Abstract
Male European starlings (Sturnus vulgaris) sing throughout the year, but the social factors that motivate singing behavior differ depending upon the context in which song is produced. In a non-breeding context (when testosterone concentrations are low) starlings form large, mixed-sex flocks and song is involved in flock cohesion and perhaps maintenance of social hierarchies. In contrast, in a breeding context (when testosterone concentrations are high) male song plays a direct role in mate attraction. How the nervous system ensures that song production occurs in an appropriate context in response to appropriate stimuli is not well understood. The song control system regulates song production, learning, and to some extent perception; however these nuclei do not appear to regulate the social context in which song is produced. A network of steroid hormone sensitive nuclei of the basal forebrain and midbrain regulates social behavior. The present study used the immediate early gene cFOS to explore possible involvement of these regions in context-dependent song production. Numbers of cFOS-labeled cells in the medial bed nucleus of the stria terminalis, anterior hypothalamus, and ventromedial nucleus of the hypothalamus related positively only to song produced in a breeding context. In contrast, numbers of cFOS-labeled cells in three zones of the lateral septum related positively only to song produced in a non-breeding context. Taken together, these data suggest differential regulation of male starling song by social behavior nuclei depending upon the breeding context in which it is produced.
Keywords: songbird, season, context, vocal communication, immediate early gene, cFOS, lateral septum, bed nucleus of the stria terminalis, anterior hypothalamus, ventromedial nucleus of the hypothalamus
INTRODUCTION
In songbirds, as in other social vertebrates, the function of vocal behavior shifts depending upon the context in which it occurs. Song production by adult male songbirds is typically used to attract mates and to defend territories and highly influenced by social and environmental factors including the presence of potential mates/rivals and season (e.g. Catchpole and Slater, 1995; Eens, 1997; Eens, Pinxten, and Verheyen, 1990). Many studies have focused on how the songbird brain regulates various aspects of singing behavior such as song learning, production, and perception (e.g. Brenowitz, Margoliash, and Nordeen, 1997; Margoliash, 1997; Nottebohm, Stokes, and Leonard, 1976; Wild, 1997). However, how the nervous system ensures that song production occurs in the appropriate context in response to appropriate stimuli has not been well examined. With this study, we explore the possibility that the proximate mechanisms regulating singing behavior in songbirds differ depending upon the context in which it occurs.
European starlings (Sturnus vulgaris) are an ideal model system in which to explore the context-dependent neural regulation of singing behavior. Male starlings sing throughout the year, but the function of song and the factors that motivate song production differ depending upon the context in which it occurs. Within a breeding context, circulating concentrations of the steroid hormone testosterone (T) are elevated (Ball and Wingfield, 1987; Riters, Eens, Pinxten, and Ball, 2002), and starling song can be highly sexually motivated. Possession of a nest site is critical for successful mate attraction (see Gwinner, Van’t Hof, and Zeman, 2002). In the absence of female conspecifics males both with and without nest sites sing at similar, relatively low, levels (Riters, Eens, Pinxten, Duffy, Balthazart, and Ball, 2000). However, only males with nest sites increase their song rate in response to the introduction of a female (Riters et al., 2000). Once these individuals pair with a female, song production occurs immediately prior to almost every copulation (Eens and Pinxten, 1990; Eens and Pinxten, 1995; Pinxten and Eens, 1997). Thus, song in males with nest sites is considered highly sexually motivated. In contrast, males without nest sites do not increase their song rate in response to the introduction of a female conspecific but continue to sing at relatively low levels (Riters et al., 2000). What motivates singing behavior in males without nest sites is not clear. However, given that female presence does not dramatically alter song rate it does not seem to be highly sexually motivated or to function in immediate mate attraction.
In a non-breeding context, in fall and winter, starlings neither occupy nor defend nest sites and the introduction of a female conspecific does not stimulate male song output (Riters et al., 2000). At this time circulating concentrations of T are basal (Ball and Wingfield, 1987; Riters et al., 2002), and song does not appear to play any direct role in mate attraction. Rather, birds are typically found feeding and roosting in large, mixed-sex flocks (Feare, 1984), and non-breeding context song is suggested to play a prominent role in flock cohesion and perhaps the establishment and/or maintenance of dominance hierarchies within the flock (Hausberger, Richardyris, Henry, Lepage, and Schmidt, 1995; Summers, Westlake, and Feare, 1987). As such, in contrast to breeding context song, singing behavior in a non-breeding context can be considered more broadly socially motivated.
In songbirds, song is controlled by a well-defined series of cytoarchitecturally distinct, interconnected brain nuclei known as the song control system. The brain regions HVC (used as a proper name), robust nucleus of the acropallium (RA), lateral magnocellular nucleus of the anterior nidopallium (lMAN), and Area X (among several others) regulate song learning, production, and to some extent perception (e.g. Brenowitz et al., 1997; Margoliash, 1997; Nottebohm et al., 1976; Wild, 1997). Data indicate that activity within the song control nuclei Area X, RA, and lMAN can be context-dependent. For example, activity within the song system can differ depending upon whether a bird directs song towards a conspecific or sings alone (Hessler and Doupe, 1999; Jarvis, Scharff, Grossman, Ramos, and Nottebohm, 1998), or whether a bird is singing within or outside a breeding context (Heimovics and Riters, 2005; Liu and Nottebohm, 2005; Riters, Teague, Schroeder, and Cummings, 2004). Taken together these studies indicate that the regulation of singing behavior by the song control system can be strongly influenced by the context in which it occurs. However, to date no data implicate song nuclei in regulating motivational aspects of vocal communication in songbirds. Lesion studies suggest that damage to song nuclei results in deficits in song production, but lesioned birds continue to adopt singing postures and display motor patterns associated with singing behavior, indicating an intact motivation to communicate (Bottjer, Miesner, and Arnold, 1984; Nordeen and Nordeen, 1993; Nottebohm et al., 1976; Sohrabji, Nordeen, and Nordeen, 1990). A growing body of research implicates nuclei outside of the classically defined song control system as being involved in regulating the motivation to sing and suggest that these regions might influence singing behavior in a context-dependent manner.
Vocal communication can be central to the mediation of social interactions between conspecifics. The lateral septum (LS), medial bed nucleus of the stria terminalis (BSTm), preoptic area (POA), anterior hypothalamus (AH), ventromedial hypothalamus (VMH), and areas of the tegmentum (including the ventral tegmental area [VTA] and midbrain central gray [GCt]) are components of a proposed vertebrate ‘social behavior network’ (Newman, 1999). These interconnected, steroid hormone sensitive nuclei appear central to the control of all types of vertebrate social behavior including communication, sexual behavior, and aggression (Goodson, 2005).
