Abstract
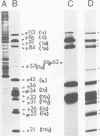
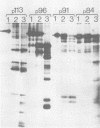
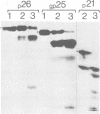
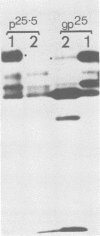
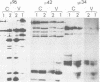
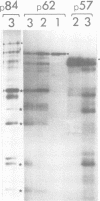
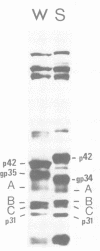
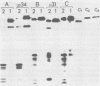
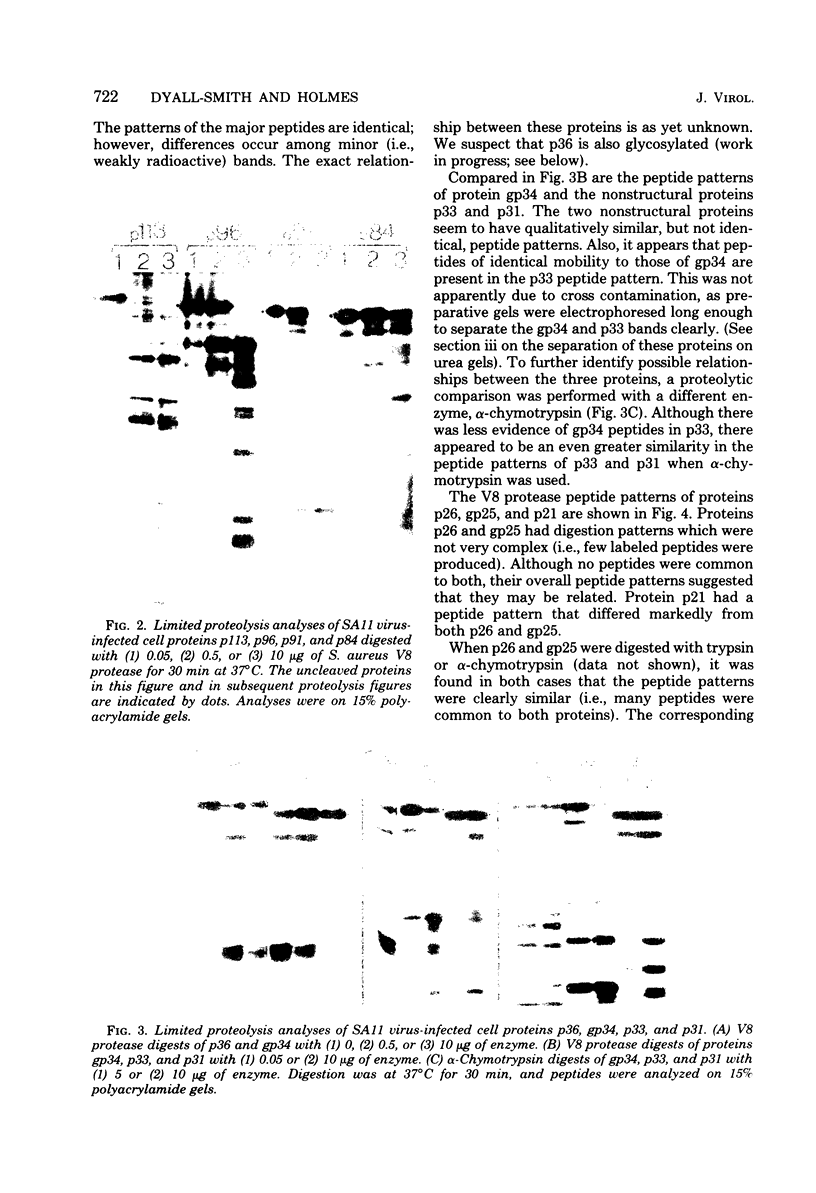
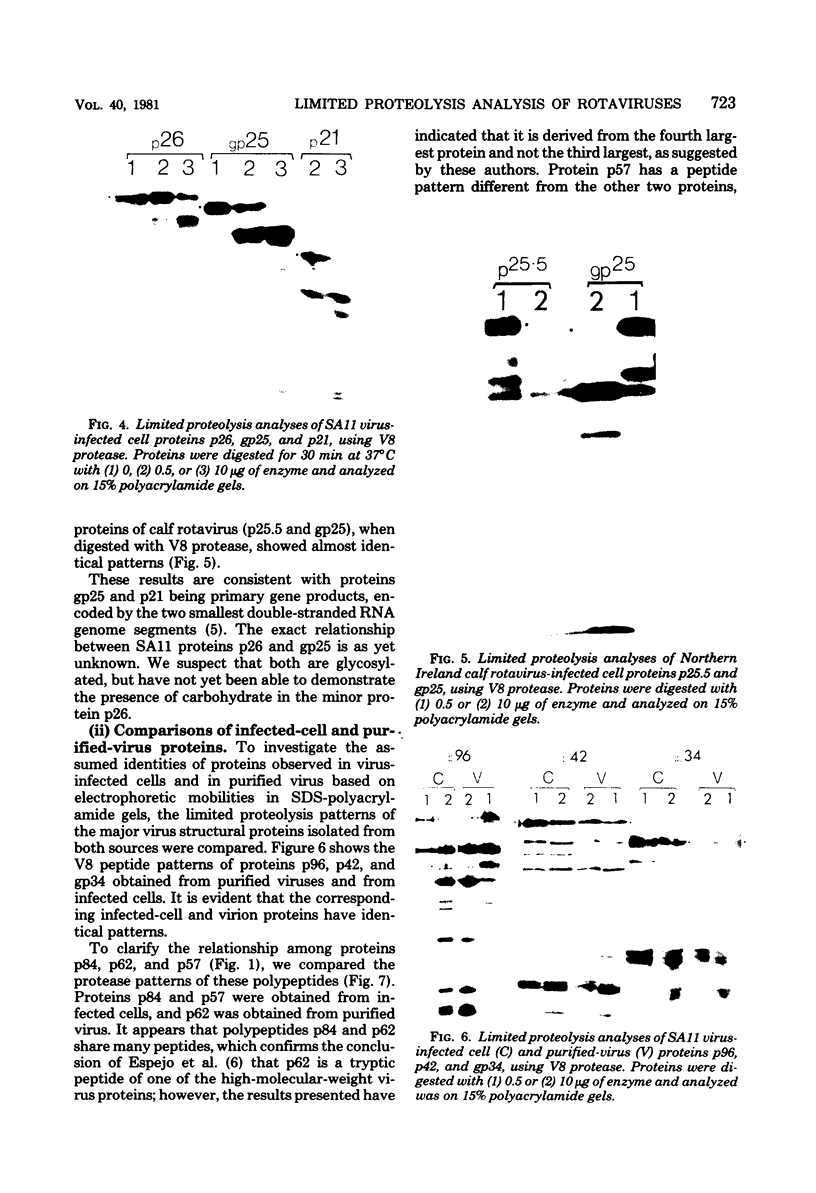
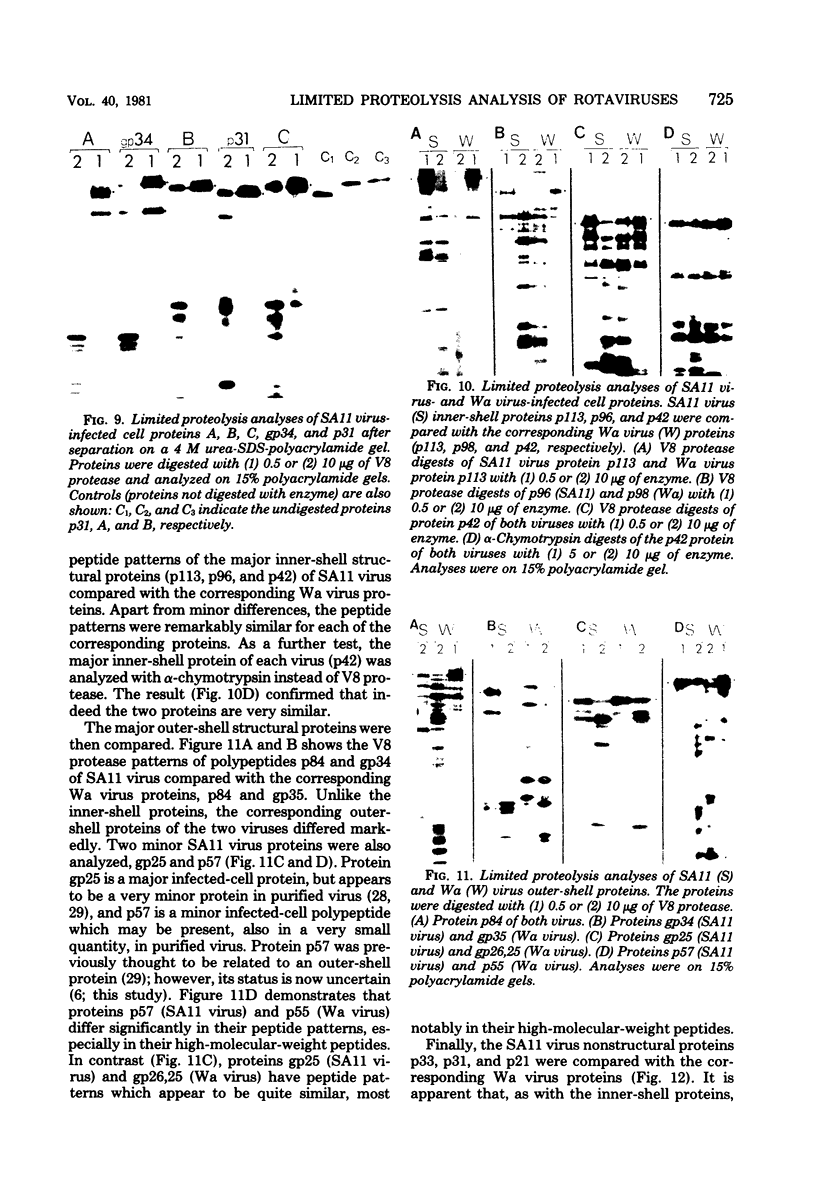
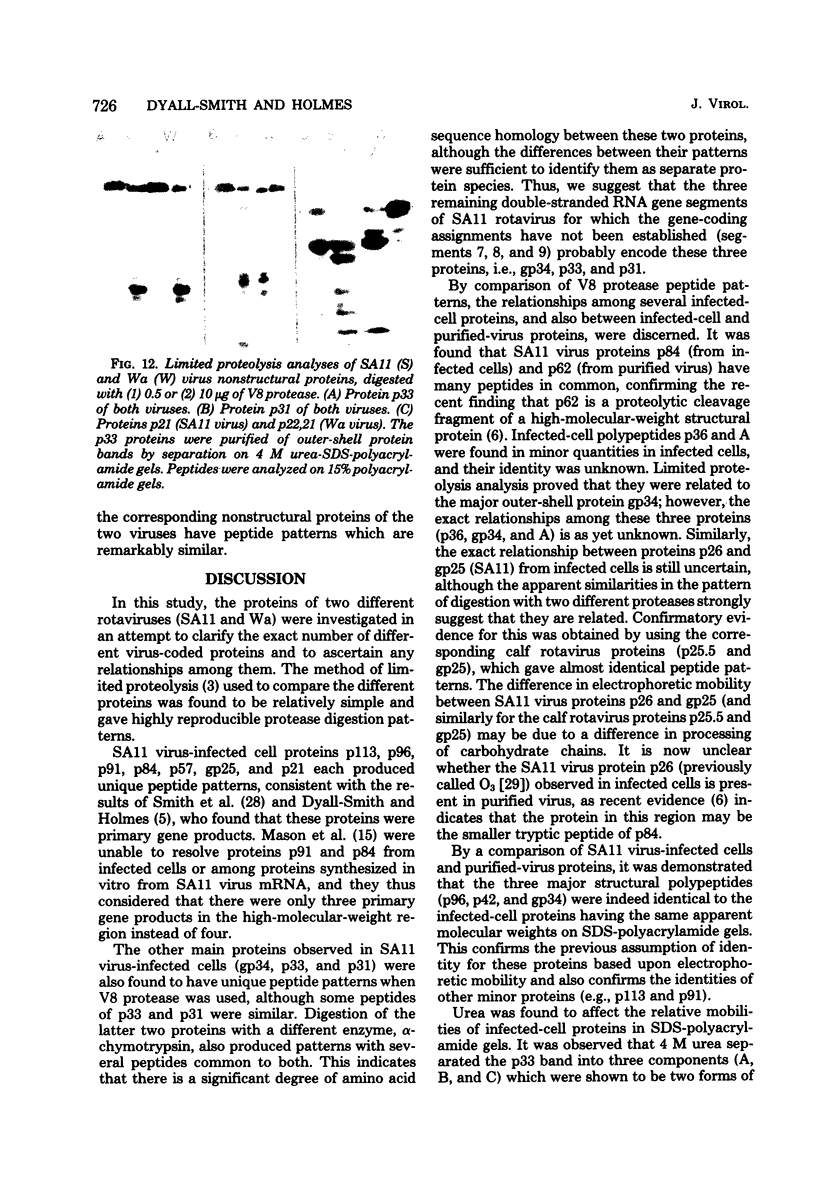
The polypeptides of SA11 rotavirus produced in virus-infected cells were analyzed by limited proteolysis, using Staphylococcus aureus V8 protease. This clearly distinguished between all the known primary gene products of the virus and allowed relationships between other infected-cell proteins, and between infected-cell and virus structural proteins, to be ascertained. A comparison of the proteolysis cleavage patterns between SA11 rotavirus and the human rotavirus Wa was also performed which demonstrated a marked conservation in the digestion patterns among nonstructural and inner-shell structural proteins, but a marked variation in the digestion patterns among outer-shell structural proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bridger J. C. Location of type-specific antigens in calf rotaviruses. J Clin Microbiol. 1978 Dec;8(6):625–628. doi: 10.1128/jcm.8.6.625-628.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge B. W., Huang A. S. Conserved peptides in the proteins of vesicular stomatitis virus. Virology. 1979 Jun;95(2):445–453. doi: 10.1016/0042-6822(79)90499-9. