly higher for cells under 20% compared with 7% O2.
Fig. 4.
Nitrotyrosine immunostaining of cortical astrocytes 4 h after OGD. Astrocyte cultures were maintained at either 20 or 7% O2 both before and after OGD. Cells were stained using 3-NT antibodies after 4 h reoxygenation. A: Representative images of immunofluorescence. B: Quantitative comparison of immunocytochemical fluorescence. Relative fluorescence values were obtained as described in Materials and Methods and are expressed as the means ± SEM from n = 6–7 different experiments. Data were analyzed using one-way ANOVA with Holm-Sidak post hock test. *P < 0.01, #P < 0.05
Fig. 5.
Immunocytochemical staining of DNA oxidation in cortical astrocytes 4 h after OGD. Astrocyte cultures were maintained at either 20 or 7% O2 both before and after OGD. Cells were stained using antibodies to 8OHdG after 4 h reoxygenation. A: Representative images of immunofluorescence. B: Quantitative comparison of immunocytochemical fluorescence. Relative fluorescence values were obtained as described in Materials and Methods and are expressed as the means ± SEM from n = 4–5 different experiments. Data were analyzed using one-way ANOVA with Holm-Sidak post hock test. *P < 0.01, #P < 0.05
DISCUSSION
The most important conclusion drawn from this study is that levels of ambient O2 ranging from what is normally used for cell culture (20%) to a level more consistent with what exists in vivo (7%) directly influence the sensitivity of astrocytes to death induced by transient exposure to O2 and glucose deprivation. While oxidative stress has been demonstrated to play a role in astrocyte death after OGD, this study is the first to demonstrate that the level of O2 present during reoxygenation of cells in vitro is an important determinant of outcome. This conclusion is based on the finding that when cells were cultured under 20% O2 prior to OGD, delayed cell death was significantly greater using reoxygenation at 20% compared to 7% O2. When cells were cultured under 7% O2 prior to OGD, there was a trend toward more cell death after reoxygenation at 20% compared to 7% O2. There was also a trend for greater cell death after reoxygenation at 20% O2 when pre-oxygenated at 7% compared to 20% O2. Although there was no difference in the background death of cells cultured at 7 or 20% O2, this observation could be explained by the possibility that cells experience greater oxidative stress when cultured at 20% compared to 7% O2, resulting in increased vulnerability to reoxygenation injury.
The conclusion that ambient O2 during reoxygenation affects cell death through promotion of oxidative stress is supported by observations that both 3-nitrotyrosine and 8-hydroxy-2-(deoxy)guanosine immunoreactivities were significantly higher at 20% compared to 7% O2 at an early period of reoxygenation (4 h), prior to when cell death matures. While our experiments do not prove a causative role for these particular forms of molecular modifications in reoxygenation-induced cell death, many other studies have demonstrated that protein, DNA, and RNA oxidation can trigger cell death cascades (Luo et al., 2007; Ohtaki et al., 2007; Pehar et al., 2002; Shan et al., 2007). Although immunostaining for 8OHdG is commonly ascribed to nuclear DNA oxidation, studies also indicate that it can reflect oxidation of both RNA and mitochondrial DNA (Clayton, 1982; Zhang et al., 1999). Studies are in progress to further identify the species and intracellular location of oxidized nucleic acids observed in these experiments.
The importance of our results obtained with an in vitro model of cerebral ischemia and reperfusion relates to the influence of astrocyte injury and death over neurologic outcome in acute neurodegenerative disorders. Astrocytes are integrally involved in metabolic and ionic homeostasis, inflammatory responses, and control of extracellular glutamate levels (Aas et al., 1993; Hertz and Zielke, 2004; Hertz et al., 1999; Storm-Mathisen et al., 1992; Torp et al., 1994). Astrocytes also produce neurotrophic factors that promote neuronal survival and provide neurons with precursors for glutathione biosyn-thesis, which is necessary to combat oxidative stress (Dringen, 2000; Drukarch et al., 1997; Makarov et al., 2002). Moreover, astrocytes release lactate, which is then used by neighboring neurons as fuel for aerobic energy metabolism (Dienel and Hertz, 2001) particularly following cerebral ischemia (Schurr, 2002; Pellerin et al., 2007). Therefore, the death or dysfunction of astrocytes in acute brain injury may compromise neuronal metabolism and survival, thereby contributing to neurologic impairment (Chen and Swanson, 2003; O'Malley et al., 1992; Takeshima et al., 1994).