Recently, brain regions within the social behavior network have been directly implicated in the regulation of singing behavior in songbirds. For example, in starlings, the numbers of cFOS-labeled cells (an indirect marker of neural activity) within the medial preoptic nucleus (POM) and VTA relate positively to song produced within, but not outside of, a breeding context (Heimovics and Riters, 2005). In song sparrows, cFOS labeling in VTA and GCt relates positively to singing behavior in response to territorial intrusion, suggesting that midbrain regions influence agonistic song production (Maney and Ball, 2003). Lesions to the lateral septum (LS) either facilitate or inhibit courtship song depending upon whether the species examined is territorial or gregarious (Goodson, Eibach, Sakata, and Adkins-Regan, 1999), suggesting septal modulation of song production is influenced by social context. Taken together, these data suggest that nuclei involved in social behavior might differentially regulate song within different social contexts.
Here, we used immunocytochemistry for the protein product of the immediate early gene (IEG) cFOS (an indirect marker of neuronal activity) to explore a possible role for these regions in the context-dependent regulation of male starling singing behavior. We compared numbers of cFOS-labeled cells within social behavior network regions of males singing within versus outside of a breeding context. Because these nuclei are known to mediate multiple forms of social behavior and are steroid hormone sensitive, the relationships between cFOS and aggression, nestbox status, and breeding condition were also examined.
METHODS
Animals
In fall 2002 and early winter 2003 forty-two adult male and four adult female starlings were captured on a single farm north of Madison, Wisconsin using fly-in traps. These are the same birds used in a previous study examining cFOS immunolabeling within a separate group of brain regions not reported here (Heimovics and Riters, 2005). Birds were immediately housed indoors after capture in single sex cages (91cm x 47cm x 47cm) in the University of Wisconsin Department of Zoology animal facilities. Photoperiodic and hormone manipulations were used to place birds into either a breeding (i.e. spring-like with high circulating testosterone (T)) or a non-breeding (i.e. fall/winter-like with low T) condition. Protocols used for bird acquisition, surgery, and behavioral testing were in adherence to guidelines approved by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (DHEW Publication 80-23, Revised 1985, Office of Science and Health Reports, DRR/ NIH, Bethesda, MD 20205) as well as the University of Wisconsin-Madison Research Animal Resource Committee (RARC).
Experimental males
All birds were captured at a time during which the outdoor photoperiod was <11L. Eighteen males were randomly assigned to the non-breeding group and housed indoors on a photoperiod matching the outdoor light/dark cycle. On December 18, 2002, when the outdoor photoperiod was ~10L, non-breeding males were subdivided into two groups (n=8 and n=10) and placed in two identical outdoor aviaries (3.7m x 2m x 2.8m). Behavioral testing began in January after a one month habituation period.
The remaining twenty-four males were assigned to the breeding condition group. To stimulate a breeding condition males were housed on 6L for two months and 11L for approximately six months, photoperiods under which males will become photosensitive and respond to testosterone with reproductive behavior (Dawson, King, Bentley, and Ball, 2001). Birds were subdivided into two groups (n=12 and n=12). Approximately two weeks prior to introduction to an outdoor aviary (identical to those used for the non-breeding groups), birds were implanted with testosterone (see below) on either September 2, when the outdoor photoperiod was ~13L, or on October 1, when the natural photoperiod was ~12L. Behavioral testing of breeding condition animals began two weeks after they were introduced to outdoor aviaries. Differences in the habituation period for breeding and non-breeding males were due to weather-related issues outside of the control of the experimenters.
Stimulus females
As described in the introduction, the presence of a female has no effect on the singing behavior of non-breeding condition males. However, to control for the possibility that perception of a novel conspecific might induce immediate early gene activity in some of the brain regions examined, stimulus females were introduced into the test aviaries of both breeding and non-breeding males. Males were presented with females whose breeding condition matched their own (i.e. breeding condition males were presented with breeding condition females and non-breeding condition males were presented with non-breeding condition females) in an attempt to maintain ecological validity of the singing behavior observed in this study. Furthermore, in unpublished studies from our lab we have found that neither males in breeding nor males in non-breeding condition discriminate behaviorally between breeding and non-breeding condition females (unpublished results Heimovics and Riters; Alger and Riters). Breeding condition stimulus females were housed indoors on an 11L photoperiod. Non-breeding condition females were housed indoors on a 6L photoperiod.
Testosterone implants
Pilot studies indicated that in our aviary setting birds often did not, or were slow to enter a breeding condition, and that T implants maximized the likelihood of observing high levels of breeding context song expression (and other courtship behaviors). Therefore, males in the breeding condition group were given T-implants two weeks prior to the introduction to an outdoor aviary (control implants were not given to non-breeding condition males because these birds had been tested prior to our decision to facilitate breeding context song through T manipulations). Breeding condition males were lightly anesthetized using isoflurane gas anesthesia and implanted subcutaneously just posterior to the last rib on the left side with two, fourteen mm lengths of silastic tubing (Dow Corning, i.d., 1.47mm; o.d. 1.96mm) packed for ten mm with crystalline testosterone proprionate (Sigma). Tubes were sealed with silastic glue. After recovering from surgery on a heated surface, males were placed back on an 11L photoperiod until being introduced into an outdoor aviary. In a study on a separate group of male starlings that is in progress in our laboratory, an enzyme assay has been used to measure plasma levels of T following this same T-implant procedure. In gonadally intact, breeding condition males implanted with T and housed on an 11L photoperiod for 4 weeks, circulating levels of T are significantly higher than those in unmanipulated, non-breeding males housed on a 6L photoperiod (breeding condition males (n=7) mean = 705.39 pg/ml, sd = 476.69); non-breeding condition males (n=7) mean = 60.58 pg/ml, sd = 91.68: Z = 3.13, p = 0.002) (Heimovics and Riters, unpublished data).
Behavioral testing
Behavioral testing took place in one of two identical outdoor aviaries (see above). The aviaries were adjacent to one another, and the birds in each group were in acoustic but not visual contact with one another. Each aviary contained six nestboxes and branches for perching. Food and water were provided ad libitum. A main goal of this study was to examine how social dynamics influence behavior and neural activity (e.g. whether or not a male acquires a nest box in spring depends on the behavioral responses of other males). Furthermore, pilot tests indicated that non-breeding condition birds do not sing when housed singly in an aviary; non-breeding song only occurs in the context of a dynamic social group (i.e. flock). Thus, we examined male behavior in groups of 8–12 individuals.
Prior to the day of behavioral testing for the present study, all birds were observed for nine days over a two-week period as part of a separate behavioral analysis. Specifically, over a nine-day period they were presented daily with females in different reproductive conditions and an observer noted their behavior for fifty minutes. Breeding and non-breeding males were exposed to identical behavioral testing procedures. This previous study might have influenced the behavioral response of the study subjects (i.e. males might have become habituated to the introduction of a novel conspecific female), but this effect would have been controlled for both breeding and non-breeding males. Observations for the present study began three days after completion of the nine-day study.