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Clinton G. M., Burge B. W., Huang A. S. Effects of phosphorylation and pH on the association of NS protein with vesicular stomatitis virus cores. J Virol. 1978 Aug;27(2):340–346. doi: 10.1128/jvi.27.2.340-346.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Gene-coding assignments of rotavirus double-stranded RNA segments 10 and 11. J Virol. 1981 Jun;38(3):1099–1103. doi: 10.1128/jvi.38.3.1099-1103.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., López S., Arias C. Structural polypeptides of simian rotavirus SA11 and the effect of trypsin. J Virol. 1981 Jan;37(1):156–160. doi: 10.1128/jvi.37.1.156-160.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H. B., Kalica A. R., Wyatt R. G., Jones R. W., Kapikian A. Z., Chanock R. M. Rescue of noncultivatable human rotavirus by gene reassortment during mixed infection with ts mutants of a cultivatable bovine rotavirus. Proc Natl Acad Sci U S A. 1981 Jan;78(1):420–424. doi: 10.1073/pnas.78.1.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes I. H. Viral gastroenteritis. Prog Med Virol. 1979;25:1–36. [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalica A. R., Sereno M. M., Wyatt R. G., Mebus C. A., Chanock R. M., Kapikian A. Z. Comparison of human and animal rotavirus strains by gel electrophoresis of viral RNA. Virology. 1978 Jun 15;87(2):247–255. doi: 10.1016/0042-6822(78)90130-7. [DOI] [PubMed] [Google Scholar]