While evidence indicates that substantial astrocyte death occurs following global cerebral ischemia, particularly in the hippocampal CA1 region known for its selective neuronal vulnerability, the effects of environmental factors, e.g., pH and O2 tension, on ischemic astrocyte cell death have not been studied. The fact that we previously found that hippocampal neuronal death following cardiac arrest is profoundly exacerbated by hyperoxic compared to normoxic resuscitation provided an additional incentive for testing the effects of ambient O2 on astrocyte cell death in an in vitro model of ischemia/reperfusion (Balan et al., 2006; Vereczki et al., 2006). A relatively high level of 20% ambient O2 was used in our experiments because it is the normal level of O2 used for maintaining cells in culture and for most cell death paradigms performed in vitro. This concentration of atmospheric O2 is equivalent to 149 mm Hg and results in a culture medium O2 concentration of ∼250 μM. Normal brain tissue pO2 ranges from around 20–30 mm Hg and can reach 75 mm Hg only when systemic arterial pO2 levels are in excess of 300 mm Hg due to ventilation of 100% O2. Therefore, the level of O2 to which cells are exposed under 95% air, 5% CO2 creates an extremely hyperoxic environment compared to what exists within the brain or, indeed, most other tissues. While even the 7% ambient O2 used for our comparative experiments corresponds to a physiologically high level of around 50 mm Hg, this was used to avoid the possibility that hypoxic conditions could be generated in the culture wells by astrocyte O2 consumption. In the absence of accurate O2 measurements at the level of the astrocyte surface, we can therefore only conclude that the 20% O2 condition corresponds to physiologic hyperoxia and that the 7% condition is closer to physiologic normoxia. Given this limitation, we can still conclude that the effects of O2 levels during reoxygenation after OGD on astrocyte oxidative stress and subsequent death are qualitatively similar to what we have observed for oxidative stress and delayed neuronal death during reperfusion after global cerebral ischemia.
The finding that ambient O2 has a substantial effect on outcome in this in vitro model of ischemia/reperfusion also indicates that the influence of other common environmental factors, e.g., concentrations of glucose, CO2, and nitric oxide, should be examined similarly. For instance, while the glucose concentrations used in our experiments are similar to those commonly employed in other models of ischemic cell death, they are higher than those present in the brain interstitium. This may result in excessive glycogen deposition, which could explain why 4 h of glucose deprivation in the presence of O2 resulted in no significant cell death. Future studies varying both glucose and O2 concentrations might also provide insight into the possible interactions between pre-ischemic hyperglycemia and postischemic oxygenation.
Oxygen is the common substrate for the generation of superoxide and nitric oxide and can therefore be the limiting factor in the production of ROS under conditions where the activities of ROS-generating processes is not saturated by the O2 concentration. The current study performed in vitro using transient OGD taken together with the in vivo studies using transient global cerebral ischemia and reperfusion indicate that avoidance of hyperoxia during reoxgenation is an effective approach to reducing prelethal oxidative stress and subsequently improving brain cell survival. Caution should be taken in applying these results to other forms of brain injury, e.g., ischemic stroke and head trauma, as systemic hyperoxia may result in brain tissue normoxia and improved outcome under conditions that can exist in these disorders where tissue oxygenation can limit aerobic cerebral energy metabolism (Menzel et al., 1999; Singhal et al., 2005).