Breeding and non-breeding males were observed on a single day for fifty minutes. Details of the methods used for behavioral testing have been published previously (Heimovics and Riters, 2005). Briefly, a point sampling technique (see Eens and Pinxten, 1990) was used to measure song production (a weak beep emitted from a timer to indicate each sixty second period and for fifty minutes it was noted at every beep whether or not each individual male was singing) and aggression (chasing, displacing, pecking and attacking) was noted continuously throughout the test period. During each behavioral observation session it was noted if and how many times any male entered a nestbox.
Tissue processing
A detailed description of tissue processing and cFOS immunocytochemistry used in this study is published elsewhere (Heimovics and Riters, 2005). Briefly, birds were sacrificed 10 minutes after the conclusion of behavioral testing, brains dissected out of their skulls, fixed in 5% acrolein, and frozen at −80°C until processing. The males included in the cFOS immunocytochemistry (ICC) presented here (breeding condition n= 14; non-breeding condition n=10) were selected from a larger group of behaviorally tested birds (described in Heimovics and Riters, 2005). Tissue from the remaining 18 individuals was used for a separate analysis not reported here.
Brains were cut in three series of 30μm sections in the coronal plane using a cryostat. cFOS ICC was run in two batches: batch one consisted of n=6 breeding and n=3 non-breeding condition males; batch two included n=8 breeding and n = 7 non-breeding condition males. Each batch contained both breeding and non-breeding birds whose singing behavior fell along a continuum (i.e. from no point samples with song to several point samples with song). Tissue was incubated a 2% NGS primary antibody solution (1:18000, cFOS Ab = K-25, sc 253 Santa Cruz) for forty-eight hours at 4°C, incubated with a 2% NGS secondary antibody solution (1:250 goat anti-rabbit Jackson ImmunoResearch Laboratories) for ninety minutes at room temperature, and visualized using diaminobenzadine (Sigma).
Quantification
A researcher blind to the reproductive condition, behavior, and nestbox status of each bird performed counts of cFOS-labeled cells. The quantification of cFOS-labeling reported here is on tissue from the same birds used in a previous study which focused on a separate group of brain regions (HVC, Area X, and RA, POM, VTA, GCt, and TnA) (Heimovics and Riters, 2005). A digital Spot camera (Diagnostic Instruments Inc.) connecting a microscope to a PC computer was used to acquire images of brain sections. To determine which cells were to be counted as labeled, Meta Vue software (Universal Imaging Corp.) was used to set a cell-count threshold for all brain regions within a batch. Thresholds were set outside the brain regions of interest. Because of differences between ICC batches that could lead to differences in density of labeling, separate thresholds were used for each batch. The numbers of labeled cells were counted on three serial sections in both hemispheres for all brain regions. In the few cases of tissue damage or lost sections counts were made on a fourth serial section.
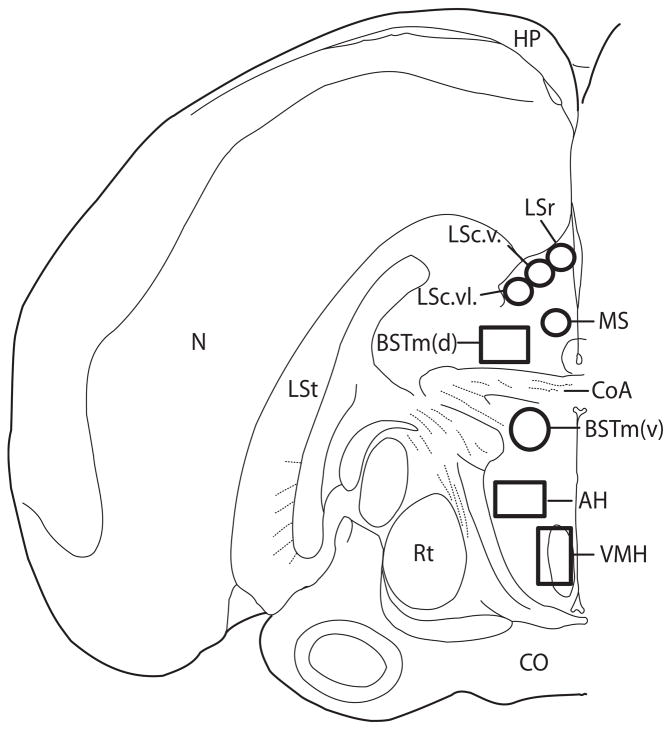
Counts were made bilaterally on serial sections containing the anterior commissure (CoA) (see Figure 1). Location and identity of social behavior network brain regions examined for this paper were based on figure published by Goodson illustrating a coronal section of the midline forebrain of a zebra finch (Goodson, Evans, Lindberg, and Allen, 2005). Research in quail has identified populations of BSTm neurons located dorsal and ventral to the CoA (Aste, Balthazart, Absil, Grossmann, Mulhbauer, Viglietti-Panzica, and Panzica, 1998). To explore the possibility that these populations are functionally distinct, counts were made separately within each sub-region. For the portion of BSTm dorsal to CoA (BSTm(d) counts were made within an area of 0.33mm X 0.66mm and for the portion of BSTm ventral to CoA (BSTm(v)) counts were made within a circle (diameter = 0.53mm). For MS, counts were made within a circle (diameter = 0.33mm); for AH, within an area of 0.27mm X 0.48mm for AH; and for VMH, within an area of.27mm X 0.48mm. In songbirds the precise location of the homologue to mammalian AH has not been identified. Here we examined numbers of cFOS labeled cells within a region referred to as anterior hypothalamus (AH) by Goodson (Goodson et al., 2005). Based on landmarks, this region appears to overlap with an area identified as a portion of lateral hypothalamus (LH) referred to as the external cellular stratum of the lateral hypothalamus (SCE) (Bottjer, Brady, and Cribbs, 2000; Foster, Mehta, and Bottjer, 1997). Cell counts within LS were made at the level of CoA within three zones of LS (caudal part, ventrolateral zone (LSc.vl); caudal part, lateral zone (LSc.v); rostral part (LSr)) within a a circle (diameter 0.33mm) based on Goodson (Goodson et al., 2005). Values from all counts for all regions were averaged and the mean value for each individual was used for analysis.
Figure 1.
Boxes indicate areas in which cFOS labeled cells were counted. Abbreviations: N, nidopallium; Rt, nucleus rotundus, LSt, lateral striatum; CO, optic chiasm; CoA, anterior commissure; LSr, lateral septum, rostral part; LSc.v., lateral septum, caudal part, lateral zone; LSc.vl., lateral septum, caudal part, ventrolateral zone; BSTm(v), medial bed nucleus of the stria terminalis ventral to CoA; BSTm(d), medial bed nucleus of the stria terminals dorsal to CoA; MS, medial septum; AH, anterior hypothalamus; VMH, ventromedial nucleus of the hypothalamus.