- Kapikian A. Z., Wyatt R. G., Greenberg H. B., Kalica A. R., Kim H. W., Brandt C. D., Rodriguez W. J., Parrott R. H., Chanock R. M. Approaches to immunization of infants and young children against gastroenteritis due to rotaviruses. Rev Infect Dis. 1980 May-Jun;2(3):459–469. doi: 10.1093/clinids/2.3.459. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Pallansch M. A., Omilianowski D. R., Rueckert R. R. Changes in three of the four coat proteins of oral polio vaccine strain derived from type 1 poliovirus. J Virol. 1980 Jan;33(1):256–263. doi: 10.1128/jvi.33.1.256-263.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Malherbe H. H., Strickland-Cholmley M. Simian virus SA11 and the related O agent. Arch Gesamte Virusforsch. 1967;22(1):235–245. doi: 10.1007/BF01240518. [DOI] [PubMed] [Google Scholar]
- Mason B. B., Graham D. Y., Estes M. K. In vitro transcription and translation of simian rotavirus SA11 gene products. J Virol. 1980 Mar;33(3):1111–1121. doi: 10.1128/jvi.33.3.1111-1121.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Kalica A. R., Kono R. Isolation of a recombinant between simian and bovine rotaviruses. J Gen Virol. 1980 May;48(1):253–256. doi: 10.1099/0022-1317-48-1-253. [DOI] [PubMed] [Google Scholar]
- Matthews R. E. The classification and nomenclature of viruses. Summary of results of meetings of the International Committee on Taxonomy of Viruses in The Hague, September 1978. Intervirology. 1979;11(3):133–135. doi: 10.1159/000149025. [DOI] [PubMed] [Google Scholar]