ACKNOWLEDGMENTS
We wish to thank Ms. Jennifer Racz, Dr. Tibor Kristian, and Dr. Carol Zielke for their assistance in these studies.
Grant sponsor: NIH; Grant numbers: NS34152, HD16596.
REFERENCES
- Aas JE, Berg-Johnsen J, Hegstad E, Laake JH, Langmoen IA, Ottersen OP. Redistribution of glutamate and glutamine in slices of human neocortex exposed to combined hypoxia and glucose deprivation in vitro. J Cereb Blood Flow Metab. 1993;13:503–515. doi: 10.1038/jcbfm.1993.65. [DOI] [PubMed] [Google Scholar]
- Balan IS, Fiskum G, Hazelton J, Cotto-Cumba C, Rosenthal RE. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke. 2006;37:3008–3013. doi: 10.1161/01.STR.0000248455.73785.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondarenko A, Chesler M. Rapid astrocyte death induced by transient hypoxia, acidosis, and extracellular ion shifts. Glia. 2001;34:134–142. doi: 10.1002/glia.1048. [DOI] [PubMed] [Google Scholar]
- Booher J, Sensenbrenner M. Growth and cultivation of dissociated neurons and glial cells from embryonic chick, rat and human brain in flask cultures. Neurobiology. 1972;2:97–105. [PubMed] [Google Scholar]
- Chen Y, Swanson RA. Astrocytes and brain injury. J Cereb Blood Flow Metab. 2003;23:137–149. doi: 10.1097/01.WCB.0000044631.80210.3C. [DOI] [PubMed] [Google Scholar]
- Clayton DA. Replication of animal mitochondrial DNA. Cell. 1982;28:693–705. doi: 10.1016/0092-8674(82)90049-6. [DOI] [PubMed] [Google Scholar]
- Dienel GA, Hertz L. Glucose and lactate metabolism during brain activation. J Neurosci Res. 2001;66:824–838. doi: 10.1002/jnr.10079. [DOI] [PubMed] [Google Scholar]
- Dringen R. Metabolism and functions of glutathione in brain. Prog Neurobiol. 2000;62:649–671. doi: 10.1016/s0301-0082(99)00060-x. [DOI] [PubMed] [Google Scholar]
- Drukarch B, Schepens E, Jongenelen CA, Stoof JC, Langeveld CH. Astrocyte-mediated enhancement of neuronal survival is abolished by glutathione deficiency. Brain Res. 1997;770:123–130. doi: 10.1016/s0006-8993(97)00790-7. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. Tissue oxygen tension and brain sensitivity to hypoxia. Respire Physiol. 2001;128:263–276. doi: 10.1016/s0034-5687(01)00306-1. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: Glutamate producers for neurons. J Neurosci Res. 1999;57:417–428. [PubMed] [Google Scholar]
- Hertz L, Zielke HR. Astrocytic control of glutamatergic activity: Astrocytes as stars of the show. Trends Neurosci. 2004;27:735–743. doi: 10.1016/j.tins.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Juurlink BH, Hertz L, Yager JY. Astrocyte maturation and susceptibility to ischaemia or substrate deprivation. Neuroreport. 1992;3:1135–1137. doi: 10.1097/00001756-199212000-00026. [DOI] [PubMed] [Google Scholar]
- Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS. Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem. 2004;279:4127–4135. doi: 10.1074/jbc.M310341200. [DOI] [PubMed] [Google Scholar]
- Liu D, Smith CL, Barone FC, Ellison JA, Lysko PG, Li K, Simpson IA. Astrocytic demise precedes delayed neuronal death in focal ischemic rat brain. Brain Res Mol Brain Res. 1999;68:29–41. doi: 10.1016/s0169-328x(99)00063-7. [DOI] [PubMed] [Google Scholar]
- Liu Y, Rosenthal RE, Haywood Y, Miljkovic-Lolic M, Vanderhoek JY, Fiskum G. Normoxic ventilation after cardiac arrest reduces oxidation of brain lipids and improves neurological outcome. Stroke. 1998;29:1679–1686. doi: 10.1161/01.str.29.8.1679. [DOI] [PubMed] [Google Scholar]