Statistical analysis
To normalize the point sampled song data, the number of point samples with singing out of 50 was arcsine transformed for each individual using the following equation: 2*arcsine square root # observed songs/50 (Lehner, 1996). Data were analyzed using Statistica 6.0 software (Stat Soft Inc., Tulsa, OK). Multiple linear regression, one-way ANOVA, and post-hoc Fisher’s LSD analyses were performed when significant differences were observed. Average number of cFOS-labeled cells per region was used as the independent variable to determine to what extent brain activity was related to male behavior, reproductive condition, and nestbox status.
RESULTS
An analysis of the behavior observed in all 42 males used for behavioral testing is published elsewhere (Heimovics and Riters, 2005). The behavior of the subset of males included in the cFOS ICC is consistent with this larger set of birds and will not be discussed here. As described in that publication, two of the breeding condition males included in the cFOS ICC had been observed exhibiting homosexual behavior (i.e. entering and exiting the same nestbox and copulating with one another). These two males were not included in the present study bringing the sample size to n=12 breeding condition males and n=10 non-breeding condition males. Of the 12 breeding condition males, 5 were observed entering and exiting nestboxes and these birds were categorized as “breeding condition with nestbox (BCbox).” The remaining 7 breeding condition males were categorized as “breeding condition without nestbox (BCno box).” In the previous publication, some non-breeding males were operationally defined as “nestbox owners” because they each entered a nestbox a single time during behavioral testing (see Heimovics and Riters, 2005). Non-breeding males neither occupy nor defend nest sites and the behavior of these individuals differs dramatically from breeding condition nestbox owners (i.e. non-breeding males rarely enter nest boxes and do not gather nest material or sing from inside a box). We now feel this terminology is misleading and in our current and future publications would like to avoid categorizing non-breeding condition males who enter boxes as “nestbox owners.” Thus, all non-breeding males (n=10) were categorized as “non-breeding condition (NBC).”
cFOS Immunolabeling and Behavior
Outliers and excluded individuals
Standard residual analysis for multiple linear regression (MLR) indicated that cell-count data for one NBC male for LSc.v. (arcsine all song: 0.97, log aggression: 1.79, LSc.v. cell count: 9.17) and two BCno box males for BSTm(d) and BSTm(v) (arcsine all song: 0.71, log aggression: 0, log BSTm(d) cell count: 1.54, log BSTm(v) cell count: 1.15; arcsine all song: 0.57, log aggression: 0.69, log BSTm(d) cell count: 6.17, log BSTm(v) cell count: 1.15) were ±2 standard deviations from the mean. However, significant linear relationships between song and cFOS immunolabeling in these three regions were detected without excluding these individuals from MLR analysis. Thus, MLR statistics reported below and accompanying scatterplots were generated using cell-counts and behavior from all males and represent a slightly more conservative analysis of the present data.
cFOS, Song, and Aggression
Counts of song and aggression were used as independent variables in MLR analysis to determine the extent to which these behaviors could account for variance in the numbers of cFOS-labeled cells within all the brain regions examined. Backward and forward analyses were performed separately for breeding and non-breeding condition males. Results of both were very similar but forward stepwise analyses had the lowest standard errors of the estimate and the highest adjusted R2 and are presented below.
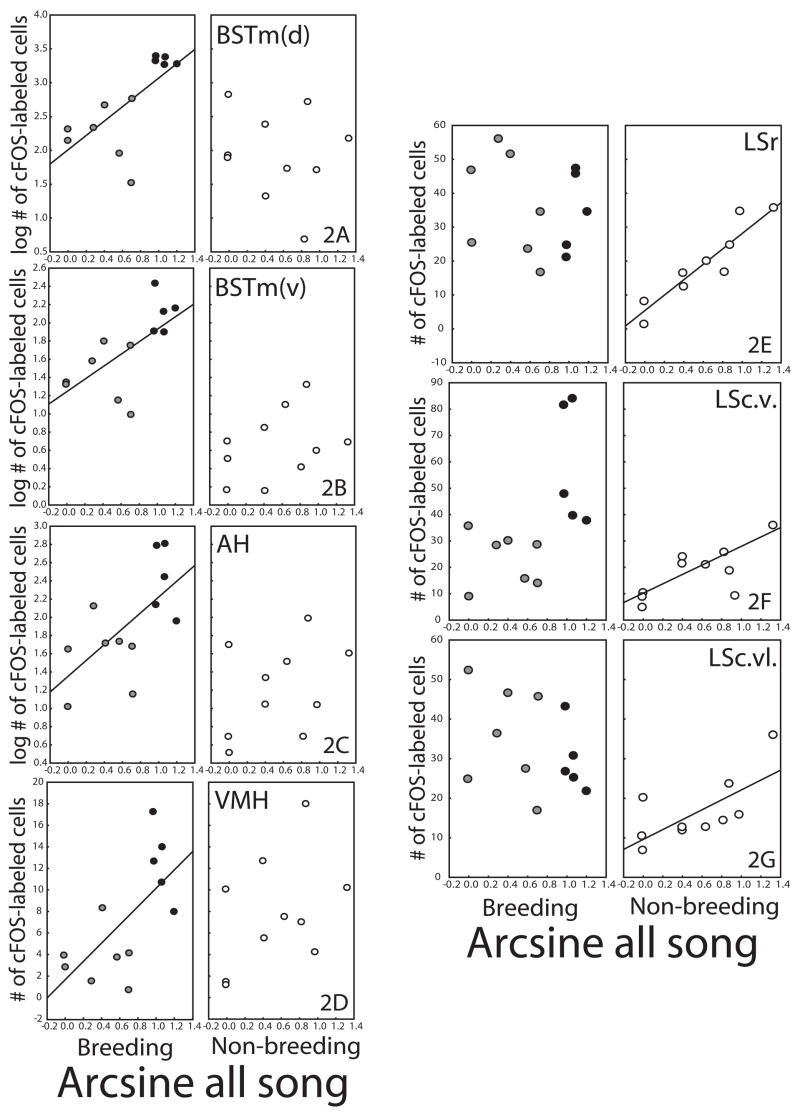
The number of cFOS-labeled cells within BSTm(d), BSTm(v), and AH were found to account for a significant portion of the variance in singing, but not aggressive behavior in breeding condition males (BSTm(d) (F1,10=8.946, p=0.014, R2=0.472, song beta=0.687; fig. 2A), BSTm(v) (F1,10=7.534, p=0.021, R2=0.429, song beta=0.656; fig. 2B), and AH (F1,10=6.922, p=0.025, R2=0.409, song beta=0.640; fig. 2C). The number of cFOS-labeled cells within VMH was found to account for a significant portion of the variance in song and aggression in breeding condition males (F2,9=10.744, p=0.004, R2=0.705, song beta=0.489, aggression beta=0.547; fig. 2D). cFOS-labeling in BSTm(d), BSTm(v), AH, and VMH could not account for variation in any behavior by non-breeding condition males.