- McCrae M. A., Faulkner-Valle G. P. Molecular biology of rotaviruses. I. Characterization of basic growth parameters and pattern of macromolecular synthesis. J Virol. 1981 Aug;39(2):490–496. doi: 10.1128/jvi.39.2.490-496.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Bishop R. F., Birch C., McLean B., Holmes I. H. Molecular epidemiology of human rotaviruses in Melbourne, Australia, from 1973 to 1979, as determined by electrophoresis of genome ribonucleic acid. J Clin Microbiol. 1981 Feb;13(2):272–278. doi: 10.1128/jcm.13.2.272-278.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Biochemical and biophysical characteristics of diarrhea viruses of human and calf origin. J Virol. 1975 Nov;16(5):1229–1235. doi: 10.1128/jvi.16.5.1229-1235.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Schnagl R. D., Holmes I. H. Further biochemical characterization, including the detection of surface glycoproteins, of human, calf, and simian rotaviruses. J Virol. 1977 Oct;24(1):91–98. doi: 10.1128/jvi.24.1.91-98.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova L. I., Tolskaya E. A., Agol V. I. Antigenic and structural relatedness among non-capsid and capsid polypeptides of polioviruses belonging to different serotypes. J Gen Virol. 1981 Feb;52(Pt 2):279–289. doi: 10.1099/0022-1317-52-2-279. [DOI] [PubMed] [Google Scholar]
- Saif L. J., Bohl E. H., Theil K. W., Cross R. F., House J. A. Rotavirus-like, calicivirus-like, and 23-nm virus-like particles associated with diarrhea in young pigs. J Clin Microbiol. 1980 Jul;12(1):105–111. doi: 10.1128/jcm.12.1.105-111.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnagl R. D., Holmes I. H. Characteristics of the genome of human infantile enteritis virus (Rotavirus). J Virol. 