- Luo J, Wang Y, Chen X, Chen H, Kintner DB, Shull GE, Philipson KD, Sun D. Increased tolerance to ischemic neuronal damage by knockdown of Na+-Ca2+ exchanger isoform 1. Ann NY Acad Sci. 2007;1099:292–305. doi: 10.1196/annals.1387.016. [DOI] [PubMed] [Google Scholar]
- Makarov PR, Wiswedel I, Augustin W, Schild L. Hypoxia/reoxygenation-induced damage to mitochondrial activity is determined by glutathione threshold in astroglia-rich cell cultures. Brain Res. 2002;933:91–97. doi: 10.1016/s0006-8993(02)02246-1. [DOI] [PubMed] [Google Scholar]
- Menzel M, Doppenberg EM, Zauner A, Soukup J, Reinert MM, Bullock R. Increased inspired oxygen concentration as a factor in improved brain tissue oxygenation and tissue lactate levels after severe human head injury. J Neurosurg. 1999;91:1–10. doi: 10.3171/jns.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- Mickel HS, Vaishnav YN, Kempski O, von Lubitz D, Weiss JF, Feuerstein G. Breathing 100% oxygen after global brain ischemia in Mongolian Gerbils results in increased lipid peroxidation and increased mortality. Stroke. 1987;18:426–430. doi: 10.1161/01.str.18.2.426. [DOI] [PubMed] [Google Scholar]
- O'Malley EK, Sieber BA, Black IB, Dreyfus CF. Mesencephalic type I astrocytes mediate the survival of substantia nigra dopaminergic neurons in culture. Brain Res. 1992;582:65–70. doi: 10.1016/0006-8993(92)90317-3. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Takeda T, Dohi K, Yofu S, Nakamachi T, Satoh K, Hiraizumi Y, Miyaoka H, Matsunaga M, Shioda S. Increased mitochondrial DNA oxidative damage after transient middle cerebral artery occlusion in mice. Neurosci Res. doi: 10.1016/j.neures.2007.04.005. in press. [DOI] [PubMed] [Google Scholar]
- Ouyang YB, Voloboueva LA, Xu LJ, Giffard RG. Selective dysfunction of hippocampal CA1 astrocytes contributes to delayed neuronal damage after transient forebrain ischemia. J Neurosci. 2007;27:4253–4260. doi: 10.1523/JNEUROSCI.0211-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaiahgari S, Kleeberger SR, Cho HY, Kalvakolanu DV, Reddy SP. NADPH oxidase and ERK signaling regulates hyperoxia-induced Nrf2-ARE transcriptional response in pulmonary epithelial cells. J Biol Chem. 2004;279:42302–42312. doi: 10.1074/jbc.M408275200. [DOI] [PubMed] [Google Scholar]
- Pehar M, Martinez-Palma L, Peluffo H, Kamaid A, Cassina P, Barbeito L. Peroxynitrite-induced cytotoxicity in cultured astrocytes is associated with morphological changes and increased nitrotyrosine immunoreactivity. Neurotox Res. 2002;4:87–93. doi: 10.1080/10298420290015818. [DOI] [PubMed] [Google Scholar]
- Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ. Activity-dependent regulataion of energy metabolism by astrocytes: An update. Glia. 2007;55:125–1262. doi: 10.1002/glia.20528. [DOI] [PubMed] [Google Scholar]
- Petito CK, Olarte JP, Roberts B, Nowak TS, Jr., Pulsinelli WA. Selective glial vulnerability following transient global ischemia in rat brain. J Neuropath Exp Neurol. 1998;57:231–238. doi: 10.1097/00005072-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Richards EM, Rosenthal RE, Kristian T, Fiskum G. Postischemic hyperoxia reduces hippocampal pyruvate dehydrogenase activity. Free Radic Biol Med. 2006;40:1960–1970. doi: 10.1016/j.freeradbiomed.2006.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal RE, Fiskum G. Oxygen: Could there be too much of a good thing? Hosp Med. 2005;66:76–77. doi: 10.12968/hmed.2005.66.2.17547. [DOI] [PubMed] [Google Scholar]