Figure 2.
Plots showing the relationships between song production and mean number of cFOS-labeled cells in (A) BSTm(d), (B) BSTm(v), (C) AH, (D) VMH, (E) LSr, (F) LSc.v., and (G) LSc.vl. in breeding and non-breeding condition males. Each point represents one individual. Black dots are breeding condition males with nestboxes; gray dots, breeding condition males without nestboxes; white dots, non-breeding condition males.
In contrast, starling singing behavior outside of a breeding context could be explained by cFOS-labeling in LS. Within all three subdivisions of this nucleus, the number of cFOS-labeled cells was found to account for a significant portion of the variance in singing, but not aggressive, behavior by non-breeding condition males (LSr (F1,8=56.005, p=0.00007, R2=0.875, song beta=0.935; fig. 2E), LSc.v. (F1,8=7.658, p=0.024, R2=0.489, song beta=0.699; fig. 2F), LSc.vl. (F1,8=7.460, p=0.025, R2=0.482, song beta=0.695; fig. 2G). cFOS in LS could not account for variation in any behavior occurring within a breeding context.
cFOS and Nestbox Possession
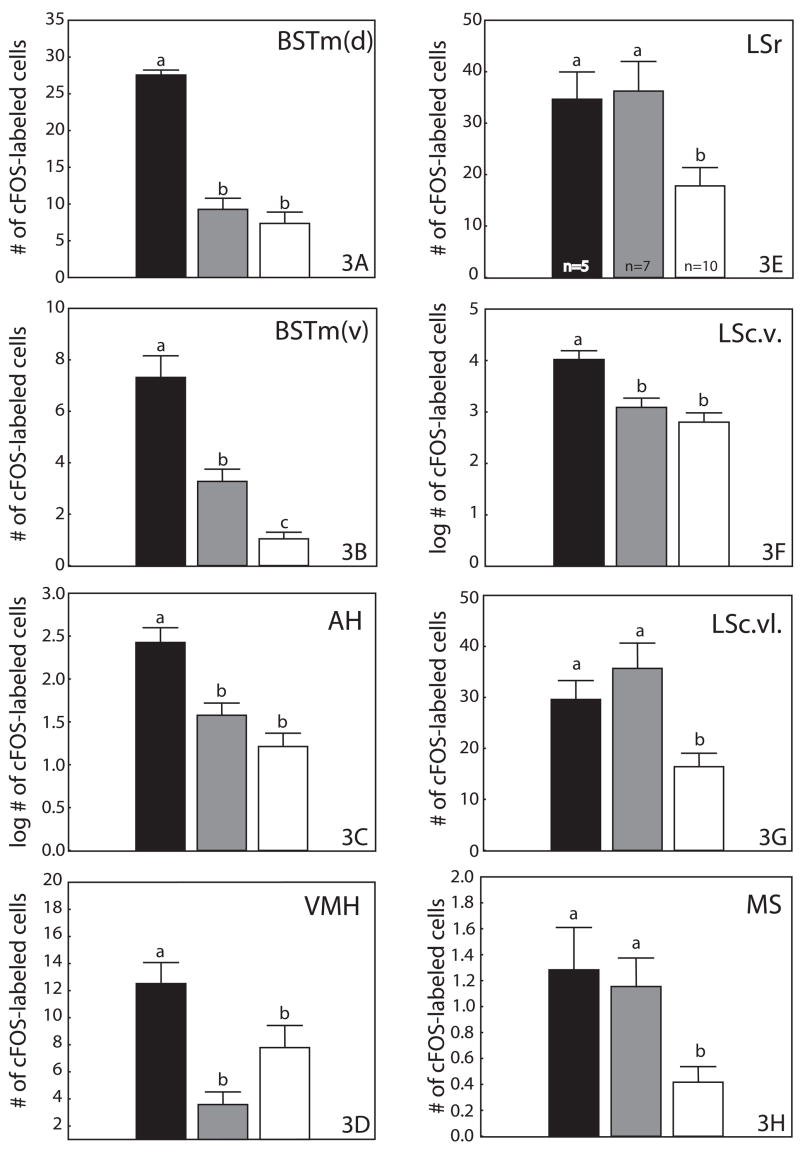
One-way ANOVA indicated that in BSTm(d) (F2,19=43.87, p=0.000000; fig. 3A), BSTm(v) (F2,19=42.13, p=0.000000; fig. 3B), AH (F2,19=10.58, p=0.0008; fig. 3C), VMH (F2,19=6.83, p=0.006; fig. 3D), and LSc.v. (F2,19=9.32, p=0.002; fig. 3F) there were significant differences in the mean numbers of cFOS-labeled cells for BCbox, BCno box, and NBC males. Post-hoc analysis (Fisher’s LSD) revealed that BCbox males had significantly greater numbers of cFOS-labeled cells in all 5 of these regions than BCno box and NBC males. Fisher’s LSD showed no significant difference between BCno box and NBC males in the numbers of cFOS-labeled cells in BSTm(d), AH, VMH, and LSc.v. (fig.s 3A, 3C, 3D, & 3F). However, this analysis indicated that the numbers of cFOS-labeled cells in BSTm(v) was significantly higher in BCno box males than in NBC males (fig. 3B).
Figure 3.
Mean numbers of cFOS-labeled cells counted in (A) BSTm(d), (B) BSTm(v), (C) AH, (D) VMH, (E) LSr, (F) LSc.v., (G) LSc.vl. and (H) MS. Different letters above the error bars indicate significant differences (Fisher’s LSD, p<0.05 following significant ANOVA) in numbers of cells counted within each region. Black bars are breeding condition males with nestboxes; gray bars, breeding condition males without nestboxes; white bars, non-breeding condition males.
cFOS and Breeding Condition
Results of one-way ANOVA also indicated a significant difference between BCbox, BCno box, and NBC males in the mean number of cFOS-labeled cells in LSr (F2,19=, p=0.0008; fig. 3E), LSc.vl. (F2,19=8.01, p=0.003; fig. 3G), and MS (F2,19=6.02, p=0.009; fig. 3H). However, the results of post-hoc analysis (Fisher’s LSD) showed that, independent of nestbox possession, the numbers of cFOS-labeled cells were significantly higher in breeding condition males than in non-breeding condition males (figs 3E, 3G, & 3H).
DISCUSSION
cFOS and song within a breeding context
In this study we observed a clear pattern of breeding context-dependent regulation of singing behavior within several brain regions of the telencephalon and hypothalamus. Specifically, the numbers of cFOS-labeled cells within BSTm(d), BSTm(v), AH, and VMH related positively to song produced within, but not outside of, a breeding context. These data, taken together with a previous report showing the numbers of cFOS-labeled cells within POM and VTA relate positively to only breeding context song (Heimovics and Riters, 2005), identify a potential network of telencephalic, diencephalic, hypothalamic, and brainstem regions in which the protein products of cFOS may be acting to specifically regulate singing behavior within a breeding context.