1976 Jul;19(1):267–270. doi: 10.1128/jvi.19.1.267-270.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtissek C., Rohde W., Von Hoyningen V., Rott R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology. 1978 Jun 1;87(1):13–20. doi: 10.1016/0042-6822(78)90153-8. [DOI] [PubMed] [Google Scholar]
- Scholtissek C. The genome of the influenza virus. Curr Top Microbiol Immunol. 1978;80:139–169. doi: 10.1007/978-3-642-66956-9_5. [DOI] [PubMed] [Google Scholar]
- Smith A. E., Smith R., Paucha E. Extraction and fingerprint analysis of simian virus 40 large and small T-antigens. J Virol. 1978 Oct;28(1):140–153. doi: 10.1128/jvi.28.1.140-153.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. L., Lazdins I., Holmes I. H. Coding assignments of double-stranded RNA segments of SA 11 rotavirus established by in vitro translation. J Virol. 1980 Mar;33(3):976–982. doi: 10.1128/jvi.33.3.976-982.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thouless M. E. Rotavirus polypeptides. J Gen Virol. 1979 Jul;44(1):187–197. doi: 10.1099/0022-1317-44-1-187. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- Yolken R. H., Barbour B., Wyatt R. G., Kalica A. R., Kapikian A. Z., Chanock R. M. Enzyme-linked immunosorbent assay for identification of rotaviruses from different animal species. Science. 1978 Jul 21;201(4352):259–262. doi: 10.1126/science.208150. [DOI] [PubMed] [Google Scholar]
- Zissis G., Lambert J. P. Different serotypes of human rotaviruses. Lancet. 1978 Jan 7;1(8054):38–39. doi: 10.1016/s0140-6736(78)90380-x. [DOI] [PubMed] [Google Scholar]