- Schurr A. Lactate, glucose and energy metabolism in the ischemic brain. Int J Mol Med. 2002;10:131–136. [PubMed] [Google Scholar]
- Shan X, Chang Y, Lin CL. Messenger RNA oxidation is an early event preceding cell death and causes reduced protein expression. FASEB J. 2007;21:2753–2764. doi: 10.1096/fj.07-8200com. [DOI] [PubMed] [Google Scholar]
- Singhal AB, Benner T, Roccatagliata L, Koroshetz WJ, Schaefer PW, Lo EH, Buonanno FS, Gonzalez RG, Sorensen AG. A pilot study of normobaric oxygen therapy in acute ischemic stroke. Stroke. 2005;36:797–802. doi: 10.1161/01.STR.0000158914.66827.2e. [DOI] [PubMed] [Google Scholar]
- Storm-Mathisen J, Danbolt NC, Rothe F, Torp R, Zhang N, Aas JE, Kanner BI, Langmoen I, Ottersen OP. Ultrastructural immunocytochemical observations on the localization, metabolism and transport of glutamate in normal and ischemic brain tissue. Prog Brain Res. 1992;94:225–241. doi: 10.1016/s0079-6123(08)61753-7. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Noshita N, Lewen A, Gasche Y, Ferrand-Drake M, Fujimura M, Morita-Fujimura Y, Chan PH. Overexpression of copper/zinc superoxide dismutase in transgenic rats protects vulnerable neurons against ischemic damage by blocking the mitochondrial pathway of caspase activation. J Neurosci. 2002;22:209–217. doi: 10.1523/JNEUROSCI.22-01-00209.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeshima T, Johnston JM, Commissiong JW. Mesencephalic type 1 astrocytes rescue dopaminergic neurons from death induced by serum deprivation. J Neurosci. 1994;14:4769–4779. doi: 10.1523/JNEUROSCI.14-08-04769.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torp R, Danbolt NC, Babaie E, Bjoras M, Seeberg E, Storm-Mathisen J, Ottersen OP. Differential expression of two glial glutamate transporters in the rat brain: An in situ hybridization study. Eur J Neurosci. 1994;6:936–942. doi: 10.1111/j.1460-9568.1994.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Turrens JF, Freeman BA, Crapo JD. Hyperoxia increases H2O2 release by lung mitochondria and microsomes. Arch Biochem Biophys. 1982;217:411–421. doi: 10.1016/0003-9861(82)90519-7. [DOI] [PubMed] [Google Scholar]
- Vereczki V, Martin E, Rosenthal RE, Hof PR, Hoffman GE, Fiskum G. Normoxic resuscitation after cardiac arrest protects against hippocampal oxidative stress, metabolic dysfunction, and neuronal death. J Cereb Blood Flow Metab. 2006;26:821–835. doi: 10.1038/sj.jcbfm.9600234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Ma JH, Giffard RG. Overexpression of copper/zinc superoxide dismutase decreases ischemia-like astrocyte injury. Free Radic Biol Med. 2005;38:1112–1118. doi: 10.1016/j.freeradbiomed.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ. Parkinson's disease is associated with oxidative damage to cytoplasmic DNA, RNA in substantia nigra neurons. Am J Pathol. 1999;154:1423–1429. doi: 10.1016/S0002-9440(10)65396-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke HR, Tildon JT, Landry ME, Max SR. Effect of 8-bromo-cAMP and dexamethasone on glutamate metabolism in rat astrocytes. Neurochem Res. 1990;15:1115–1122. doi: 10.1007/BF01101713. [DOI] [PubMed] [Google Scholar]