Across vertebrate taxa, BSTm, AH, and VMH have been implicated in the neural control of both male sexual behavior and aggression (Ball and Balthazart, 2004; Coolen, Peters, and Veening, 1997; Davis and Marler, 2004; Delville, De Vries, and Ferris, 2000; Edwards, Kriegsfeld, and Crews, 2004; Ferris, Melloni, Koppel, Perry, Fuller, and Delville, 1997; Goodson et al., 2005; Jasnow, Davis, and Huhman, 2004; Kollack-Walker and Newman, 1995; Paredes, 2003; Pfaus and Heeb, 1997; Powers, Newman, and Bergondy, 1987). In starlings, the results of multiple studies indicate that the primary function of song within a breeding context is mate attraction and, to date, little direct evidence supports a role for breeding context starling song in territory defense (see Eens, 1997 for review). Play-back studies have shown that male starlings preferentially approach nestboxes broadcasting natural starling song over silent nestboxes (Mountjoy and Lemon, 1991); findings contrary to the notion that male song is used as a mechanism for nestbox defense. However, in this same study males tended to avoid nestboxes broadcasting synthetic complex song versus those broadcasting synthetic simple song (Mountjoy and Lemon, 1991), suggesting song complexity may be used to assess potential competitors. The introduction of a conspecific male has no effect on the singing behavior of males with nest sites, although one study has reported a slight, but non-significant, trend towards an increase in singing activity in response to a male intruder (Eens et al., 1990; Eens, Pinxten, and Verheyen, 1993). Thus, positive linear relationships between song and numbers of cFOS-labeled cells in BSTm, AH, and VMH likely reflect a role for these regions in the regulation of sexually motivated singing behavior in a breeding context. The extent to which these regions differentially regulate song used in the context of mate attraction versus inter-male aggression within a breeding context should be addressed in future research.
cFOS and song in a non-breeding context
In contrast to BSTm, AH, and VMH, the protein products of cFOS within LS related positively to more broadly socially motivated, non-breeding context, song. No linear relationship between cFOS in LS and sexually motivated song was observed. Septal modulation of singing behavior has been reported in other species and appears to be strongly influenced by social factors (Goodson, 1998; Goodson et al., 1999; Maney, Erwin, and Goode, 2005). Lesions to LS increase singing behavior in the territorial field sparrow but decrease singing behavior in the colonial zebra finch (Goodson et al., 1999). One interpretation of these findings is that LS activity inhibits song in asocial (i.e. territorial) individuals but facilitates song in highly social individuals (i.e. animals living in large social groups). This interpretation is consistent with the present data showing that LS activity was strongly linked to song produced within a non-breeding context when starlings feed and roost large social groups, but not to song within the breeding context, when males possess and defend nest boxes.
It is important to note that cFOS is not expressed by all neurons. Thus the present data do not preclude a role for BSTm, AH, and VMH in the regulation of song produced in a non-breeding context, or LS in breeding context song. Rather, the results presented here suggest that these social behavior network regions differentially regulate sexually motivated versus more broadly socially motivated starling song.
Regulation of aggression within a breeding context
Very little overt aggression (i.e. chasing, displacing, pecking, and attacking) was observed in the males included in this study (behavioral data reported in Heimovics and Riters, 2005). Furthermore, neither breeding condition nor nestbox status appeared to influence the propensity to behave aggressively. Nevertheless, a positive linear relationship between aggression observed in a breeding context and numbers of cFOS-labeled cells was found within VMH, but not other social behavior network regions. VMH has been previously implicated in mediating agonistic encounters in mammals (e.g. Kollack-Walker and Newman, 1995) and these data suggest a role for VMH in the modulation of breeding context aggression in birds.
cFOS labeling and breeding condition
All breeding condition males, independent of whether or not they possessed nest boxes, had significantly higher numbers of cFOS-labeled cells within LSr, LSc.vl. and MS compared to non-breeding condition males. This effect might be due to physiological and/or environmental factors that differentiate the breeding season from the non-breeding season. Non-breeding males have low levels of circulating testosterone (T); breeding males, high levels of T. Non-breeding males were exposed to short days and cool temperatures; breeding males, long days and warm temperatures. Additionally, the effect of breeding season on patterns of cFOS-labeling within these regions might also be due to social factors differing between non-breeding and breeding condition males. During the non-breeding season starlings are found in large mixed-sex flocks, whereas during the breeding season flocks disperse and males compete over a limited number of appropriate nest sites (Eens, 1997). Finally, male starlings sing slightly longer songs during compared to outside a breeding context (Riters et al., 2000), thus differences in temporal and/or spectral qualities of song may also contribute to patterns of cFOS-labeling in LSr, LSc.vl., and MS observed in this study.
cFOS labeling and nest box possession
In contrast, to LSr, LSc.vl. and MS, the patterns of cFOS labeling within BSTm, AH, VMH, and LSc.v. related to nest box status rather than breeding condition. Breeding males with nestboxes had significantly more cFOS-labeled cells within these regions compared to either breeding males without nestboxes or non-breeding males. These effects were not due to differences in T concentrations, as all breeding condition males (those with and without nestboxes) were T treated. Rather, this pattern of neural activation might be due to overall differences in the social behavior of males that have acquired nest boxes versus those who have not. First, breeding condition males with nest boxes were the most highly sexually motivated individuals in this study. They sang the most, were the only birds observed wing-waving, and collected the majority of nest-material (detailed in Heimovics and Riters, 2005). Relative to males without nest boxes, nest box owners can also be considered territorial, defending the area immediate surrounding the nest box opening (Feare, 1984; Kessel, 1957), and to be the most dominant animals within a population (e.g. Sartor and Ball, 2005). Thus, higher numbers of cFOS-labeled cells in BSTm, AH, VMH, and LSc.v of males with nestboxes may relate to territoriality or dominance status. This interpretation is consistent with data in a study of closely related songbirds species in which territorial songbirds had higher immediate early gene responses within BSTm, AH, VMH, and LS after exposure to a same-sex conspecific than gregarious species (Goodson et al., 2005), and in rodents implicating these regions in dominance/subordinance relationships (e.g. Ferris, Axelson, Martin, and Roberge, 1989; Kollack-Walker and Newman, 1995; Kollack-Walker, Watson, and Akil, 1997).
Regional specificity within BSTm and LS
Interestingly, activity in both subregions of BSTm appeared to regulate breeding context song. However, the overall pattern of neural activation in BSTm(d) appeared to be only influenced by nestbox status, whereas in BSTm(v) activity appeared to be influenced by both nestbox status and breeding condition. These results suggest that these subdivisions of BSTm may be functionally distinct. To the best of our knowledge, this is the first time regional specificity within BSTm has been reported and the significance of these findings should be explored with future research. In songbirds, distinct subregions of LS have been identified (Goodson, Evans, and Lindberg, 2004) and previous reports suggest that zones within LS might differentially regulate social behavior (Goodson et al., 2005). In the present study, we observed similar linear relationships between non-breeding context song and cFOS labeling within LSr, LSc.v., and LSc.vl, but found different patterns in these three zones of LS relating to nestbox status and/or breeding condition. Activity within LSr and LSc.v. appeared to be influenced by breeding condition whereas LSc.v. appeared to be influenced by nestbox status. Future research examining the role of LS in mediating starling behavior will help to elucidate the significance of these results.
Social brain regions and the song control system
Data from tract tracing studies point to several routes by which nuclei of the social behavior network might influence activity within the song control system. Based on landmarks, the area presently referred to as AH overlaps with an area referred to by Foster and collaborators (Foster et al., 1997) as the external cellular stratum (SCE) of the lateral hypothalamus which projects to HVC via the dorsomedial thalamus (DMP) and the medial portion of the medial magnocellular nucleus of the anterior nidopallium. In starlings, the POM has been found to connect reciprocally with VTA, GCt, the locus coeruleus (LoC), and each of the regions examined here (Riters and Alger, 2004). VTA, GCt, and LoC all send direct projections to the song control system (Appeltants, Absil, Balthazart, and Ball, 2000; Appeltants, Ball, and Balthazart, 2002). Thus, these are several indirect routs through which social behavior network regions may influence the song control system. Overall, the data presented in this paper lend further support to the idea of a social network of telencephalic, diencephalic, and hypothalamic brain regions working in concert with the song control system to regulate singing behavior in male starlings (Goodson, 2005; Newman, 1999).
Acknowledgments
The data presented in this paper are based upon work supported by grants from NIMH (R01-MH 65645) to LVR and a graduate research fellowship from NSF to SAH. We gratefully acknowledge Jandra Morrow for assistance in starling capture; Kate Skogen, Jeff Alexander, Chris Elliot, John Irwin, and Martin Lund for animal care taking; Gina Bower, Nate Good, Cindi Kelm, Sarah Schlachet and Hollie Wingate for their help with tissue processing; and Billy Feeny for assistance with illustrations.
References
- Appeltants D, Absil P, Balthazart J, Ball GF. Identification of the origin of catecholaminergic inputs to HVc in canaries by retrograde tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Chem Neuroanat. 2000;18(3):117–33. doi: 10.1016/s0891-0618(99)00054-x. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002;13(5):649–53. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- Aste N, Balthazart J, Absil P, Grossmann R, Mulhbauer E, Viglietti-Panzica C, Panzica GC. Anatomical and neurochemical definition of the nucleus of the stria terminalis in Japanese quail (Coturnix japonica) J Comp Neurol. 1998;396(2):141–57. [PubMed] [Google Scholar]
- Ball GF, Balthazart J. Hormonal regulation of brain circuits mediating male sexual behavior in birds. Physiol Behav. 2004;83(2):329–46. doi: 10.1016/j.physbeh.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Ball GF, Wingfield JC. Changes in Plasma-Levels of Luteinizing-Hormone and Sex Steroid-Hormones in Relation to Multiple-Broodedness and Nest-Site Density in Male Starlings. Physiological Zoology. 1987;60(2):191–199. [Google Scholar]
- Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol. 2000;420(2):244–60. [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224(4651):901–3. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Margoliash D, Nordeen KW. An introduction to birdsong and the avian song system. J Neurobiol. 1997;33(5):495–500. [PubMed] [Google Scholar]
- Catchpole C, Slater PJB. Bird song : biological themes and variations. New York NY USA: Cambridge, England: Cambridge University Press; 1995. [Google Scholar]
- Coolen LM, Peters HJ, Veening JG. Distribution of Fos immunoreactivity following mating versus anogenital investigation in the male rat brain. Neuroscience. 1997;77(4):1151–61. doi: 10.1016/s0306-4522(96)00542-8. [DOI] [PubMed] [Google Scholar]
- Davis ES, Marler CA. c-fos Changes following an aggressive encounter in female California mice: a synthesis of behavior, hormone changes and neural activity. Neuroscience. 2004;127(3):611–24. doi: 10.1016/j.neuroscience.2004.05.034. [DOI] [PubMed] [Google Scholar]
- Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. J Biol Rhythms. 2001;16(4):365–80. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- Delville Y, De Vries GJ, Ferris CF. Neural connections of the anterior hypothalamus and agonistic behavior in golden hamsters. Brain Behav Evol. 2000;55(2):53–76. doi: 10.1159/000006642. [DOI] [PubMed] [Google Scholar]
- Edwards N, Kriegsfeld L, Crews D. Neural substrates for sexual and thermoregulatory behavior in the male leopard gecko, Eublepharis macularius. Brain Res. 2004;1029(1):77–83. doi: 10.1016/j.brainres.2004.09.028. [DOI] [PubMed] [Google Scholar]
- Eens M. Understanding the Complex Song of the European Starling: An Integrated Ethological Approach. Advances in the Study of Behavior. 1997;26:255–435. [Google Scholar]
- Eens M, Pinxten R. Extra-Pair Courtship in the Starling Sturnus-Vulgaris. Ibis. 1990;132(4):618–619. [Google Scholar]
- Eens M, Pinxten R. Intersexual Conflicts over Copulations in the European Starling - Evidence for the Female Mate Guarding Hypothesis. Behavioral Ecology and Sociobiology. 1995;36(2):71–81. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. On the Function of Singing and Wing-Waving in the European Starling Sturnus-Vulgaris. Bird Study. 1990;37:48–52. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Function of the Song and Song Repertoire in the European Starling (Sturnus-Vulgaris) - an Aviary Experiment. Behaviour. 1993;125:51–66. [Google Scholar]
- Feare C. The starling. Oxford University Press; Oxford [Oxfordshire]: New York: 1984. [Google Scholar]
- Ferris CF, Axelson JF, Martin AM, Roberge LF. Vasopressin immunoreactivity in the anterior hypothalamus is altered during the establishment of dominant/subordinate relationships between hamsters. Neuroscience. 1989;29(3):675–83. doi: 10.1016/0306-4522(89)90140-1. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Jr, Koppel G, Perry KW, Fuller RW, Delville Y. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J Neurosci. 1997;17(11):4331–40. doi: 10.1523/JNEUROSCI.17-11-04331.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster EF, Mehta RP, Bottjer SW. Axonal connections of the medial magnocellular nucleus of the anterior neostriatum in zebra finches. J Comp Neurol. 1997;382(3):364–81. doi: 10.1002/cne.903820305. [DOI] [PubMed] [Google Scholar]
- Goodson JL. Territorial aggression and dawn song are modulated by septal vasotocin and vasoactive intestinal polypeptide in male field sparrows (Spizella pusilla) Horm Behav. 1998;34(1):67–77. doi: 10.1006/hbeh.1998.1467. [DOI] [PubMed] [Google Scholar]
- Goodson JL. The vertebrate social behavior network: evolutionary themes and variations. Horm Behav. 2005;48(1):11–22. doi: 10.1016/j.yhbeh.2005.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Eibach R, Sakata J, Adkins-Regan E. Effect of septal lesions on male song and aggression in the colonial zebra finch (Taeniopygia guttata) and the territorial field sparrow (Spizella pusilla) Behav Brain Res. 1999;101(1):167–80. [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L. Chemoarchitectonic subdivisions of the songbird septum and a comparative overview of septum chemical anatomy in jawed vertebrates. J Comp Neurol. 2004;473(3):293–314. doi: 10.1002/cne.20061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodson JL, Evans AK, Lindberg L, Allen CD. Neuro-evolutionary patterning of sociality. Proc R Soc B. 2005;272:227–235. doi: 10.1098/rspb.2004.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinner H, Van’t Hof T, Zeman M. Hormonal and behavioral responses of starlings during a confrontation with males or females at nest boxes during the reproductive season. Horm Behav. 2002;42(1):21–31. doi: 10.1006/hbeh.2002.1795. [DOI] [PubMed] [Google Scholar]
- Hausberger M, Richardyris MA, Henry L, Lepage L, Schmidt I. Song Sharing Reflects the Social-Organization in a Captive Group of European Starlings (Sturnus-Vulgaris) Journal of Comparative Psychology. 1995;109(3):222–241. [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65(3):207–24. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing-related neural activity in the songbird forebrain. Nat Neurosci. 1999;2(3):209–11. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: context-dependent gene expression. Neuron. 1998;21(4):775–88. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Jasnow AM, Davis M, Huhman KL. Involvement of central amygdalar and bed nucleus of the stria terminalis corticotropin-releasing factor in behavioral responses to social defeat. Behav Neurosci. 2004;118(5):1052–61. doi: 10.1037/0735-7044.118.5.1052. [DOI] [PubMed] [Google Scholar]
- Kessel B. A study of the breeding biology of the European starling (Sturnus vulgaris L.) in North America. American Midland Naturalist. 1957;58:257–331. [Google Scholar]
- Kollack-Walker S, Newman SW. Mating and agonistic behavior produce different patterns of Fos immunolabeling in the male Syrian hamster brain. Neuroscience. 1995;66(3):721–36. doi: 10.1016/0306-4522(94)00563-k. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Watson SJ, Akil H. Social stress in hamsters: defeat activates specific neurocircuits within the brain. J Neurosci. 1997;17(22):8842–55. doi: 10.1523/JNEUROSCI.17-22-08842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner PN. Handbook of ethological methods. 2. Cambridge University Press; Cambridge: New York: 1996. [Google Scholar]
- Liu WC, Nottebohm F. Variable rate of singing and variable song duration are associated with high immediate early gene expression in two anterior forebrain song nuclei. Proc Natl Acad Sci U S A. 2005;102(30):10724–9. doi: 10.1073/pnas.0504677102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maney DL, Ball GF. Fos-like immunoreactivity in catecholaminergic brain nuclei after territorial behavior in free-living song sparrows. J Neurobiol. 2003;56(2):163–70. doi: 10.1002/neu.10227. [DOI] [PubMed] [Google Scholar]
- Maney DL, Erwin KL, Goode CT. Neuroendocrine correlates of behavioral polymorphism in white-throated sparrows. Horm Behav. 2005;48(2):196–206. doi: 10.1016/j.yhbeh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Functional organization of forebrain pathways for song production and perception. J Neurobiol. 1997;33(5):671–93. doi: 10.1002/(sici)1097-4695(19971105)33:5<671::aid-neu12>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Mountjoy DJ, Lemon RE. Song as an Attractant for Male and Female European Starlings, and the Influence of Song Complexity on Their Response. Behavioral Ecology and Sociobiology. 1991;28(2):97–100. [Google Scholar]
- Newman SW. The medial extended amygdala in male reproductive behavior. A node in the mammalian social behavior network. Ann N Y Acad Sci. 1999;877:242–57. doi: 10.1111/j.1749-6632.1999.tb09271.x. [DOI] [PubMed] [Google Scholar]
- Nordeen KW, Nordeen EJ. Long-term maintenance of song in adult zebra finches is not affected by lesions of a forebrain region involved in song learning. Behav Neural Biol. 1993;59(1):79–82. doi: 10.1016/0163-1047(93)91215-9. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165(4):457–86. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Paredes RG. Medial preoptic area/anterior hypothalamus and sexual motivation. Scand J Psychol. 2003;44(3):203–12. doi: 10.1111/1467-9450.00337. [DOI] [PubMed] [Google Scholar]
- Pfaus JG, Heeb MM. Implications of immediate-early gene induction in the brain following sexual stimulation of female and male rodents. Brain Res Bull. 1997;44(4):397–407. doi: 10.1016/s0361-9230(97)00219-0. [DOI] [PubMed] [Google Scholar]
- Pinxten R, Eens M. Copulation and mate-guarding patterns in polygynous European starlings. Animal Behaviour. 1997;54:45–58. doi: 10.1006/anbe.1996.0432. [DOI] [PubMed] [Google Scholar]
- Powers JB, Newman SW, Bergondy ML. MPOA and BNST lesions in male Syrian hamsters: differential effects on copulatory and chemoinvestigatory behaviors. Behav Brain Res. 1987;23(3):181–95. doi: 10.1016/0166-4328(87)90019-2. [DOI] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tissue Res. 2004;316(1):35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha(2)-noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. Journal of Comparative Neurology. 2002;444(1):63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38(4):250–61. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Teague DP, Schroeder MB, Cummings SE. Vocal production in different social contexts relates to variation in immediate early gene immunoreactivity within and outside of the song control system. Behavioural Brain Research. 2004;155(2):307–318. doi: 10.1016/j.bbr.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Sartor JJ, Ball GF. Social suppression of song is associated with a reduction in volume of a song-control nucleus in European starlings (Sturnus vulgaris) Behav Neurosci. 2005;119(1):233–44. doi: 10.1037/0735-7044.119.1.233. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53(1):51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Summers RW, Westlake GE, Feare CJ. Differences in the Ages, Sexes and Physical Condition of Starlings Sturnus-Vulgaris at the Center and Periphery of Roosts. Ibis. 1987;129(1):96–102. [Google Scholar]
- Wild JM. Neural pathways for the control of birdsong production. J Neurobiol. 1997;33(5):653–70. doi: 10.1002/(sici)1097-4695(19971105)33:5<653::aid-neu11>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]